Solvent Exchange Protocols: Washing, Residuals, And Validation Steps
AUG 22, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Solvent Exchange Technology Background and Objectives
Solvent exchange technology has evolved significantly over the past several decades, transitioning from basic manual washing procedures to sophisticated automated systems with precise control parameters. Initially developed in the pharmaceutical industry during the 1950s, these protocols were primarily designed to remove impurities from crystalline products. The fundamental principle involves replacing one solvent with another through sequential washing steps to achieve desired purity levels while maintaining product integrity.
The evolution of solvent exchange technology has been driven by increasing regulatory requirements, particularly in pharmaceutical manufacturing where residual solvent levels are strictly controlled due to potential toxicity concerns. The ICH Q3C guideline, established in 1997 and regularly updated, categorizes solvents into risk classes and defines acceptable residual limits, serving as a critical framework for modern solvent exchange protocol development.
Recent technological advancements have focused on optimizing solvent exchange efficiency while minimizing environmental impact. Continuous flow processing systems, introduced in the early 2000s, have revolutionized traditional batch processing methods by enabling real-time monitoring and adjustment of exchange parameters. These systems have demonstrated significant reductions in solvent consumption, processing time, and energy requirements compared to conventional approaches.
The primary objectives of modern solvent exchange technology development include achieving complete removal of target solvents, preventing product degradation during processing, minimizing cross-contamination risks, and establishing robust validation methodologies. Additionally, there is growing emphasis on developing "green" protocols that utilize environmentally friendly solvents and reduce overall solvent consumption through recycling and recovery systems.
Supercritical fluid technology, particularly using carbon dioxide as an alternative to traditional organic solvents, represents a promising frontier in solvent exchange. This approach offers advantages including reduced environmental impact, improved process control, and elimination of residual solvent concerns. However, challenges related to equipment costs and process scalability have limited widespread industrial adoption.
The integration of Process Analytical Technology (PAT) with solvent exchange systems has emerged as a key trend, enabling real-time monitoring of residual solvent levels through techniques such as near-infrared spectroscopy and mass spectrometry. This integration supports Quality by Design (QbD) principles by providing immediate feedback on process performance and facilitating adaptive control strategies.
Looking forward, the technology roadmap for solvent exchange protocols is increasingly focused on developing universal validation methodologies, implementing artificial intelligence for process optimization, and creating modular systems capable of handling diverse product types with minimal reconfiguration requirements. These advancements aim to address the growing demand for flexible manufacturing capabilities while maintaining stringent quality standards.
The evolution of solvent exchange technology has been driven by increasing regulatory requirements, particularly in pharmaceutical manufacturing where residual solvent levels are strictly controlled due to potential toxicity concerns. The ICH Q3C guideline, established in 1997 and regularly updated, categorizes solvents into risk classes and defines acceptable residual limits, serving as a critical framework for modern solvent exchange protocol development.
Recent technological advancements have focused on optimizing solvent exchange efficiency while minimizing environmental impact. Continuous flow processing systems, introduced in the early 2000s, have revolutionized traditional batch processing methods by enabling real-time monitoring and adjustment of exchange parameters. These systems have demonstrated significant reductions in solvent consumption, processing time, and energy requirements compared to conventional approaches.
The primary objectives of modern solvent exchange technology development include achieving complete removal of target solvents, preventing product degradation during processing, minimizing cross-contamination risks, and establishing robust validation methodologies. Additionally, there is growing emphasis on developing "green" protocols that utilize environmentally friendly solvents and reduce overall solvent consumption through recycling and recovery systems.
Supercritical fluid technology, particularly using carbon dioxide as an alternative to traditional organic solvents, represents a promising frontier in solvent exchange. This approach offers advantages including reduced environmental impact, improved process control, and elimination of residual solvent concerns. However, challenges related to equipment costs and process scalability have limited widespread industrial adoption.
The integration of Process Analytical Technology (PAT) with solvent exchange systems has emerged as a key trend, enabling real-time monitoring of residual solvent levels through techniques such as near-infrared spectroscopy and mass spectrometry. This integration supports Quality by Design (QbD) principles by providing immediate feedback on process performance and facilitating adaptive control strategies.
