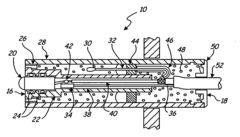
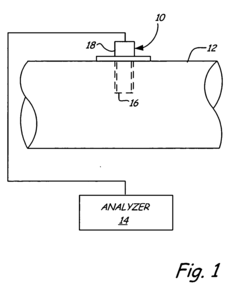
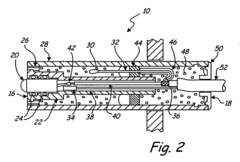
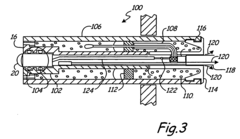
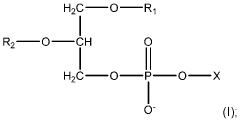
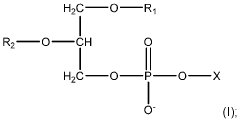
Process Analytical Technology (PAT) For Purification: Sensors And Control Loops
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PAT Evolution and Purification Objectives
Process Analytical Technology (PAT) emerged in the early 2000s as a regulatory framework initiated by the FDA to encourage pharmaceutical manufacturers to implement innovative systems for process monitoring and control. The evolution of PAT has been characterized by a shift from traditional quality-by-testing approaches to quality-by-design methodologies, emphasizing real-time monitoring and process understanding. Initially focused on primary manufacturing processes, PAT applications have gradually expanded into downstream purification operations, which represent critical steps in biopharmaceutical production.
The purification phase presents unique challenges for PAT implementation due to complex fluid dynamics, multiple separation mechanisms, and the sensitivity of biological molecules. Early PAT tools in purification were limited to offline analytics with significant time delays, but technological advancements have enabled the development of sophisticated inline and online monitoring capabilities that provide immediate feedback on critical process parameters.
Recent technological breakthroughs in sensor miniaturization, multivariate data analysis, and machine learning algorithms have accelerated PAT adoption in purification processes. These innovations allow for more precise monitoring of product attributes and impurity profiles during chromatography, filtration, and other separation techniques. The integration of spectroscopic methods such as UV-Vis, Raman, and FTIR has been particularly transformative, enabling non-destructive, real-time analysis of complex biological mixtures.
The primary objectives of implementing PAT in purification processes include enhancing process understanding, reducing batch-to-batch variability, minimizing product loss, and enabling continuous processing. By establishing robust control strategies based on real-time data, manufacturers aim to optimize yield while maintaining consistent product quality. Additionally, PAT implementation supports regulatory compliance by providing comprehensive documentation of process conditions and demonstrating process control.
Looking forward, the evolution of PAT in purification is moving toward fully integrated systems that combine multiple sensor technologies with advanced control algorithms. The ultimate goal is to achieve autonomous purification operations where process parameters are automatically adjusted based on real-time measurements of critical quality attributes. This vision aligns with broader industry trends toward digitalization, process intensification, and continuous manufacturing in biopharmaceutical production.
The convergence of PAT with emerging technologies such as artificial intelligence and digital twins promises to further revolutionize purification processes by enabling predictive control strategies and real-time decision support systems. These developments represent the next frontier in ensuring consistent product quality while maximizing operational efficiency in biopharmaceutical manufacturing.
The purification phase presents unique challenges for PAT implementation due to complex fluid dynamics, multiple separation mechanisms, and the sensitivity of biological molecules. Early PAT tools in purification were limited to offline analytics with significant time delays, but technological advancements have enabled the development of sophisticated inline and online monitoring capabilities that provide immediate feedback on critical process parameters.
Recent technological breakthroughs in sensor miniaturization, multivariate data analysis, and machine learning algorithms have accelerated PAT adoption in purification processes. These innovations allow for more precise monitoring of product attributes and impurity profiles during chromatography, filtration, and other separation techniques. The integration of spectroscopic methods such as UV-Vis, Raman, and FTIR has been particularly transformative, enabling non-destructive, real-time analysis of complex biological mixtures.
