Continuous Versus Batch Purification: When To Choose Which Mode
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Purification Technology Evolution and Objectives
Purification processes have evolved significantly over the past century, transitioning from simple filtration and crystallization methods to sophisticated chromatographic and membrane-based techniques. The historical trajectory shows a clear progression from predominantly batch-oriented approaches toward more continuous methodologies, particularly in industries requiring high throughput and consistent product quality.
In the early 20th century, batch purification dominated industrial processes due to its simplicity and controllability. The 1950s marked a turning point with the introduction of chromatographic techniques, which initially operated in batch mode but laid the groundwork for continuous applications. By the 1970s, continuous chromatography concepts emerged, though implementation remained limited by technological constraints.
The pharmaceutical industry's evolution has been particularly influential in driving purification technology advancement. Traditional batch processing, while reliable and well-established in regulatory frameworks, faced increasing pressure to improve efficiency and reduce costs. This catalyzed research into continuous alternatives, especially for high-value biopharmaceuticals where purification can represent up to 80% of production costs.
Recent technological breakthroughs in process analytical technology (PAT) and automation have accelerated the shift toward continuous purification. The development of simulated moving bed (SMB) chromatography, periodic counter-current chromatography (PCC), and integrated continuous bioprocessing platforms has demonstrated significant advantages in productivity, footprint reduction, and operational flexibility.
The primary objectives driving purification technology evolution include increasing productivity while maintaining product quality, reducing manufacturing costs through improved resource utilization, enhancing process robustness through real-time monitoring and control, and meeting sustainability goals through reduced solvent usage and waste generation.
Regulatory perspectives have also evolved, with agencies like the FDA encouraging continuous manufacturing adoption through initiatives such as the Quality by Design (QbD) framework. This has provided a pathway for validating continuous purification processes while ensuring product safety and efficacy.
Current research focuses on overcoming remaining technical challenges, including developing more robust continuous chromatography media, improving in-line monitoring capabilities for real-time release testing, and creating integrated continuous platforms that seamlessly connect upstream and downstream processes. The ultimate goal is to establish flexible, scalable purification technologies that can adapt to changing production demands while maintaining consistent product quality.
In the early 20th century, batch purification dominated industrial processes due to its simplicity and controllability. The 1950s marked a turning point with the introduction of chromatographic techniques, which initially operated in batch mode but laid the groundwork for continuous applications. By the 1970s, continuous chromatography concepts emerged, though implementation remained limited by technological constraints.
The pharmaceutical industry's evolution has been particularly influential in driving purification technology advancement. Traditional batch processing, while reliable and well-established in regulatory frameworks, faced increasing pressure to improve efficiency and reduce costs. This catalyzed research into continuous alternatives, especially for high-value biopharmaceuticals where purification can represent up to 80% of production costs.
Recent technological breakthroughs in process analytical technology (PAT) and automation have accelerated the shift toward continuous purification. The development of simulated moving bed (SMB) chromatography, periodic counter-current chromatography (PCC), and integrated continuous bioprocessing platforms has demonstrated significant advantages in productivity, footprint reduction, and operational flexibility.
The primary objectives driving purification technology evolution include increasing productivity while maintaining product quality, reducing manufacturing costs through improved resource utilization, enhancing process robustness through real-time monitoring and control, and meeting sustainability goals through reduced solvent usage and waste generation.
Regulatory perspectives have also evolved, with agencies like the FDA encouraging continuous manufacturing adoption through initiatives such as the Quality by Design (QbD) framework. This has provided a pathway for validating continuous purification processes while ensuring product safety and efficacy.
Current research focuses on overcoming remaining technical challenges, including developing more robust continuous chromatography media, improving in-line monitoring capabilities for real-time release testing, and creating integrated continuous platforms that seamlessly connect upstream and downstream processes. The ultimate goal is to establish flexible, scalable purification technologies that can adapt to changing production demands while maintaining consistent product quality.
Market Demand Analysis for Purification Technologies
The global purification technology market is experiencing robust growth, driven primarily by increasing demand in pharmaceutical, biotechnology, and food and beverage industries. Market analysis indicates that the purification technology sector is expected to reach $36.5 billion by 2026, growing at a CAGR of 8.2% from 2021. This growth trajectory is significantly influenced by the ongoing debate between continuous and batch purification methodologies.
