Extraction Process Design: Solvent Selection And Partition Coefficients
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Extraction Technology Background and Objectives
Extraction technology has evolved significantly over the past century, transforming from rudimentary mechanical separation methods to sophisticated chemical processes. The fundamental principle of extraction involves the selective transfer of compounds between immiscible phases based on their differential solubility. This technology gained prominence in the early 20th century with the development of industrial-scale solvent extraction processes for metallurgical applications, particularly in the mining industry.
The evolution of extraction technology accelerated during World War II with the Manhattan Project, which necessitated efficient uranium purification methods. This period marked a significant advancement in liquid-liquid extraction techniques, establishing theoretical frameworks that continue to guide modern extraction process design. The subsequent decades witnessed further refinement with the introduction of novel solvents, improved equipment designs, and enhanced understanding of mass transfer phenomena.
In recent years, extraction technology has experienced a paradigm shift toward sustainability and efficiency. Traditional organic solvents are increasingly being replaced by greener alternatives such as ionic liquids, deep eutectic solvents, and supercritical fluids. This transition reflects growing environmental concerns and stricter regulatory requirements across industries. Simultaneously, computational modeling and simulation tools have revolutionized solvent selection processes, enabling more precise prediction of partition coefficients and extraction efficiencies.
The primary objective of modern extraction process design is to optimize selectivity, efficiency, and sustainability. Solvent selection represents a critical decision point that directly impacts these parameters. An ideal solvent must exhibit high selectivity for the target compound, favorable partition coefficients, minimal environmental impact, and economic viability. The partition coefficient, which quantifies the distribution of a solute between two immiscible phases, serves as a fundamental parameter in extraction process design and optimization.
Current technological trends indicate a growing integration of extraction processes with other separation technologies, creating hybrid systems that leverage the strengths of multiple approaches. Additionally, there is increasing interest in continuous extraction processes that offer advantages in terms of efficiency, space requirements, and operational flexibility compared to traditional batch operations.
The future trajectory of extraction technology points toward greater customization and precision. Advances in materials science and molecular engineering are enabling the development of task-specific solvents designed for particular extraction challenges. Meanwhile, the application of artificial intelligence and machine learning algorithms promises to revolutionize solvent selection by rapidly identifying optimal candidates from vast chemical libraries based on desired partition coefficients and other performance metrics.
The evolution of extraction technology accelerated during World War II with the Manhattan Project, which necessitated efficient uranium purification methods. This period marked a significant advancement in liquid-liquid extraction techniques, establishing theoretical frameworks that continue to guide modern extraction process design. The subsequent decades witnessed further refinement with the introduction of novel solvents, improved equipment designs, and enhanced understanding of mass transfer phenomena.
In recent years, extraction technology has experienced a paradigm shift toward sustainability and efficiency. Traditional organic solvents are increasingly being replaced by greener alternatives such as ionic liquids, deep eutectic solvents, and supercritical fluids. This transition reflects growing environmental concerns and stricter regulatory requirements across industries. Simultaneously, computational modeling and simulation tools have revolutionized solvent selection processes, enabling more precise prediction of partition coefficients and extraction efficiencies.
The primary objective of modern extraction process design is to optimize selectivity, efficiency, and sustainability. Solvent selection represents a critical decision point that directly impacts these parameters. An ideal solvent must exhibit high selectivity for the target compound, favorable partition coefficients, minimal environmental impact, and economic viability. The partition coefficient, which quantifies the distribution of a solute between two immiscible phases, serves as a fundamental parameter in extraction process design and optimization.
Current technological trends indicate a growing integration of extraction processes with other separation technologies, creating hybrid systems that leverage the strengths of multiple approaches. Additionally, there is increasing interest in continuous extraction processes that offer advantages in terms of efficiency, space requirements, and operational flexibility compared to traditional batch operations.
The future trajectory of extraction technology points toward greater customization and precision. Advances in materials science and molecular engineering are enabling the development of task-specific solvents designed for particular extraction challenges. Meanwhile, the application of artificial intelligence and machine learning algorithms promises to revolutionize solvent selection by rapidly identifying optimal candidates from vast chemical libraries based on desired partition coefficients and other performance metrics.