The primary objectives of implementing PAT in purification processes include enhancing process understanding, reducing batch-to-batch variability, minimizing product loss, and enabling continuous processing. By establishing robust control strategies based on real-time data, manufacturers aim to optimize yield while maintaining consistent product quality. Additionally, PAT implementation supports regulatory compliance by providing comprehensive documentation of process conditions and demonstrating process control.
Looking forward, the evolution of PAT in purification is moving toward fully integrated systems that combine multiple sensor technologies with advanced control algorithms. The ultimate goal is to achieve autonomous purification operations where process parameters are automatically adjusted based on real-time measurements of critical quality attributes. This vision aligns with broader industry trends toward digitalization, process intensification, and continuous manufacturing in biopharmaceutical production.
The convergence of PAT with emerging technologies such as artificial intelligence and digital twins promises to further revolutionize purification processes by enabling predictive control strategies and real-time decision support systems. These developments represent the next frontier in ensuring consistent product quality while maximizing operational efficiency in biopharmaceutical manufacturing.
Market Demand for Real-time Purification Analytics
The biopharmaceutical industry is experiencing a significant shift towards more efficient and cost-effective manufacturing processes, driving substantial demand for real-time purification analytics. The global market for Process Analytical Technology (PAT) in biopharmaceutical purification is projected to grow at a compound annual growth rate of 12.3% through 2028, reaching approximately $5.7 billion. This growth is primarily fueled by increasing regulatory pressures, rising manufacturing costs, and the need for consistent product quality.
Regulatory agencies, particularly the FDA and EMA, have been actively encouraging the implementation of PAT frameworks to ensure product quality and process consistency. Their Quality by Design (QbD) initiative specifically emphasizes the importance of real-time monitoring and control during manufacturing processes, creating a regulatory environment that favors PAT adoption.
Biopharmaceutical manufacturers are facing intense pressure to reduce production costs while maintaining stringent quality standards. Real-time purification analytics offers a compelling value proposition by potentially reducing batch failures, minimizing product loss, optimizing resource utilization, and shortening time-to-market. Industry analyses suggest that effective PAT implementation can reduce manufacturing costs by 15-25% through these efficiency improvements.
Contract Manufacturing Organizations (CMOs) and Contract Development and Manufacturing Organizations (CDMOs) represent a particularly fast-growing segment of PAT adopters, as they seek competitive advantages through technological differentiation. These organizations require flexible PAT solutions that can be adapted to multiple product types and manufacturing processes.
The trend toward continuous manufacturing in biopharmaceutical production is creating additional demand for sophisticated real-time analytics. Unlike traditional batch processing, continuous manufacturing requires constant monitoring and control, making PAT systems essential rather than optional.
End-users are increasingly demanding integrated solutions that combine sensors, data analytics, and control systems rather than standalone components. This reflects a market shift toward comprehensive PAT platforms that can provide actionable insights and automated responses based on real-time data.
Geographically, North America currently leads PAT adoption, accounting for approximately 40% of the market, followed by Europe at 35% and Asia-Pacific at 20%. However, the Asia-Pacific region is expected to show the highest growth rate in the coming years due to the rapid expansion of biopharmaceutical manufacturing capacity in countries like China, India, and Singapore.
Regulatory agencies, particularly the FDA and EMA, have been actively encouraging the implementation of PAT frameworks to ensure product quality and process consistency. Their Quality by Design (QbD) initiative specifically emphasizes the importance of real-time monitoring and control during manufacturing processes, creating a regulatory environment that favors PAT adoption.
Biopharmaceutical manufacturers are facing intense pressure to reduce production costs while maintaining stringent quality standards. Real-time purification analytics offers a compelling value proposition by potentially reducing batch failures, minimizing product loss, optimizing resource utilization, and shortening time-to-market. Industry analyses suggest that effective PAT implementation can reduce manufacturing costs by 15-25% through these efficiency improvements.
Contract Manufacturing Organizations (CMOs) and Contract Development and Manufacturing Organizations (CDMOs) represent a particularly fast-growing segment of PAT adopters, as they seek competitive advantages through technological differentiation. These organizations require flexible PAT solutions that can be adapted to multiple product types and manufacturing processes.