Pharmaceutical manufacturing represents the largest market segment for purification technologies, accounting for approximately 40% of the total market share. Within this segment, there is a notable shift towards continuous purification processes, particularly for high-volume products and biologics manufacturing. This transition is driven by regulatory pressures for improved quality control and the economic benefits of reduced production footprints.
Biotechnology applications follow closely behind pharmaceuticals, with a growing demand for purification solutions in monoclonal antibody production, recombinant proteins, and vaccine development. Market research indicates that over 65% of new biotech facilities are incorporating some form of continuous purification capability, highlighting the industry's recognition of its advantages in certain applications.
Regional analysis reveals that North America dominates the purification technology market with approximately 38% market share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is demonstrating the fastest growth rate at 10.5% annually, driven by expanding pharmaceutical manufacturing capabilities in China and India, and increasing adoption of advanced purification technologies.
Consumer demand trends are also reshaping the purification technology landscape. End-users increasingly prioritize solutions that offer flexibility between batch and continuous operations, recognizing that hybrid approaches often provide optimal efficiency. This has led to a 27% increase in demand for modular purification systems that can be reconfigured based on production requirements.
Environmental considerations are becoming increasingly important market drivers, with water and energy consumption reduction emerging as key selection criteria. Continuous purification systems typically demonstrate 30-40% lower resource consumption compared to equivalent batch processes, making them particularly attractive in regions with stringent environmental regulations or resource constraints.
Market forecasts suggest that while batch purification will maintain dominance in certain applications requiring high flexibility and smaller production volumes, continuous purification technologies will see accelerated adoption rates of approximately 15% annually through 2026, particularly in high-throughput applications where process intensification delivers significant economic advantages.
Pharmaceutical manufacturing represents the largest market segment for purification technologies, accounting for approximately 40% of the total market share. Within this segment, there is a notable shift towards continuous purification processes, particularly for high-volume products and biologics manufacturing. This transition is driven by regulatory pressures for improved quality control and the economic benefits of reduced production footprints.
Biotechnology applications follow closely behind pharmaceuticals, with a growing demand for purification solutions in monoclonal antibody production, recombinant proteins, and vaccine development. Market research indicates that over 65% of new biotech facilities are incorporating some form of continuous purification capability, highlighting the industry's recognition of its advantages in certain applications.
Regional analysis reveals that North America dominates the purification technology market with approximately 38% market share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is demonstrating the fastest growth rate at 10.5% annually, driven by expanding pharmaceutical manufacturing capabilities in China and India, and increasing adoption of advanced purification technologies.
Consumer demand trends are also reshaping the purification technology landscape. End-users increasingly prioritize solutions that offer flexibility between batch and continuous operations, recognizing that hybrid approaches often provide optimal efficiency. This has led to a 27% increase in demand for modular purification systems that can be reconfigured based on production requirements.
Environmental considerations are becoming increasingly important market drivers, with water and energy consumption reduction emerging as key selection criteria. Continuous purification systems typically demonstrate 30-40% lower resource consumption compared to equivalent batch processes, making them particularly attractive in regions with stringent environmental regulations or resource constraints.
Market forecasts suggest that while batch purification will maintain dominance in certain applications requiring high flexibility and smaller production volumes, continuous purification technologies will see accelerated adoption rates of approximately 15% annually through 2026, particularly in high-throughput applications where process intensification delivers significant economic advantages.
Current Landscape and Challenges in Purification Modes
The purification landscape is currently experiencing a significant shift from traditional batch processing toward continuous manufacturing methodologies. Batch purification has dominated the biopharmaceutical industry for decades due to its established regulatory acceptance, operational familiarity, and process flexibility. However, this mode faces inherent limitations including equipment size constraints, batch-to-batch variability, and inefficient resource utilization during non-productive cleaning and setup periods.
Continuous purification technologies have gained substantial momentum over the past decade, with major pharmaceutical companies investing heavily in their development. These systems offer compelling advantages including reduced footprint requirements (typically 30-50% smaller than equivalent batch systems), improved process consistency, and enhanced productivity through elimination of hold times. Recent market analyses indicate that approximately 25% of new purification installations now incorporate some continuous processing elements, representing a threefold increase from just five years ago.