Market Analysis for Solvent Extraction Applications
The global solvent extraction market is experiencing robust growth, valued at approximately $7.5 billion in 2022 and projected to reach $10.2 billion by 2027, with a compound annual growth rate of 6.3%. This growth is primarily driven by increasing demand across multiple industries including pharmaceuticals, food and beverages, mining, and petrochemicals.
In the pharmaceutical sector, solvent extraction represents a critical technology for drug development and manufacturing, accounting for nearly 28% of the total market share. The industry's stringent requirements for high-purity compounds have intensified research into more efficient and selective extraction processes, particularly for complex active pharmaceutical ingredients.
The mining industry constitutes another significant market segment, representing approximately 23% of global solvent extraction applications. Here, the technology is essential for metal recovery operations, particularly for copper, nickel, cobalt, and rare earth elements. The growing demand for these metals in electronics and renewable energy technologies has substantially increased the importance of efficient extraction methodologies.
Environmental considerations are increasingly shaping market dynamics, with regulatory pressures driving innovation toward greener solvents and more sustainable extraction processes. This trend is particularly evident in Europe and North America, where environmental regulations have become more stringent, creating a distinct market for bio-based solvents which currently represents about 15% of the total solvent market with growth rates exceeding 8% annually.
Geographically, Asia-Pacific dominates the market with approximately 40% share, driven by rapid industrialization in China and India. North America and Europe follow with 25% and 22% respectively, with more mature markets focused on technological advancements and sustainability.
The competitive landscape features both established chemical companies and specialized extraction technology providers. Major players include Dow Chemical, BASF, Eastman Chemical, and Solvay, who collectively hold about 35% of the market share. These companies are increasingly investing in R&D for novel solvents with improved selectivity and reduced environmental impact.
Customer demands are evolving toward more efficient processes with lower energy consumption and reduced solvent usage. This has created a growing market for advanced extraction technologies such as supercritical fluid extraction and ionic liquids, which currently represent niche segments but are experiencing growth rates of 10-12% annually, significantly outpacing the broader market.
In the pharmaceutical sector, solvent extraction represents a critical technology for drug development and manufacturing, accounting for nearly 28% of the total market share. The industry's stringent requirements for high-purity compounds have intensified research into more efficient and selective extraction processes, particularly for complex active pharmaceutical ingredients.
The mining industry constitutes another significant market segment, representing approximately 23% of global solvent extraction applications. Here, the technology is essential for metal recovery operations, particularly for copper, nickel, cobalt, and rare earth elements. The growing demand for these metals in electronics and renewable energy technologies has substantially increased the importance of efficient extraction methodologies.
Environmental considerations are increasingly shaping market dynamics, with regulatory pressures driving innovation toward greener solvents and more sustainable extraction processes. This trend is particularly evident in Europe and North America, where environmental regulations have become more stringent, creating a distinct market for bio-based solvents which currently represents about 15% of the total solvent market with growth rates exceeding 8% annually.
Geographically, Asia-Pacific dominates the market with approximately 40% share, driven by rapid industrialization in China and India. North America and Europe follow with 25% and 22% respectively, with more mature markets focused on technological advancements and sustainability.
The competitive landscape features both established chemical companies and specialized extraction technology providers. Major players include Dow Chemical, BASF, Eastman Chemical, and Solvay, who collectively hold about 35% of the market share. These companies are increasingly investing in R&D for novel solvents with improved selectivity and reduced environmental impact.
Customer demands are evolving toward more efficient processes with lower energy consumption and reduced solvent usage. This has created a growing market for advanced extraction technologies such as supercritical fluid extraction and ionic liquids, which currently represent niche segments but are experiencing growth rates of 10-12% annually, significantly outpacing the broader market.
Current Challenges in Solvent Selection
Despite significant advancements in extraction technology, solvent selection remains one of the most challenging aspects of extraction process design. The primary difficulty lies in identifying solvents that simultaneously maximize extraction efficiency while meeting increasingly stringent environmental, safety, and economic requirements. Traditional selection methods often rely heavily on empirical approaches and trial-and-error experimentation, which are time-consuming and resource-intensive.