The trend toward continuous manufacturing in biopharmaceutical production is creating additional demand for sophisticated real-time analytics. Unlike traditional batch processing, continuous manufacturing requires constant monitoring and control, making PAT systems essential rather than optional.
End-users are increasingly demanding integrated solutions that combine sensors, data analytics, and control systems rather than standalone components. This reflects a market shift toward comprehensive PAT platforms that can provide actionable insights and automated responses based on real-time data.
Geographically, North America currently leads PAT adoption, accounting for approximately 40% of the market, followed by Europe at 35% and Asia-Pacific at 20%. However, the Asia-Pacific region is expected to show the highest growth rate in the coming years due to the rapid expansion of biopharmaceutical manufacturing capacity in countries like China, India, and Singapore.
Current PAT Sensor Technologies and Limitations
Process Analytical Technology (PAT) in purification processes currently employs a range of sensor technologies, each with specific capabilities and limitations. Spectroscopic sensors, including Near-Infrared (NIR), Raman, and UV-Vis spectroscopy, offer real-time, non-destructive monitoring of chemical compositions and concentrations. While these technologies provide excellent molecular specificity, they often struggle with complex mixtures and require sophisticated chemometric models for accurate interpretation. Additionally, their performance can be compromised by sample turbidity, bubbles, or particulate matter in process streams.
Electrochemical sensors, such as pH, conductivity, and dissolved oxygen probes, are widely deployed for their robustness and relative simplicity. However, these sensors frequently suffer from drift issues during extended operations and may require regular calibration, interrupting continuous monitoring capabilities. Fouling of sensor surfaces in biopharmaceutical processes remains a significant challenge, often necessitating cleaning protocols that can disrupt production.
Chromatography detectors, including refractive index, UV absorbance, and multi-angle light scattering detectors, provide crucial information during purification steps. Their limitations primarily revolve around sensitivity to flow rate variations and temperature fluctuations, which can introduce measurement errors if not properly controlled. Furthermore, these detectors typically operate at single points in the process, limiting their ability to provide comprehensive process understanding.
Mass spectrometry offers exceptional specificity and sensitivity for complex biological samples but faces substantial challenges in implementation for real-time monitoring due to sample preparation requirements, high costs, and complexity of operation. The integration of mass spectrometry into continuous processing remains technically challenging despite its analytical power.
Optical sensors for particle characterization and turbidity measurement provide valuable insights into aggregation and particulate formation during purification. However, these technologies often lack the ability to distinguish between different types of particles or to accurately size heterogeneous populations, limiting their diagnostic value in complex purification environments.
A significant limitation across all PAT sensor technologies is the challenge of data integration and interpretation. The volume and complexity of data generated by multiple sensors require advanced algorithms and computational approaches. Current systems often struggle with real-time data processing and the extraction of actionable insights, particularly when dealing with the biological variability inherent in biopharmaceutical processes.
Sensor robustness in aseptic environments presents another critical limitation. Many advanced sensors cannot withstand sterilization procedures or maintain performance under the stringent cleaning regimes required in GMP manufacturing, restricting their application in commercial production settings.
Electrochemical sensors, such as pH, conductivity, and dissolved oxygen probes, are widely deployed for their robustness and relative simplicity. However, these sensors frequently suffer from drift issues during extended operations and may require regular calibration, interrupting continuous monitoring capabilities. Fouling of sensor surfaces in biopharmaceutical processes remains a significant challenge, often necessitating cleaning protocols that can disrupt production.
Chromatography detectors, including refractive index, UV absorbance, and multi-angle light scattering detectors, provide crucial information during purification steps. Their limitations primarily revolve around sensitivity to flow rate variations and temperature fluctuations, which can introduce measurement errors if not properly controlled. Furthermore, these detectors typically operate at single points in the process, limiting their ability to provide comprehensive process understanding.
Mass spectrometry offers exceptional specificity and sensitivity for complex biological samples but faces substantial challenges in implementation for real-time monitoring due to sample preparation requirements, high costs, and complexity of operation. The integration of mass spectrometry into continuous processing remains technically challenging despite its analytical power.