Despite these advances, the industry faces significant technical challenges in fully implementing continuous purification. Integration complexity remains a primary obstacle, as connecting multiple unit operations into a seamless continuous process requires sophisticated control systems and real-time monitoring capabilities. Current continuous chromatography platforms like SMCC (Sequential Multi-Column Chromatography) and PCC (Periodic Counter-Current Chromatography) demonstrate promising performance but struggle with operational robustness in manufacturing environments.
Regulatory uncertainty presents another substantial barrier. While regulatory agencies have expressed support for continuous manufacturing, the approval pathway remains less defined than for traditional batch processes. Companies must navigate evolving guidelines and often invest in extensive validation studies to demonstrate process equivalence and product quality consistency.
Scale-up challenges persist across both modes but manifest differently. Batch processes face economic limitations at very large scales due to vessel size constraints and handling difficulties. Continuous systems encounter challenges in maintaining consistent residence time distribution and preventing process drift during extended operation periods, particularly for high-throughput applications exceeding 100 kg/year production targets.
The talent gap represents an often-overlooked challenge, with industry surveys indicating that over 60% of biopharmaceutical companies report difficulty recruiting personnel with expertise in continuous processing technologies. This skills shortage has slowed implementation timelines and increased reliance on technology vendors and consultants for system design and optimization.
Regional differences in adoption are notable, with European manufacturers generally leading implementation of continuous purification technologies, followed by North American companies, while Asian manufacturers have predominantly maintained batch processing approaches with incremental improvements in automation and process intensification.
Continuous purification technologies have gained substantial momentum over the past decade, with major pharmaceutical companies investing heavily in their development. These systems offer compelling advantages including reduced footprint requirements (typically 30-50% smaller than equivalent batch systems), improved process consistency, and enhanced productivity through elimination of hold times. Recent market analyses indicate that approximately 25% of new purification installations now incorporate some continuous processing elements, representing a threefold increase from just five years ago.
Despite these advances, the industry faces significant technical challenges in fully implementing continuous purification. Integration complexity remains a primary obstacle, as connecting multiple unit operations into a seamless continuous process requires sophisticated control systems and real-time monitoring capabilities. Current continuous chromatography platforms like SMCC (Sequential Multi-Column Chromatography) and PCC (Periodic Counter-Current Chromatography) demonstrate promising performance but struggle with operational robustness in manufacturing environments.
Regulatory uncertainty presents another substantial barrier. While regulatory agencies have expressed support for continuous manufacturing, the approval pathway remains less defined than for traditional batch processes. Companies must navigate evolving guidelines and often invest in extensive validation studies to demonstrate process equivalence and product quality consistency.
Scale-up challenges persist across both modes but manifest differently. Batch processes face economic limitations at very large scales due to vessel size constraints and handling difficulties. Continuous systems encounter challenges in maintaining consistent residence time distribution and preventing process drift during extended operation periods, particularly for high-throughput applications exceeding 100 kg/year production targets.
The talent gap represents an often-overlooked challenge, with industry surveys indicating that over 60% of biopharmaceutical companies report difficulty recruiting personnel with expertise in continuous processing technologies. This skills shortage has slowed implementation timelines and increased reliance on technology vendors and consultants for system design and optimization.
Regional differences in adoption are notable, with European manufacturers generally leading implementation of continuous purification technologies, followed by North American companies, while Asian manufacturers have predominantly maintained batch processing approaches with incremental improvements in automation and process intensification.
Comparative Analysis of Batch vs Continuous Purification Methods
01 Membrane-based purification technologies
Membrane-based purification technologies offer high efficiency for separating various substances. These systems utilize selective membranes that allow certain molecules to pass through while blocking others based on size, charge, or other properties. Advanced membrane configurations can significantly reduce energy consumption while increasing throughput. Innovations in membrane materials and designs have led to improved fouling resistance and longer operational lifespans, making these systems increasingly cost-effective for industrial applications.- Membrane-based purification technologies: Membrane-based purification technologies utilize selective barriers to separate components based on size, charge, or other physical properties. These processes offer high efficiency with lower energy consumption compared to traditional methods. Advanced membrane materials and configurations enhance separation performance while reducing fouling issues. Innovations in this area include composite membranes, surface modifications, and integrated membrane systems that significantly improve purification efficiency across various industries.