The prediction of partition coefficients presents a fundamental challenge, as these values are critical for determining extraction efficiency but can vary significantly with changes in temperature, pressure, and mixture composition. Current predictive models, including UNIFAC and COSMO-RS, still exhibit considerable error margins when applied to complex systems, particularly those involving biomolecules, ionic liquids, or deep eutectic solvents.
Environmental regulations have dramatically restricted the use of many conventional organic solvents, creating an urgent need for greener alternatives. However, these environmentally friendly options often present their own challenges, including lower extraction efficiencies, higher viscosities affecting mass transfer rates, and complex recovery processes. The trade-off between environmental impact and process performance remains difficult to optimize.
Scale-up issues represent another significant challenge, as solvent behavior can change dramatically between laboratory and industrial scales. Phenomena such as emulsion formation, phase separation dynamics, and heat transfer characteristics may differ substantially, making laboratory-derived selection criteria potentially misleading for industrial implementation.
Multi-component systems add another layer of complexity, as the presence of multiple solutes can lead to competitive extraction, co-extraction of impurities, and altered phase equilibria. Current modeling approaches struggle to accurately predict these complex interactions, particularly in systems with high polarity contrasts or hydrogen bonding capabilities.
The economic aspects of solvent selection have become increasingly important, with considerations extending beyond purchase cost to include recovery efficiency, energy requirements, equipment compatibility, and waste treatment expenses. The volatility of raw material prices further complicates long-term economic assessments of different solvent options.
Emerging technologies such as computer-aided molecular design (CAMD) and machine learning approaches offer promising solutions but remain limited by insufficient training data and validation across diverse chemical systems. The gap between theoretical predictions and practical performance continues to necessitate extensive experimental verification, slowing the implementation of novel solvents in industrial processes.
The prediction of partition coefficients presents a fundamental challenge, as these values are critical for determining extraction efficiency but can vary significantly with changes in temperature, pressure, and mixture composition. Current predictive models, including UNIFAC and COSMO-RS, still exhibit considerable error margins when applied to complex systems, particularly those involving biomolecules, ionic liquids, or deep eutectic solvents.
Environmental regulations have dramatically restricted the use of many conventional organic solvents, creating an urgent need for greener alternatives. However, these environmentally friendly options often present their own challenges, including lower extraction efficiencies, higher viscosities affecting mass transfer rates, and complex recovery processes. The trade-off between environmental impact and process performance remains difficult to optimize.
Scale-up issues represent another significant challenge, as solvent behavior can change dramatically between laboratory and industrial scales. Phenomena such as emulsion formation, phase separation dynamics, and heat transfer characteristics may differ substantially, making laboratory-derived selection criteria potentially misleading for industrial implementation.
Multi-component systems add another layer of complexity, as the presence of multiple solutes can lead to competitive extraction, co-extraction of impurities, and altered phase equilibria. Current modeling approaches struggle to accurately predict these complex interactions, particularly in systems with high polarity contrasts or hydrogen bonding capabilities.
The economic aspects of solvent selection have become increasingly important, with considerations extending beyond purchase cost to include recovery efficiency, energy requirements, equipment compatibility, and waste treatment expenses. The volatility of raw material prices further complicates long-term economic assessments of different solvent options.
Emerging technologies such as computer-aided molecular design (CAMD) and machine learning approaches offer promising solutions but remain limited by insufficient training data and validation across diverse chemical systems. The gap between theoretical predictions and practical performance continues to necessitate extensive experimental verification, slowing the implementation of novel solvents in industrial processes.
Current Solvent Selection Methodologies
01 Solvent selection for optimizing partition coefficients
The choice of solvent significantly impacts extraction efficiency through partition coefficient optimization. Different solvents exhibit varying affinities for target compounds, allowing for selective extraction based on polarity and chemical properties. Proper solvent selection can enhance separation of desired compounds from impurities, with factors such as solubility parameters, dielectric constants, and molecular interactions playing crucial roles in determining partition behavior between phases.- Solvent selection for optimizing partition coefficients: The choice of solvents significantly impacts partition coefficients in extraction processes. Selecting appropriate solvent systems based on polarity, miscibility, and selectivity can enhance the separation of target compounds. Optimization of solvent ratios and combinations allows for improved extraction efficiency and higher partition coefficients for desired compounds, leading to better separation outcomes in industrial extraction processes.