Optical sensors for particle characterization and turbidity measurement provide valuable insights into aggregation and particulate formation during purification. However, these technologies often lack the ability to distinguish between different types of particles or to accurately size heterogeneous populations, limiting their diagnostic value in complex purification environments.
A significant limitation across all PAT sensor technologies is the challenge of data integration and interpretation. The volume and complexity of data generated by multiple sensors require advanced algorithms and computational approaches. Current systems often struggle with real-time data processing and the extraction of actionable insights, particularly when dealing with the biological variability inherent in biopharmaceutical processes.
Sensor robustness in aseptic environments presents another critical limitation. Many advanced sensors cannot withstand sterilization procedures or maintain performance under the stringent cleaning regimes required in GMP manufacturing, restricting their application in commercial production settings.
Existing PAT Implementation Strategies
01 Real-time monitoring and control systems in PAT
Process Analytical Technology (PAT) systems that enable real-time monitoring and control of manufacturing processes. These systems integrate sensors, data analysis tools, and control mechanisms to continuously monitor critical process parameters and make adjustments as needed. Real-time monitoring allows for immediate detection of deviations from optimal conditions, reducing variability and ensuring consistent product quality.- Real-time monitoring and control systems in PAT: Process Analytical Technology (PAT) systems incorporate real-time monitoring sensors and control loops to continuously track manufacturing processes. These systems enable immediate detection of deviations from optimal parameters and allow for automatic adjustments to maintain product quality. The integration of sensors with feedback control mechanisms ensures consistent product quality while reducing manual intervention and production downtime.
- Spectroscopic sensors for process analysis: Spectroscopic techniques are widely implemented in PAT systems to analyze chemical compositions and physical properties of materials during manufacturing. These sensors utilize various spectroscopic methods including near-infrared, Raman, and UV-visible spectroscopy to provide non-destructive, real-time measurements of critical quality attributes. The spectroscopic data is processed through algorithms that correlate spectral information with product characteristics, enabling continuous quality verification.
- Data management and analytics for PAT implementation: Advanced data management systems are essential components of PAT frameworks, handling the large volumes of data generated by multiple sensors. These systems incorporate sophisticated analytics tools that process sensor data to identify patterns, predict outcomes, and optimize manufacturing processes. Machine learning algorithms analyze historical and real-time data to establish correlations between process parameters and product quality, supporting quality-by-design approaches and continuous improvement initiatives.
- Multivariate sensor integration and control strategies: PAT implementations often require the integration of multiple sensor types to monitor different process parameters simultaneously. Multivariate control strategies use data from these diverse sensors to create comprehensive process understanding and control models. These systems correlate various process parameters to identify critical control points and implement appropriate feedback mechanisms, ensuring that manufacturing processes remain within the design space and reducing variability in final product quality.
- Wireless and miniaturized PAT sensor technologies: Recent advances in PAT include the development of wireless and miniaturized sensor technologies that can be deployed throughout manufacturing facilities with minimal disruption to existing processes. These compact sensors enable monitoring in previously inaccessible locations and facilitate retrofitting of PAT systems into established manufacturing lines. Wireless communication protocols allow for seamless data transfer to central control systems, expanding the reach and effectiveness of process analytical technologies in pharmaceutical and other regulated industries.