- Adsorption and chromatographic purification methods: Adsorption and chromatographic techniques utilize specialized materials to selectively capture target compounds from mixtures. These methods employ adsorbents with high surface area and specific binding properties to achieve efficient separation. Recent developments include novel adsorbent materials, optimized flow patterns, and continuous processing systems that enhance purification efficiency. These approaches are particularly valuable for high-purity requirements in pharmaceutical, biotechnology, and fine chemical applications.
- Chemical and catalytic purification processes: Chemical and catalytic purification processes involve reactions that transform impurities into more easily removable forms or directly convert raw materials into desired products with fewer byproducts. These methods utilize specific reagents or catalysts to enhance reaction selectivity and efficiency. Innovations focus on catalyst design, reaction optimization, and process intensification to reduce energy consumption and waste generation while improving yield and purity of the final products.
- Energy-efficient separation technologies: Energy-efficient separation technologies focus on reducing the power consumption of purification processes while maintaining or improving separation performance. These include advanced distillation configurations, heat integration systems, pressure-swing processes, and hybrid separation methods. Innovations in equipment design, process control, and operating strategies enable significant energy savings. These technologies are particularly important for large-scale industrial applications where energy costs represent a substantial portion of operational expenses.
- Automated and continuous purification systems: Automated and continuous purification systems replace traditional batch processes with integrated, continuously operating equipment that offers improved efficiency and consistency. These systems incorporate real-time monitoring, feedback control, and process optimization algorithms to maintain optimal performance. Advanced designs include modular units, flow-through reactors, and integrated separation stages that minimize intermediate handling and storage. The continuous operation reduces labor requirements, equipment footprint, and processing time while improving product quality and yield.
02 Chemical precipitation and crystallization methods
Chemical precipitation and crystallization techniques are widely used for purifying compounds by converting dissolved substances into solid forms that can be easily separated. These methods involve controlling parameters such as temperature, pH, and concentration to optimize crystal formation and growth. Recent advancements focus on improving crystal purity, size distribution, and recovery rates. The efficiency of these processes can be enhanced through precise control of nucleation and growth kinetics, resulting in higher purity products with reduced processing steps.Expand Specific Solutions03 Adsorption and ion exchange purification systems
Adsorption and ion exchange systems utilize specialized materials to selectively capture target compounds from mixtures. These technologies rely on the affinity between adsorbents and specific molecules or ions. Efficiency improvements have been achieved through the development of novel adsorbent materials with higher capacity, selectivity, and regeneration capabilities. Process innovations include continuous operation systems, optimized flow patterns, and advanced regeneration methods that minimize waste and energy consumption while maximizing purification efficiency.Expand Specific Solutions04 Distillation and thermal separation enhancements
Advanced distillation and thermal separation techniques have been developed to improve purification efficiency. These include innovations in column design, packing materials, and heat integration systems that significantly reduce energy requirements. Multi-effect distillation, vapor recompression, and dividing-wall columns represent major advancements that increase separation efficiency while decreasing operational costs. Process intensification approaches combine multiple separation steps into single units, further enhancing overall system performance and reducing equipment footprint.Expand Specific Solutions05 Continuous flow purification processes
Continuous flow purification processes offer advantages over batch operations through improved consistency, reduced labor requirements, and higher throughput. These systems maintain steady-state conditions that optimize separation parameters and product quality. Recent developments include modular designs that allow for flexible scaling and reconfiguration based on production needs. Integration of real-time monitoring and control systems enables automatic adjustments to process parameters, ensuring consistent purification efficiency even with varying feed compositions.Expand Specific Solutions
Key Industry Players and Technology Providers
The continuous versus batch purification landscape is evolving rapidly, with the market transitioning from early adoption to growth phase. The global market for continuous bioprocessing is expanding at approximately 23% CAGR, driven by efficiency demands in biopharmaceutical manufacturing. Technologically, continuous purification is maturing but remains less established than traditional batch methods. Leading players like Merck, Cytiva, and Sartorius are advancing continuous chromatography technologies, while academic institutions such as Swiss Federal Institute of Technology and North Carolina State University contribute fundamental research. Pharmaceutical manufacturers including Amgen and Bayer are implementing hybrid approaches, balancing the established reliability of batch processing with the economic advantages of continuous operations in specific applications.