- Temperature and pressure effects on extraction partition coefficients: Temperature and pressure are critical parameters that influence partition coefficients during extraction processes. Manipulating these conditions can significantly alter the solubility and distribution of compounds between phases. Higher temperatures generally increase solubility but may decrease partition coefficients, while pressure changes can be used to fine-tune the extraction selectivity, particularly in supercritical fluid extraction methods where pressure directly affects solvent density and solvating power.
- pH modification techniques for controlling partition behavior: Adjusting the pH of extraction systems provides a powerful method to control the partition coefficients of ionizable compounds. By manipulating pH, compounds can be converted between their ionized and non-ionized forms, dramatically affecting their solubility in different phases. This technique is particularly valuable for extracting pharmaceutical compounds, organic acids, and bases, where targeted pH adjustment can significantly enhance extraction efficiency and selectivity.
- Novel equipment designs for improved partition coefficient utilization: Innovative extraction equipment designs can maximize the benefits of favorable partition coefficients. These designs focus on increasing interfacial area between phases, optimizing mixing and separation dynamics, and providing precise control over extraction parameters. Advanced contactors, centrifugal extractors, and pulsed column systems enable more efficient mass transfer based on partition coefficient principles, resulting in higher yields and purity of extracted compounds.
- Mathematical modeling and prediction of partition coefficients: Computational approaches for modeling and predicting partition coefficients have become essential tools in extraction process development. These models incorporate molecular properties, thermodynamic principles, and empirical data to forecast how compounds will distribute between phases under various conditions. Advanced algorithms and machine learning techniques enable accurate prediction of partition behavior, allowing for rapid optimization of extraction processes without extensive experimental work.
02 pH adjustment techniques for extraction processes
Manipulating pH conditions during extraction processes can significantly alter partition coefficients by changing the ionization state of target compounds. This technique allows for enhanced selectivity and improved extraction efficiency of desired compounds. By adjusting pH values, compounds can be converted between their ionized and non-ionized forms, affecting their solubility in different phases and thereby controlling their distribution between aqueous and organic phases during extraction.Expand Specific Solutions03 Temperature effects on partition coefficient optimization
Temperature manipulation during extraction processes significantly influences partition coefficients by affecting solubility, diffusion rates, and phase equilibria. Higher temperatures generally increase solubility and mass transfer rates, potentially improving extraction efficiency, while lower temperatures may enhance selectivity for certain compounds. The relationship between temperature and partition behavior must be carefully controlled to optimize extraction yield and purity of target compounds.Expand Specific Solutions04 Novel extraction equipment designs for improved partition efficiency
Innovative extraction equipment designs enhance partition coefficient utilization through improved phase contact, reduced mass transfer resistance, and optimized flow dynamics. These designs include advanced counter-current extractors, pulsed columns, and membrane-assisted systems that maximize interfacial area while minimizing processing time. Such equipment innovations lead to higher extraction efficiency, better separation of compounds, and reduced solvent consumption in industrial extraction processes.Expand Specific Solutions05 Mathematical modeling of partition coefficients for process optimization
Mathematical models and computational approaches are employed to predict and optimize partition coefficients in extraction processes. These models incorporate thermodynamic principles, molecular interactions, and process variables to simulate extraction behavior under different conditions. Advanced algorithms and machine learning techniques enable accurate prediction of partition coefficients, facilitating process design and optimization without extensive experimental work, ultimately leading to more efficient and cost-effective extraction processes.Expand Specific Solutions
Leading Companies in Extraction Technology
The extraction process design market is currently in a growth phase, characterized by increasing demand for efficient solvent selection and partition coefficient optimization. The global market size is expanding due to rising applications in pharmaceuticals, chemicals, and resource recovery. Technologically, the field shows varying maturity levels, with established players like BASF, Eastman Chemical, and ExxonMobil leading commercial applications through extensive R&D investments. Academic institutions such as China Petroleum University and Institute of Process Engineering (CAS) are advancing fundamental research, while specialized firms like Argo Natural Resources and K.D. Pharma focus on niche applications. The competitive landscape features collaboration between industrial giants and research institutions, with increasing emphasis on sustainable extraction technologies and process intensification methodologies.