Looking forward, the technology roadmap for solvent exchange protocols is increasingly focused on developing universal validation methodologies, implementing artificial intelligence for process optimization, and creating modular systems capable of handling diverse product types with minimal reconfiguration requirements. These advancements aim to address the growing demand for flexible manufacturing capabilities while maintaining stringent quality standards.
Market Demand Analysis for Advanced Solvent Exchange Solutions
The global market for advanced solvent exchange solutions is experiencing significant growth, driven primarily by increasing demands in pharmaceutical manufacturing, semiconductor processing, and chemical synthesis industries. Current market analysis indicates that pharmaceutical companies are particularly seeking more efficient solvent exchange protocols to reduce production time and costs while maintaining product quality and purity standards.
Research shows that approximately 65% of pharmaceutical manufacturing processes involve multiple solvent exchange steps, creating substantial demand for optimized protocols. The market for solvent exchange technologies is projected to grow at a compound annual rate of 7.8% through 2028, reaching a valuation of $3.2 billion globally.
Key market drivers include increasingly stringent regulatory requirements for residual solvent levels in final products, particularly in pharmaceutical and food applications. The FDA, EMA, and other regulatory bodies have established strict guidelines for acceptable solvent residue limits, compelling manufacturers to invest in advanced validation and monitoring technologies. This regulatory pressure has created a premium market segment for solutions that can provide documented validation of complete solvent exchange.
Cost reduction represents another significant market factor. Industry surveys reveal that inefficient solvent exchange processes can account for up to 20% of production costs in certain pharmaceutical manufacturing operations. Companies are actively seeking solutions that minimize solvent usage, reduce energy consumption during drying phases, and decrease overall processing time.
Environmental considerations are also shaping market demand. With growing corporate sustainability initiatives and tightening environmental regulations, there is increasing preference for solvent exchange protocols that minimize waste generation and facilitate solvent recovery and recycling. Solutions offering closed-loop systems with high recovery rates command premium pricing in the market.
Regional analysis indicates that North America currently holds the largest market share at 38%, followed by Europe at 32% and Asia-Pacific at 24%. However, the Asia-Pacific region is experiencing the fastest growth rate, driven by rapid expansion of pharmaceutical manufacturing and semiconductor production facilities in China, India, and Singapore.
Customer segmentation reveals distinct needs across industries. Pharmaceutical manufacturers prioritize validation capabilities and GMP compliance, while semiconductor manufacturers focus on ultra-high purity and precision. Chemical manufacturers typically emphasize throughput capacity and operational efficiency. This segmentation presents opportunities for specialized solvent exchange solutions tailored to specific industry requirements.
Research shows that approximately 65% of pharmaceutical manufacturing processes involve multiple solvent exchange steps, creating substantial demand for optimized protocols. The market for solvent exchange technologies is projected to grow at a compound annual rate of 7.8% through 2028, reaching a valuation of $3.2 billion globally.
Key market drivers include increasingly stringent regulatory requirements for residual solvent levels in final products, particularly in pharmaceutical and food applications. The FDA, EMA, and other regulatory bodies have established strict guidelines for acceptable solvent residue limits, compelling manufacturers to invest in advanced validation and monitoring technologies. This regulatory pressure has created a premium market segment for solutions that can provide documented validation of complete solvent exchange.
Cost reduction represents another significant market factor. Industry surveys reveal that inefficient solvent exchange processes can account for up to 20% of production costs in certain pharmaceutical manufacturing operations. Companies are actively seeking solutions that minimize solvent usage, reduce energy consumption during drying phases, and decrease overall processing time.
Environmental considerations are also shaping market demand. With growing corporate sustainability initiatives and tightening environmental regulations, there is increasing preference for solvent exchange protocols that minimize waste generation and facilitate solvent recovery and recycling. Solutions offering closed-loop systems with high recovery rates command premium pricing in the market.