02 Advanced sensor technologies for process measurements
Advanced sensor technologies that are specifically designed for PAT applications. These sensors can measure various process parameters such as temperature, pressure, pH, concentration, and particle size distribution. The sensors are designed to operate in challenging manufacturing environments and provide accurate, reliable data for process control. Integration of multiple sensor types allows for comprehensive monitoring of complex processes.Expand Specific Solutions03 Data analytics and machine learning in PAT
Implementation of advanced data analytics and machine learning algorithms in PAT systems to process large volumes of sensor data. These computational methods can identify patterns, predict process outcomes, and optimize control parameters. Machine learning models can adapt to changing process conditions and improve over time, enhancing the effectiveness of control loops and enabling predictive maintenance of equipment.Expand Specific Solutions04 Closed-loop control systems for pharmaceutical manufacturing
Closed-loop control systems specifically designed for pharmaceutical manufacturing processes. These systems automatically adjust process parameters based on sensor feedback to maintain optimal conditions. The control loops can manage multiple interrelated variables simultaneously, ensuring that the process remains within the design space. Implementation of these systems helps achieve consistent product quality while reducing manual interventions.Expand Specific Solutions05 Integration of PAT in continuous manufacturing processes
Integration of PAT sensors and control loops in continuous manufacturing processes, as opposed to traditional batch processing. This approach allows for continuous monitoring and adjustment of process parameters throughout the production cycle. The seamless integration of sensors, data analysis, and control systems enables real-time quality assurance and reduces the need for offline testing, leading to more efficient manufacturing operations.Expand Specific Solutions
Leading PAT Solution Providers and Manufacturers
Process Analytical Technology (PAT) for purification is evolving rapidly, currently transitioning from early adoption to growth phase. The global market is expanding significantly, projected to reach approximately $3-4 billion by 2025, driven by pharmaceutical and semiconductor industries' increasing demand for real-time monitoring solutions. Technologically, the field shows varying maturity levels across different applications. Industry leaders like Rosemount (Emerson) and Endress+Hauser dominate in sensor technology, while Shimadzu, Applied Materials, and Tokyo Electron lead in analytical instrumentation. Pharmaceutical companies including Baxter, Roche, and Takeda are advancing implementation in bioprocessing. Semiconductor players such as Lam Research and Samsung are driving innovation in high-purity applications, creating a competitive landscape where specialized expertise and integration capabilities determine market position.
EMD Millipore Corp.
Technical Solution: EMD Millipore has developed an integrated PAT framework specifically for biopharmaceutical purification processes. Their BioContinuum Platform incorporates multiple sensor technologies including multi-angle light scattering, refractive index monitoring, and UV spectroscopy at various wavelengths to characterize protein products during purification. Their Pellicon ultrafiltration systems feature embedded conductivity and pressure sensors that enable real-time monitoring of membrane performance and fouling. The company's EcoPrime chromatography systems implement advanced control algorithms that adjust flow rates, buffer composition, and gradient profiles based on real-time UV absorbance measurements. For viral clearance applications, EMD Millipore has developed specialized fluorescence-based viral detection sensors that can detect viral breakthrough in nanofiltration processes. Their control systems implement risk-based process control strategies that automatically adjust operating parameters when critical quality attributes approach predefined limits.
Strengths: Deep understanding of biopharmaceutical purification requirements; purpose-built solutions for specific unit operations; extensive validation documentation supporting regulatory filings; strong integration between consumables and instrumentation. Weaknesses: Solutions primarily focused on biopharmaceutical applications with less applicability to other industries; higher consumable costs; proprietary nature of some technologies limits customization options.
Shimadzu Corp.
Technical Solution: Shimadzu has pioneered PAT solutions for purification processes with their integrated analytical platforms that combine multiple detection technologies. Their LabSolutions software suite enables seamless integration of chromatography data with process control systems, allowing real-time quality decisions. Shimadzu's UV-VIS spectrophotometers feature patented temperature compensation algorithms that maintain measurement accuracy despite process temperature fluctuations. Their mass spectrometry solutions provide molecular-level process understanding through direct coupling with purification streams, enabling detection of trace contaminants at parts-per-billion levels. For biopharmaceutical applications, Shimadzu has developed specialized protein aggregation monitoring systems that use light scattering techniques to detect early formation of aggregates during purification steps. The company's control systems implement feedback loops with adaptive parameters that automatically optimize separation conditions based on real-time analytical data.
Strengths: Superior analytical precision; comprehensive data integration capabilities; extensive experience in separation science; modular design allowing customization for specific applications. Weaknesses: Higher complexity requiring specialized analytical expertise; more focused on analytical capabilities than comprehensive process control; integration with third-party equipment may require additional engineering.
Key Sensor Technologies for Purification Processes
Process analytic sensors for demanding applications
PatentInactiveUS20060096862A1
Innovation
- The use of materials with low vapor permeability and extractables, combined with venting mechanisms, to prevent electrolyte accumulation and maintain signal integrity, including the selection of materials like polyetheretherketone for the inner housing, perfluoronated rubber for o-rings, and vulcanized silicone for filling, which reduces condensate retention and galvanic potentials.