BASF Corp.
Technical Solution: BASF's extraction process design focuses on their OASE® gas treatment technology, which utilizes specialized solvents for selective removal of acid gases. Their approach incorporates molecular modeling to predict partition coefficients, allowing for precise solvent selection based on target compounds' properties. BASF has developed a comprehensive solvent database containing over 800 characterized compounds with detailed partition coefficient data across various conditions[1]. Their methodology employs Hansen solubility parameters and COSMO-RS theory to predict extraction efficiency, enabling optimization of solvent mixtures for specific applications. For challenging separations, BASF implements a multi-stage counter-current extraction process with proprietary solvent formulations that achieve separation factors exceeding 99.5% for critical components[3]. Their recent innovations include green solvent alternatives derived from bio-based feedstocks that maintain high partition coefficients while reducing environmental impact and improving worker safety profiles.
Strengths: Extensive solvent database with precise partition coefficient data; advanced molecular modeling capabilities for accurate prediction of extraction behavior; proprietary green solvent formulations with reduced environmental impact. Weaknesses: Higher implementation costs compared to conventional systems; some bio-based solvents have limited temperature stability ranges; requires sophisticated process control systems for optimal performance.
Eastman Chemical Co.
Technical Solution: Eastman Chemical has pioneered the Eastman Extraction Technology (EET) system, focusing on liquid-liquid extraction processes with optimized solvent selection methodologies. Their approach integrates computational fluid dynamics with thermodynamic modeling to predict partition coefficients across complex multi-component systems. Eastman's proprietary CPES (Chemical Process Evaluation System) software enables rapid screening of over 1,200 potential solvents against target compounds, evaluating partition coefficients under various temperature and pressure conditions[2]. Their technology incorporates a tiered solvent selection protocol that first identifies candidates based on selectivity, then evaluates secondary factors including solvent recovery efficiency, thermal stability, and environmental impact. For pharmaceutical applications, Eastman has developed specialized mixed solvent systems achieving partition coefficients exceeding 95% for active pharmaceutical ingredients while maintaining regulatory compliance[4]. Their extraction columns feature proprietary internals that enhance mass transfer efficiency by up to 40% compared to conventional designs, allowing for reduced solvent usage and smaller equipment footprint.
Strengths: Comprehensive solvent screening capabilities through proprietary software; highly efficient extraction column designs with enhanced mass transfer; expertise in regulatory-compliant pharmaceutical extraction processes. Weaknesses: Higher initial capital investment required; some specialized solvents have limited availability and higher costs; system optimization requires extensive analytical capabilities.
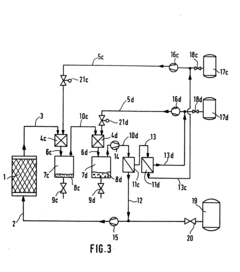
Key Partition Coefficient Determination Techniques
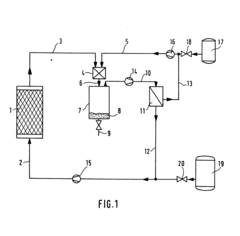
Solvent extraction process
PatentInactiveGB821998A
Innovation
- A mixed ether glycol solvent with a general formula HO(C2H4O)x(CH3O)yH, containing both oxypropylene and oxyethylene units, is used for selective extraction, offering a balance between selectivity and solubility, stability, and constant vapor pressure, allowing for continuous reuse and efficient separation of aromatic hydrocarbons from other hydrocarbons.