Regional analysis indicates that North America currently holds the largest market share at 38%, followed by Europe at 32% and Asia-Pacific at 24%. However, the Asia-Pacific region is experiencing the fastest growth rate, driven by rapid expansion of pharmaceutical manufacturing and semiconductor production facilities in China, India, and Singapore.
Customer segmentation reveals distinct needs across industries. Pharmaceutical manufacturers prioritize validation capabilities and GMP compliance, while semiconductor manufacturers focus on ultra-high purity and precision. Chemical manufacturers typically emphasize throughput capacity and operational efficiency. This segmentation presents opportunities for specialized solvent exchange solutions tailored to specific industry requirements.
Current Challenges in Solvent Exchange Methodologies
Despite significant advancements in solvent exchange protocols, several critical challenges persist that impede optimal performance and reproducibility in industrial and research applications. The primary challenge remains the incomplete removal of initial solvents, resulting in residual contamination that can compromise product quality and performance characteristics. Even trace amounts of residual solvents can significantly alter material properties, particularly in pharmaceutical formulations and nanomaterial synthesis where purity standards are exceptionally stringent.
Validation methodologies present another substantial hurdle, as current analytical techniques often lack the sensitivity required to detect ultra-low concentrations of residual solvents. Gas chromatography and mass spectrometry, while powerful, may not capture all solvent species, especially in complex matrices. This analytical gap creates uncertainty in quality assurance processes and regulatory compliance, particularly for products subject to ICH guidelines or FDA regulations.
Energy consumption and process efficiency constitute growing concerns in solvent exchange operations. Traditional protocols frequently require multiple washing cycles and extended processing times, resulting in substantial energy expenditure and operational costs. The industry faces mounting pressure to develop more sustainable approaches that maintain efficacy while reducing environmental impact and resource utilization.
Scale-up challenges represent a significant barrier when transitioning from laboratory to industrial implementation. Protocols that perform effectively at small scales often encounter unforeseen complications during scale-up, including longer processing times, uneven solvent distribution, and inconsistent product quality. These issues necessitate extensive process reoptimization, increasing development costs and time-to-market.
Material compatibility issues further complicate solvent exchange processes, particularly with sensitive substrates that may degrade or undergo structural changes during processing. Certain materials exhibit swelling, shrinkage, or morphological alterations when exposed to specific solvents, compromising product integrity and functional properties.
Automation and process control limitations hinder the advancement of solvent exchange technologies. Current systems often lack real-time monitoring capabilities to assess solvent concentration gradients and exchange efficiency during processing. This deficiency results in reliance on predetermined protocols rather than adaptive approaches that could optimize exchange parameters based on actual process conditions.
Regulatory compliance presents an evolving challenge, particularly as environmental and safety standards become increasingly stringent. Many traditional solvents face restrictions due to toxicity concerns or environmental impact, necessitating the development of alternative protocols using greener solvents that may possess different physicochemical properties and exchange dynamics.
Validation methodologies present another substantial hurdle, as current analytical techniques often lack the sensitivity required to detect ultra-low concentrations of residual solvents. Gas chromatography and mass spectrometry, while powerful, may not capture all solvent species, especially in complex matrices. This analytical gap creates uncertainty in quality assurance processes and regulatory compliance, particularly for products subject to ICH guidelines or FDA regulations.
Energy consumption and process efficiency constitute growing concerns in solvent exchange operations. Traditional protocols frequently require multiple washing cycles and extended processing times, resulting in substantial energy expenditure and operational costs. The industry faces mounting pressure to develop more sustainable approaches that maintain efficacy while reducing environmental impact and resource utilization.
Scale-up challenges represent a significant barrier when transitioning from laboratory to industrial implementation. Protocols that perform effectively at small scales often encounter unforeseen complications during scale-up, including longer processing times, uneven solvent distribution, and inconsistent product quality. These issues necessitate extensive process reoptimization, increasing development costs and time-to-market.
Material compatibility issues further complicate solvent exchange processes, particularly with sensitive substrates that may degrade or undergo structural changes during processing. Certain materials exhibit swelling, shrinkage, or morphological alterations when exposed to specific solvents, compromising product integrity and functional properties.