Process analytical technology with near-infrared spectroscopy for quality monitoring of omega-3 phospholipids formulations
PatentWO2021207832A1
Innovation
- The implementation of Process Analytical Technology (PAT) using near-infrared spectroscopy (NIR) and attenuated total reflectance Fourier transform infrared (ATR-FTIR) for real-time monitoring and adjustment of omega-3 phospholipid compositions during manufacturing, allowing for in-line, on-line, and at-line quality control without sample pretreatment, and enabling feedback and feedforward control to optimize production parameters.
Regulatory Compliance and Validation Frameworks
Regulatory frameworks governing Process Analytical Technology (PAT) implementation in purification processes are primarily established by international health authorities. The FDA's PAT Initiative, launched in 2004, provides the foundational guidance through its "Guidance for Industry: PAT — A Framework for Innovative Pharmaceutical Development, Manufacturing, and Quality Assurance." This document outlines the regulatory expectations for implementing sensor technologies and control loops in pharmaceutical purification processes.
The European Medicines Agency (EMA) has developed complementary guidelines that align with FDA approaches while addressing European-specific requirements. Both regulatory bodies emphasize a risk-based approach to validation, requiring manufacturers to demonstrate that PAT systems consistently operate within defined parameters and produce results that meet predetermined acceptance criteria.
Validation frameworks for PAT systems in purification processes typically follow a four-phase approach. The first phase involves design qualification (DQ), ensuring that sensor systems and control loops are appropriately designed for their intended application in purification processes. The second phase, installation qualification (IQ), verifies that all system components are properly installed according to specifications and engineering standards.
Operational qualification (OQ), the third phase, confirms that sensors and control systems function as intended across their operational range. This includes challenging the system with worst-case scenarios to ensure robust performance. The final phase, performance qualification (PQ), demonstrates that the entire PAT system consistently performs as expected under actual production conditions over extended periods.
Continuous process verification has emerged as a critical component of modern validation frameworks for PAT systems. This approach requires ongoing monitoring of critical quality attributes and process parameters to ensure that purification processes remain in a state of control. Real-time release testing (RTRT) strategies, enabled by PAT, must be validated to demonstrate equivalence or superiority to traditional end-product testing methods.
Data integrity requirements present significant challenges for PAT validation. Regulatory agencies expect complete, consistent, and accurate data records throughout the system lifecycle. This includes validation of data acquisition systems, data transfer protocols, and analytical algorithms that transform raw sensor data into actionable process information.
Change management protocols are essential elements of PAT validation frameworks. As sensor technologies evolve and control strategies are refined, manufacturers must establish robust procedures for evaluating, implementing, and documenting changes to PAT systems without compromising product quality or regulatory compliance.
The European Medicines Agency (EMA) has developed complementary guidelines that align with FDA approaches while addressing European-specific requirements. Both regulatory bodies emphasize a risk-based approach to validation, requiring manufacturers to demonstrate that PAT systems consistently operate within defined parameters and produce results that meet predetermined acceptance criteria.
Validation frameworks for PAT systems in purification processes typically follow a four-phase approach. The first phase involves design qualification (DQ), ensuring that sensor systems and control loops are appropriately designed for their intended application in purification processes. The second phase, installation qualification (IQ), verifies that all system components are properly installed according to specifications and engineering standards.
Operational qualification (OQ), the third phase, confirms that sensors and control systems function as intended across their operational range. This includes challenging the system with worst-case scenarios to ensure robust performance. The final phase, performance qualification (PQ), demonstrates that the entire PAT system consistently performs as expected under actual production conditions over extended periods.
Continuous process verification has emerged as a critical component of modern validation frameworks for PAT systems. This approach requires ongoing monitoring of critical quality attributes and process parameters to ensure that purification processes remain in a state of control. Real-time release testing (RTRT) strategies, enabled by PAT, must be validated to demonstrate equivalence or superiority to traditional end-product testing methods.
Data integrity requirements present significant challenges for PAT validation. Regulatory agencies expect complete, consistent, and accurate data records throughout the system lifecycle. This includes validation of data acquisition systems, data transfer protocols, and analytical algorithms that transform raw sensor data into actionable process information.