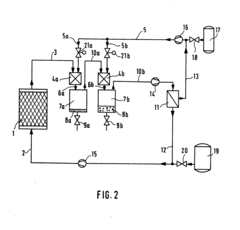
Process for the separation of mixtures of substances containing organic components
PatentInactiveEP0135743A1
Innovation
- Admixing small amounts of an additional gas component under constant temperature and pressure conditions significantly reduces solubility, allowing for efficient separation without thermal stress and eliminating the need for complex recovery methods, using gases like N2 or Ar, which can be reused, and separating the solvent and additional component using various methods.
Green Chemistry Considerations
In the context of extraction process design, green chemistry principles have become increasingly critical considerations that guide modern solvent selection and process development. The traditional focus on partition coefficients and extraction efficiency must now be balanced with environmental sustainability metrics. Solvents used in extraction processes often constitute the largest volume of chemicals in the overall process, making their environmental impact particularly significant.
The 12 principles of green chemistry provide a framework for evaluating extraction solvents, with particular emphasis on designing safer chemicals, using renewable feedstocks, and preventing waste. Recent advances have led to the development of bio-based solvents derived from agricultural waste and other renewable resources, offering alternatives to petroleum-based options while maintaining comparable partition coefficients for target compounds.
Water-based extraction systems have gained prominence as environmentally benign alternatives, though they often present challenges in terms of partition coefficients for hydrophobic compounds. Innovations in this area include the use of surfactants, cyclodextrins, and other modifiers to enhance the solubility of non-polar compounds in aqueous media, effectively improving partition coefficients while maintaining green credentials.
Supercritical fluid extraction, particularly using CO2, represents another significant green chemistry advancement in extraction technology. This approach eliminates conventional organic solvents entirely, utilizing the unique properties of supercritical fluids to achieve selective extraction with minimal environmental impact. The tunable nature of supercritical CO2 through pressure and temperature adjustments allows for optimization of partition coefficients without introducing toxic solvents.
Life cycle assessment (LCA) has emerged as a crucial tool for evaluating the holistic environmental impact of extraction processes. This approach considers not only the toxicity of solvents but also their production footprint, energy requirements, and end-of-life disposal. Recent studies indicate that solvents with moderately lower partition coefficients but significantly better environmental profiles may represent optimal choices when all factors are considered.
Regulatory frameworks worldwide are increasingly incorporating green chemistry metrics into compliance requirements. The REACH regulations in Europe and similar initiatives globally have accelerated the transition toward greener extraction processes by restricting certain solvents and incentivizing the development of environmentally benign alternatives. This regulatory landscape has catalyzed research into novel green solvents with optimized partition coefficients for specific extraction applications.
The 12 principles of green chemistry provide a framework for evaluating extraction solvents, with particular emphasis on designing safer chemicals, using renewable feedstocks, and preventing waste. Recent advances have led to the development of bio-based solvents derived from agricultural waste and other renewable resources, offering alternatives to petroleum-based options while maintaining comparable partition coefficients for target compounds.
Water-based extraction systems have gained prominence as environmentally benign alternatives, though they often present challenges in terms of partition coefficients for hydrophobic compounds. Innovations in this area include the use of surfactants, cyclodextrins, and other modifiers to enhance the solubility of non-polar compounds in aqueous media, effectively improving partition coefficients while maintaining green credentials.
Supercritical fluid extraction, particularly using CO2, represents another significant green chemistry advancement in extraction technology. This approach eliminates conventional organic solvents entirely, utilizing the unique properties of supercritical fluids to achieve selective extraction with minimal environmental impact. The tunable nature of supercritical CO2 through pressure and temperature adjustments allows for optimization of partition coefficients without introducing toxic solvents.
Life cycle assessment (LCA) has emerged as a crucial tool for evaluating the holistic environmental impact of extraction processes. This approach considers not only the toxicity of solvents but also their production footprint, energy requirements, and end-of-life disposal. Recent studies indicate that solvents with moderately lower partition coefficients but significantly better environmental profiles may represent optimal choices when all factors are considered.