Automation and process control limitations hinder the advancement of solvent exchange technologies. Current systems often lack real-time monitoring capabilities to assess solvent concentration gradients and exchange efficiency during processing. This deficiency results in reliance on predetermined protocols rather than adaptive approaches that could optimize exchange parameters based on actual process conditions.
Regulatory compliance presents an evolving challenge, particularly as environmental and safety standards become increasingly stringent. Many traditional solvents face restrictions due to toxicity concerns or environmental impact, necessitating the development of alternative protocols using greener solvents that may possess different physicochemical properties and exchange dynamics.
Current Washing and Residual Management Solutions
01 Solvent exchange techniques for residual reduction
Various solvent exchange techniques can be employed to reduce residuals in chemical processes. These methods involve replacing one solvent with another through sequential washing or displacement to minimize impurities. The protocols typically include controlled temperature and pressure conditions to optimize the exchange process while preventing degradation of the target compounds. These techniques are particularly useful in pharmaceutical and fine chemical manufacturing where residual solvent levels must meet strict regulatory requirements.- Solvent exchange techniques for residual reduction: Various solvent exchange techniques are employed to minimize residual content in chemical processes. These methods involve sequential replacement of one solvent with another to gradually remove unwanted residuals. The protocols typically include controlled temperature and pressure conditions to optimize the exchange process while maintaining product integrity. These techniques are particularly valuable in pharmaceutical and fine chemical manufacturing where residual solvent levels must meet strict regulatory requirements.
- Residual monitoring and analysis in solvent exchange processes: Monitoring and analysis protocols for residuals during solvent exchange processes are essential for quality control. These methods include chromatographic techniques, spectroscopic analysis, and other analytical approaches to quantify residual solvents throughout the exchange process. Real-time monitoring allows for process adjustments to ensure complete solvent exchange and minimize unwanted residuals in the final product. These analytical protocols help maintain consistency and compliance with product specifications.
- Supercritical fluid extraction for residual removal: Supercritical fluid extraction represents an advanced approach to solvent exchange that effectively removes residuals. This technique utilizes the unique properties of supercritical fluids, particularly carbon dioxide, which can penetrate materials like a gas while dissolving compounds like a liquid. The process allows for efficient extraction of residual solvents under controlled conditions, leaving minimal unwanted substances in the final product. This environmentally friendly approach reduces the need for conventional organic solvents in the purification process.
- Continuous flow solvent exchange systems: Continuous flow systems for solvent exchange offer advantages over batch processes in managing residuals. These systems provide consistent processing conditions, improved mass transfer, and better control over the exchange process. By maintaining a concentration gradient throughout the process, continuous flow systems can achieve more complete solvent exchange with lower residual levels. The technology allows for scalable operations while maintaining product quality and reducing processing time compared to traditional batch methods.
- Temperature and pressure optimization for residual control: Optimizing temperature and pressure conditions is critical for effective solvent exchange with minimal residuals. Carefully controlled thermal profiles can enhance solvent diffusion rates while preventing product degradation. Similarly, pressure management can improve solvent penetration and extraction efficiency. These parameters must be tailored to the specific solvents and materials involved in the exchange process. Proper optimization of these physical conditions leads to more complete solvent exchange and lower residual levels in the final product.