Change management protocols are essential elements of PAT validation frameworks. As sensor technologies evolve and control strategies are refined, manufacturers must establish robust procedures for evaluating, implementing, and documenting changes to PAT systems without compromising product quality or regulatory compliance.
Data Management and Digital Twin Applications
The integration of data management systems with digital twin technology represents a transformative approach in Process Analytical Technology (PAT) for purification processes. Modern purification operations generate vast amounts of sensor data that require sophisticated management infrastructures to extract meaningful insights. Cloud-based data platforms have emerged as the preferred solution, offering scalable storage capabilities and advanced analytics tools that can process real-time sensor data from multiple purification stages simultaneously.
Digital twins provide virtual representations of physical purification systems, enabling comprehensive simulation and optimization without disrupting ongoing operations. These models incorporate historical process data, real-time sensor inputs, and predictive algorithms to create dynamic representations that evolve alongside the physical system. For purification processes, digital twins can model complex phenomena such as membrane fouling, chromatography column performance degradation, and filter capacity reduction over time.
The implementation of digital twins for PAT applications requires robust data pipelines that can handle diverse data types from various sensors. Standardized data formats and communication protocols are essential for ensuring seamless integration between different system components. Organizations implementing these technologies typically adopt frameworks like OPC UA (Open Platform Communications Unified Architecture) or MQTT (Message Queuing Telemetry Transport) to facilitate consistent data exchange across their purification infrastructure.
Advanced analytics capabilities within these digital environments enable pattern recognition across historical purification runs, identifying optimal operating conditions and predicting potential quality deviations before they occur. Machine learning algorithms trained on comprehensive datasets can detect subtle correlations between process parameters and product quality attributes that might otherwise remain hidden in traditional analysis approaches.
Security considerations are paramount when implementing data management systems for PAT applications. Pharmaceutical and biotechnology companies must ensure compliance with regulatory requirements while protecting proprietary process information. Multi-layered security architectures incorporating encryption, access controls, and audit trails have become standard practice in industry-leading implementations.
Return on investment for digital twin implementations in purification processes typically manifests through reduced development cycles, decreased batch failures, and optimized resource utilization. Organizations report 15-30% reductions in purification development timelines and up to 25% improvements in process yields following successful digital twin deployments. These benefits are particularly significant for high-value biopharmaceutical products where purification efficiency directly impacts overall manufacturing economics.
Digital twins provide virtual representations of physical purification systems, enabling comprehensive simulation and optimization without disrupting ongoing operations. These models incorporate historical process data, real-time sensor inputs, and predictive algorithms to create dynamic representations that evolve alongside the physical system. For purification processes, digital twins can model complex phenomena such as membrane fouling, chromatography column performance degradation, and filter capacity reduction over time.
The implementation of digital twins for PAT applications requires robust data pipelines that can handle diverse data types from various sensors. Standardized data formats and communication protocols are essential for ensuring seamless integration between different system components. Organizations implementing these technologies typically adopt frameworks like OPC UA (Open Platform Communications Unified Architecture) or MQTT (Message Queuing Telemetry Transport) to facilitate consistent data exchange across their purification infrastructure.
Advanced analytics capabilities within these digital environments enable pattern recognition across historical purification runs, identifying optimal operating conditions and predicting potential quality deviations before they occur. Machine learning algorithms trained on comprehensive datasets can detect subtle correlations between process parameters and product quality attributes that might otherwise remain hidden in traditional analysis approaches.
Security considerations are paramount when implementing data management systems for PAT applications. Pharmaceutical and biotechnology companies must ensure compliance with regulatory requirements while protecting proprietary process information. Multi-layered security architectures incorporating encryption, access controls, and audit trails have become standard practice in industry-leading implementations.
Return on investment for digital twin implementations in purification processes typically manifests through reduced development cycles, decreased batch failures, and optimized resource utilization. Organizations report 15-30% reductions in purification development timelines and up to 25% improvements in process yields following successful digital twin deployments. These benefits are particularly significant for high-value biopharmaceutical products where purification efficiency directly impacts overall manufacturing economics.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!