Amgen, Inc.
Technical Solution: Amgen has developed a comprehensive approach to continuous versus batch purification as part of their Next-Generation Biomanufacturing initiative. Their strategy involves a hybrid continuous processing platform that integrates continuous upstream perfusion culture with advanced downstream purification technologies. For continuous purification, Amgen has implemented multi-column chromatography systems that operate in a continuous countercurrent mode, allowing for constant loading and processing of product. Their proprietary Process Analytical Technology (PAT) framework provides real-time monitoring of critical quality attributes throughout the purification process, enabling automated control and adjustment. Amgen has reported productivity improvements of up to 3-fold when transitioning from batch to continuous purification, along with a 60% reduction in buffer consumption and a 40% smaller facility footprint[1]. Their approach emphasizes flexibility, with manufacturing facilities designed to operate in batch, continuous, or hybrid modes depending on product requirements. Amgen has successfully implemented this technology for commercial production of several biopharmaceuticals, demonstrating consistent product quality while achieving significant operational efficiencies.
Strengths: Integrated approach connecting upstream and downstream continuous processing; extensive experience implementing continuous manufacturing at commercial scale; demonstrated regulatory success with continuous processing. Weaknesses: Proprietary systems with limited external availability; significant investment required for implementation; complex technology transfer to contract manufacturing organizations.
Sartorius Chromatography Equipment SAS
Technical Solution: Sartorius Chromatography Equipment SAS has developed the BioSC® platform, a modular system specifically designed to address the continuous versus batch purification challenge. This technology enables seamless switching between batch and continuous operations based on process requirements. Their continuous chromatography solution implements a multi-column approach that maximizes resin capacity utilization while maintaining product quality. The BioSC® system incorporates inline dilution capabilities to reduce buffer storage requirements by up to 70% compared to traditional batch processes[1]. Their proprietary software provides real-time process monitoring and control, with automated column switching based on UV, conductivity, and pH measurements. The system can be configured for various continuous chromatography techniques including SMCC (Sequential Multi-Column Chromatography) and CaptureSMB™, allowing for optimization based on specific purification challenges. Independent evaluations have shown that their continuous chromatography solutions can increase productivity by 30-80% while reducing buffer consumption by 40-60% compared to batch operations[2].
Strengths: Modular design allows for flexible implementation of continuous processing; proprietary software simplifies operation and monitoring; significant reduction in buffer consumption and facility footprint. Weaknesses: Higher initial capital investment compared to batch systems; requires specialized training for operators; more complex validation and regulatory documentation.
Critical Technologies and Patents in Modern Purification
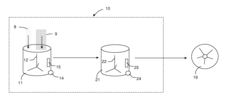
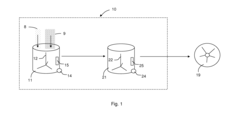
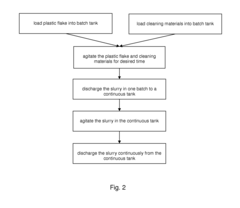
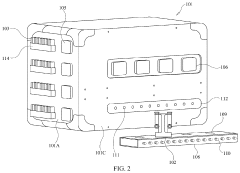
Method and Apparatus for Washing Plastic Bottle Flakes Combining Batch- and Continuous-Mode Processing
PatentInactiveUS20100243004A1
Innovation
- A hybrid system combining batch- and continuous-mode reactor vessels, where plastic flakes and washing liquid are loaded in batches but discharged continuously, ensuring each flake receives a minimum washing time and optimizing downstream equipment usage by acting as a surge tank.