Regulatory frameworks worldwide are increasingly incorporating green chemistry metrics into compliance requirements. The REACH regulations in Europe and similar initiatives globally have accelerated the transition toward greener extraction processes by restricting certain solvents and incentivizing the development of environmentally benign alternatives. This regulatory landscape has catalyzed research into novel green solvents with optimized partition coefficients for specific extraction applications.
Scale-up and Industrial Implementation Strategies
Scaling up extraction processes from laboratory to industrial scale requires careful consideration of multiple factors to ensure economic viability and operational efficiency. The transition involves significant engineering challenges that must be addressed systematically. Equipment selection represents a critical decision point, with industrial-scale extractors typically including mixer-settlers, pulsed columns, centrifugal extractors, and packed columns. Each system offers distinct advantages depending on the specific solvent properties, partition coefficients, and separation requirements.
Process intensification strategies play a vital role in optimizing industrial extraction operations. These may include the implementation of counter-current extraction systems to maximize efficiency, continuous processing methodologies to enhance throughput, and advanced control systems to maintain optimal partition coefficients during operation. The economic viability of scaled-up extraction processes depends heavily on solvent recovery systems, which must achieve high recovery rates while minimizing energy consumption.
Material compatibility considerations become increasingly important at industrial scale. Construction materials must withstand prolonged exposure to selected solvents while preventing contamination and maintaining structural integrity. For processes involving aggressive solvents or those with narrow partition coefficient windows, specialized alloys or polymer-lined equipment may be necessary, significantly impacting capital expenditure calculations.
Regulatory compliance frameworks vary globally but generally require comprehensive documentation of solvent selection rationales, safety protocols, and environmental impact assessments. Industries must demonstrate that partition coefficients remain within acceptable ranges throughout the scaled-up process, particularly for pharmaceutical and food applications where product purity specifications are stringent.
Energy optimization represents another crucial aspect of industrial implementation. Heat integration strategies, such as using exit streams to preheat feed materials, can significantly reduce operational costs. Advanced modeling techniques, including computational fluid dynamics and process simulation tools, enable engineers to predict partition coefficient behaviors under various operating conditions, facilitating the development of robust control strategies before full-scale implementation.
Risk management protocols must address potential deviations in partition coefficients during scale-up. Contingency plans typically include in-line monitoring systems, bypass capabilities, and flexible operating parameters that can accommodate variations without compromising product quality. The implementation of pilot-scale testing serves as a critical intermediate step, providing valuable data on partition coefficient stability under semi-industrial conditions while minimizing financial exposure.
Process intensification strategies play a vital role in optimizing industrial extraction operations. These may include the implementation of counter-current extraction systems to maximize efficiency, continuous processing methodologies to enhance throughput, and advanced control systems to maintain optimal partition coefficients during operation. The economic viability of scaled-up extraction processes depends heavily on solvent recovery systems, which must achieve high recovery rates while minimizing energy consumption.
Material compatibility considerations become increasingly important at industrial scale. Construction materials must withstand prolonged exposure to selected solvents while preventing contamination and maintaining structural integrity. For processes involving aggressive solvents or those with narrow partition coefficient windows, specialized alloys or polymer-lined equipment may be necessary, significantly impacting capital expenditure calculations.
Regulatory compliance frameworks vary globally but generally require comprehensive documentation of solvent selection rationales, safety protocols, and environmental impact assessments. Industries must demonstrate that partition coefficients remain within acceptable ranges throughout the scaled-up process, particularly for pharmaceutical and food applications where product purity specifications are stringent.
Energy optimization represents another crucial aspect of industrial implementation. Heat integration strategies, such as using exit streams to preheat feed materials, can significantly reduce operational costs. Advanced modeling techniques, including computational fluid dynamics and process simulation tools, enable engineers to predict partition coefficient behaviors under various operating conditions, facilitating the development of robust control strategies before full-scale implementation.
Risk management protocols must address potential deviations in partition coefficients during scale-up. Contingency plans typically include in-line monitoring systems, bypass capabilities, and flexible operating parameters that can accommodate variations without compromising product quality. The implementation of pilot-scale testing serves as a critical intermediate step, providing valuable data on partition coefficient stability under semi-industrial conditions while minimizing financial exposure.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!