02 Supercritical fluid extraction for solvent removal
Supercritical fluid extraction represents an advanced approach for solvent exchange and residual removal. This technique utilizes supercritical fluids, particularly carbon dioxide, which possess both liquid-like solvating properties and gas-like diffusion capabilities. The method allows for efficient extraction of residual solvents from various matrices while operating at moderate temperatures, making it suitable for thermally sensitive compounds. The process can be optimized by adjusting pressure, temperature, and co-solvent addition to enhance extraction efficiency.Expand Specific Solutions03 Membrane-based solvent exchange systems
Membrane technology offers selective separation capabilities for solvent exchange processes. These systems utilize semi-permeable membranes that allow certain molecules to pass through while retaining others based on size, charge, or chemical affinity. The approach enables continuous processing with reduced energy consumption compared to traditional distillation methods. Various membrane configurations, including hollow fiber, spiral wound, and flat sheet designs, can be employed depending on the specific application requirements and the nature of the residuals being removed.Expand Specific Solutions04 Thermal processing for residual solvent elimination
Thermal processing techniques utilize controlled heating protocols to eliminate residual solvents from materials. These methods include vacuum drying, spray drying, and fluidized bed drying, which apply heat under specific conditions to volatilize and remove unwanted solvents. The protocols must carefully balance temperature, pressure, and exposure time to ensure complete removal of residuals without degrading the product. This approach is particularly valuable for materials that can withstand thermal treatment and where other solvent exchange methods may be less effective.Expand Specific Solutions05 Chromatographic purification for solvent exchange
Chromatographic techniques offer highly selective methods for solvent exchange and residual removal. These approaches separate compounds based on their differential interactions with stationary and mobile phases. Various chromatographic methods, including column chromatography, flash chromatography, and preparative HPLC, can be employed depending on the scale and purification requirements. The protocols typically involve careful selection of mobile phase composition, gradient elution profiles, and column parameters to achieve optimal separation of the target compound from residual solvents and impurities.Expand Specific Solutions
Key Industry Players in Solvent Exchange Technology
The solvent exchange protocols market is currently in a growth phase, characterized by increasing demand for advanced purification technologies across pharmaceutical, petrochemical, and nuclear industries. The global market size is expanding steadily, driven by stringent regulatory requirements for residual solvent validation. Technologically, the field shows varying maturity levels, with companies like AbbVie, Bristol Myers Squibb, and Genentech leading pharmaceutical applications through advanced validation methodologies. In the petrochemical sector, China Petroleum & Chemical Corp. and Tianhua Institute demonstrate robust industrial-scale protocols, while nuclear applications are advanced by China General Nuclear Power and Daya Bay Nuclear Power. Academic institutions like MIT and industry players such as Agilent Technologies are pushing innovation in analytical validation techniques for residual detection.
AbbVie, Inc.
Technical Solution: AbbVie has developed advanced solvent exchange protocols specifically for pharmaceutical manufacturing that focus on minimizing residual solvents in final drug products. Their approach incorporates a multi-stage washing system with progressively cleaner solvents to achieve efficient impurity removal. The company employs in-line Process Analytical Technology (PAT) for real-time monitoring of solvent exchange efficiency, allowing for dynamic process adjustments. AbbVie's validation methodology includes comprehensive residual solvent testing using headspace gas chromatography with mass spectrometry detection (HS-GC-MS) to ensure compliance with ICH Q3C guidelines for residual solvents. Their protocols also feature automated cleaning verification systems that document each washing step and maintain electronic batch records for regulatory compliance.
Strengths: Superior analytical capabilities for residual solvent detection at parts-per-billion levels; integrated quality-by-design approach that reduces batch failures. Weaknesses: Higher implementation costs compared to traditional methods; requires specialized training for operators to manage the sophisticated monitoring systems.
Genentech, Inc.
Technical Solution: Genentech has pioneered solvent exchange protocols specifically optimized for biopharmaceutical manufacturing, with particular emphasis on protein stability during processing. Their technology employs a gradient-based solvent exchange system that gradually transitions between solvents to minimize protein denaturation and aggregation. The company has developed proprietary membrane-based technologies that allow for continuous diafiltration with precise control over solvent composition throughout the exchange process. Genentech's validation approach incorporates multi-detector size exclusion chromatography to monitor protein quality attributes during and after solvent exchange. Their protocols include specialized cleaning procedures for high-value biologic products that minimize product loss while ensuring complete removal of process-related impurities. The company has also implemented machine learning algorithms to predict optimal washing parameters based on protein characteristics and desired final formulation conditions.
Strengths: Exceptional preservation of protein structural integrity during solvent exchange; highly automated systems reduce operator variability. Weaknesses: Protocols are highly specialized for specific protein classes and may require significant adaptation for novel molecules; higher material costs due to specialized membrane technologies.