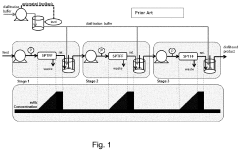
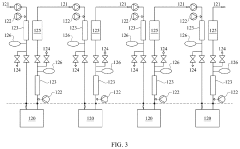
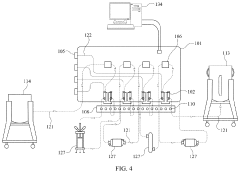
End-to-End Continuous Purification System
PatentPendingUS20220168668A1
Innovation
- A continuous protein purification system that integrates four interchangeable purification stages with a control system, pinch valves, and sensors, allowing for simultaneous operation and automated control of mass flow rates, reducing complexity and space needs.
Scale-up Considerations and Process Economics
Scale-up considerations for purification processes represent a critical decision point in the transition from laboratory to commercial production. When evaluating continuous versus batch purification modes, the economics of scale-up follow distinctly different patterns. Batch processes typically demonstrate a scale-up factor of 0.6-0.7, meaning capital costs increase less than proportionally with capacity. Continuous processes, however, often exhibit more favorable economics with scale-up factors approaching 0.4-0.5, offering significant advantages for large-scale operations.
Equipment utilization rates present another key economic consideration. Batch systems frequently operate at 60-70% utilization due to cleaning, setup, and changeover requirements. Continuous systems can achieve utilization rates of 85-95%, substantially improving return on capital investment. This difference becomes particularly pronounced when calculating the effective cost per unit of product purified over the equipment lifetime.
Labor requirements also scale differently between the two modes. Batch operations typically require staffing proportional to the number of units in operation, while continuous processes can maintain relatively constant labor needs despite increasing throughput. Studies indicate labor costs per unit product can be 30-50% lower in continuous operations at commercial scale.
Facility footprint considerations further impact economics. Continuous purification systems generally require 40-60% less floor space than equivalent batch systems, reducing both capital expenditure for facility construction and ongoing operational costs for utilities, maintenance, and compliance. This space efficiency becomes increasingly valuable as production scales increase.
Energy and resource consumption patterns differ significantly between modes. Batch processes often experience higher peak demand for utilities but lower average consumption. Continuous processes maintain more consistent utility demands, allowing for optimized energy recovery systems and reduced overall consumption. Analysis of multiple case studies indicates continuous operations can reduce energy costs by 20-35% compared to equivalent batch processes.
Risk management also factors into economic evaluations. Batch processes contain risk within discrete units, limiting potential product loss from system failures. Continuous processes require sophisticated monitoring and control systems to mitigate risks, representing additional capital investment but potentially reducing long-term operational variability and associated costs.
Return on investment timelines typically favor batch processes for smaller scales and shorter product lifecycles, while continuous processes demonstrate superior economics for high-volume, long-lifecycle products. The crossover point where continuous becomes economically advantageous typically occurs at annual production volumes of 100-500 kg for high-value biologics and 5-20 metric tons for small molecule pharmaceuticals.
Equipment utilization rates present another key economic consideration. Batch systems frequently operate at 60-70% utilization due to cleaning, setup, and changeover requirements. Continuous systems can achieve utilization rates of 85-95%, substantially improving return on capital investment. This difference becomes particularly pronounced when calculating the effective cost per unit of product purified over the equipment lifetime.
Labor requirements also scale differently between the two modes. Batch operations typically require staffing proportional to the number of units in operation, while continuous processes can maintain relatively constant labor needs despite increasing throughput. Studies indicate labor costs per unit product can be 30-50% lower in continuous operations at commercial scale.
Facility footprint considerations further impact economics. Continuous purification systems generally require 40-60% less floor space than equivalent batch systems, reducing both capital expenditure for facility construction and ongoing operational costs for utilities, maintenance, and compliance. This space efficiency becomes increasingly valuable as production scales increase.
Energy and resource consumption patterns differ significantly between modes. Batch processes often experience higher peak demand for utilities but lower average consumption. Continuous processes maintain more consistent utility demands, allowing for optimized energy recovery systems and reduced overall consumption. Analysis of multiple case studies indicates continuous operations can reduce energy costs by 20-35% compared to equivalent batch processes.
Risk management also factors into economic evaluations. Batch processes contain risk within discrete units, limiting potential product loss from system failures. Continuous processes require sophisticated monitoring and control systems to mitigate risks, representing additional capital investment but potentially reducing long-term operational variability and associated costs.