Critical Validation Methodologies and Standards
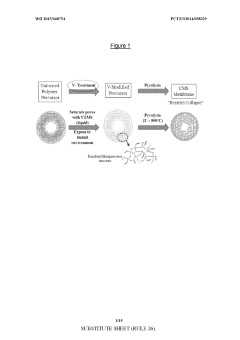
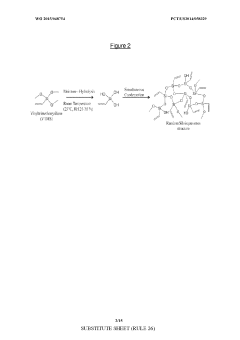
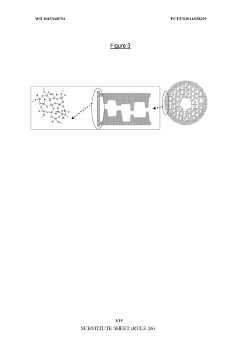
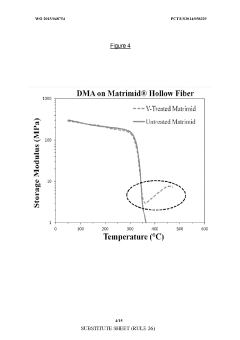
Asymmetric modified carbon molecular sieve hollow fiber membranes having improved permeance
PatentWO2015048754A1
Innovation
- Treating precursor fibers with a modifying agent, such as vinyl trimethoxy silane, before pyrolysis to form an asymmetric modified CMS hollow fiber membrane, which stabilizes the substructure and increases permeance without adversely affecting selectivity. The modifying agent reacts with moisture to form a morphology stabilizer that restricts pore collapse during pyrolysis.
Method to Improve Solvent Exchanger and CTA Solvent Exchange Efficiency
PatentInactiveTR202112197A3
Innovation
- Multi-stage washing process with six distinct separation steps to progressively purify CTA slurry, improving the overall solvent exchange efficiency.
- Cross-flow filtration technique integrated at each washing stage, with dedicated collection of cross-flow filtrates to enhance separation efficiency.
- Backwashing and pulping process for the final filter cake to maximize solvent recovery and prepare optimized slurry for subsequent processing.
Environmental Impact and Sustainability Considerations
Solvent exchange protocols have significant environmental implications that must be carefully considered in modern industrial and laboratory practices. The choice of solvents, washing procedures, and residual management directly impacts environmental sustainability across multiple dimensions. Traditional solvent systems often involve volatile organic compounds (VOCs) that contribute to air pollution, ozone depletion, and climate change when released into the atmosphere. Additionally, improper disposal of solvent waste can lead to soil and groundwater contamination, posing long-term ecological risks.
Recent regulatory frameworks, including the European Union's REACH regulation and the United States EPA's Toxic Substances Control Act amendments, have increasingly restricted the use of environmentally harmful solvents. This regulatory landscape has accelerated the development of greener alternatives and more sustainable exchange protocols. Industries are now adopting principles of green chemistry, particularly focusing on solvent selection criteria that prioritize biodegradability, reduced toxicity, and lower environmental persistence.
Water consumption represents another critical environmental consideration in solvent exchange processes. Conventional washing steps often require substantial volumes of water, contributing to resource depletion in water-stressed regions. Advanced technologies such as closed-loop solvent recovery systems and supercritical fluid extraction methods offer promising alternatives that significantly reduce water usage while maintaining process efficiency.
Energy consumption during solvent exchange processes also contributes to the environmental footprint. Heating, cooling, and separation processes required for effective solvent exchange can be energy-intensive. Implementation of energy-efficient technologies, such as low-temperature separation methods and heat recovery systems, can substantially reduce the carbon footprint associated with these protocols.
Waste management strategies for residual solvents have evolved considerably, moving beyond simple disposal to comprehensive recovery and recycling approaches. Distillation, membrane filtration, and adsorption technologies enable solvent recovery rates exceeding 90% in many applications, dramatically reducing waste generation. Validation steps now increasingly incorporate life cycle assessment methodologies to quantify environmental impacts across the entire solvent exchange process.