Return on investment timelines typically favor batch processes for smaller scales and shorter product lifecycles, while continuous processes demonstrate superior economics for high-volume, long-lifecycle products. The crossover point where continuous becomes economically advantageous typically occurs at annual production volumes of 100-500 kg for high-value biologics and 5-20 metric tons for small molecule pharmaceuticals.
Regulatory Compliance and Quality Assurance Frameworks
Regulatory frameworks governing pharmaceutical manufacturing processes have evolved significantly to accommodate both batch and continuous purification methodologies. The FDA's Quality by Design (QbD) initiative provides a structured approach for implementing either purification mode while ensuring product quality and consistency. This framework emphasizes understanding critical process parameters and establishing appropriate control strategies, which becomes particularly important when transitioning between batch and continuous operations.
For continuous purification processes, regulatory bodies have developed specialized guidelines addressing the unique challenges of real-time monitoring and process validation. The FDA's Process Analytical Technology (PAT) framework specifically supports continuous manufacturing by enabling real-time quality assurance rather than relying solely on end-product testing. This approach aligns perfectly with continuous purification's inherent capability for ongoing quality monitoring.
ICH guidelines, particularly Q8 (Pharmaceutical Development), Q9 (Quality Risk Management), and Q10 (Pharmaceutical Quality System), provide comprehensive frameworks applicable to both purification modes. These guidelines emphasize risk-based approaches to quality assurance that can be tailored to the specific challenges of each purification methodology. For continuous processes, this often means implementing more sophisticated in-line monitoring systems.
Validation requirements differ significantly between batch and continuous purification. Batch processes typically follow traditional validation protocols with clearly defined beginning and end points. Continuous processes, however, require state-of-control demonstrations over extended operational periods and during startup/shutdown phases. Regulatory agencies increasingly accept continuous process validation approaches that demonstrate consistent quality over time rather than batch-by-batch testing.
Documentation requirements also vary between the two modes. Batch records document discrete manufacturing events, while continuous processing requires comprehensive data management systems that track process parameters continuously. Electronic batch records have evolved to accommodate the unique documentation needs of continuous processes, including data handling for extended production campaigns.
Quality risk management strategies must be tailored to each purification mode. Batch processes focus on identifying and mitigating risks at discrete processing steps, while continuous processes require dynamic risk assessment frameworks that address potential drift and disturbances in real-time. Regulatory expectations increasingly emphasize robust risk management regardless of the chosen purification mode.
For continuous purification processes, regulatory bodies have developed specialized guidelines addressing the unique challenges of real-time monitoring and process validation. The FDA's Process Analytical Technology (PAT) framework specifically supports continuous manufacturing by enabling real-time quality assurance rather than relying solely on end-product testing. This approach aligns perfectly with continuous purification's inherent capability for ongoing quality monitoring.
ICH guidelines, particularly Q8 (Pharmaceutical Development), Q9 (Quality Risk Management), and Q10 (Pharmaceutical Quality System), provide comprehensive frameworks applicable to both purification modes. These guidelines emphasize risk-based approaches to quality assurance that can be tailored to the specific challenges of each purification methodology. For continuous processes, this often means implementing more sophisticated in-line monitoring systems.
Validation requirements differ significantly between batch and continuous purification. Batch processes typically follow traditional validation protocols with clearly defined beginning and end points. Continuous processes, however, require state-of-control demonstrations over extended operational periods and during startup/shutdown phases. Regulatory agencies increasingly accept continuous process validation approaches that demonstrate consistent quality over time rather than batch-by-batch testing.
Documentation requirements also vary between the two modes. Batch records document discrete manufacturing events, while continuous processing requires comprehensive data management systems that track process parameters continuously. Electronic batch records have evolved to accommodate the unique documentation needs of continuous processes, including data handling for extended production campaigns.
Quality risk management strategies must be tailored to each purification mode. Batch processes focus on identifying and mitigating risks at discrete processing steps, while continuous processes require dynamic risk assessment frameworks that address potential drift and disturbances in real-time. Regulatory expectations increasingly emphasize robust risk management regardless of the chosen purification mode.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!