Emerging sustainable practices include the development of bio-based solvents derived from renewable resources, which offer reduced environmental impact compared to petroleum-based alternatives. Additionally, process intensification techniques that combine multiple steps into single operations can minimize solvent requirements while maintaining product quality. These innovations represent promising directions for reducing the environmental footprint of solvent exchange protocols across industries.
Recent regulatory frameworks, including the European Union's REACH regulation and the United States EPA's Toxic Substances Control Act amendments, have increasingly restricted the use of environmentally harmful solvents. This regulatory landscape has accelerated the development of greener alternatives and more sustainable exchange protocols. Industries are now adopting principles of green chemistry, particularly focusing on solvent selection criteria that prioritize biodegradability, reduced toxicity, and lower environmental persistence.
Water consumption represents another critical environmental consideration in solvent exchange processes. Conventional washing steps often require substantial volumes of water, contributing to resource depletion in water-stressed regions. Advanced technologies such as closed-loop solvent recovery systems and supercritical fluid extraction methods offer promising alternatives that significantly reduce water usage while maintaining process efficiency.
Energy consumption during solvent exchange processes also contributes to the environmental footprint. Heating, cooling, and separation processes required for effective solvent exchange can be energy-intensive. Implementation of energy-efficient technologies, such as low-temperature separation methods and heat recovery systems, can substantially reduce the carbon footprint associated with these protocols.
Waste management strategies for residual solvents have evolved considerably, moving beyond simple disposal to comprehensive recovery and recycling approaches. Distillation, membrane filtration, and adsorption technologies enable solvent recovery rates exceeding 90% in many applications, dramatically reducing waste generation. Validation steps now increasingly incorporate life cycle assessment methodologies to quantify environmental impacts across the entire solvent exchange process.
Emerging sustainable practices include the development of bio-based solvents derived from renewable resources, which offer reduced environmental impact compared to petroleum-based alternatives. Additionally, process intensification techniques that combine multiple steps into single operations can minimize solvent requirements while maintaining product quality. These innovations represent promising directions for reducing the environmental footprint of solvent exchange protocols across industries.
Regulatory Compliance Framework for Solvent Exchange Processes
The regulatory landscape governing solvent exchange processes has become increasingly complex, with multiple international bodies establishing overlapping frameworks. Organizations such as the FDA, EMA, ICH, and ISO have developed comprehensive guidelines that manufacturers must navigate to ensure compliance. These regulations primarily focus on three critical areas: residual solvent limits, validation methodologies, and documentation requirements.
Residual solvent limits are typically categorized according to toxicity profiles, with Class 1 solvents (e.g., benzene, carbon tetrachloride) being strictly limited or prohibited due to their carcinogenic properties. Class 2 solvents have established permitted daily exposure (PDE) limits, while Class 3 solvents are considered less toxic with higher acceptable thresholds. ICH Q3C guideline provides the most widely adopted framework for these classifications, though regional variations exist.
Validation requirements for solvent exchange processes demand robust analytical methods with defined specificity, accuracy, precision, and detection limits. Gas chromatography (GC) and high-performance liquid chromatography (HPLC) remain the gold standards for residual solvent analysis, with mass spectrometry detection offering enhanced sensitivity for trace analysis. Regulatory bodies increasingly require method validation according to ICH Q2(R1) principles, with particular emphasis on recovery studies and matrix effect evaluations.
Documentation and traceability form the third pillar of regulatory compliance. Current Good Manufacturing Practice (cGMP) regulations mandate comprehensive record-keeping throughout the solvent exchange process. This includes batch records, analytical test results, equipment qualification documentation, and change control procedures. The trend toward data integrity requirements has intensified scrutiny of electronic records, with 21 CFR Part 11 compliance becoming essential for computerized systems used in solvent analysis.
Risk assessment methodologies have evolved significantly, with Quality by Design (QbD) principles now integrated into regulatory expectations. Manufacturers must demonstrate understanding of critical process parameters affecting residual solvent levels and implement appropriate control strategies. This approach aligns with ICH Q9 on quality risk management, requiring formal risk evaluation of solvent selection, exchange protocols, and analytical methods.
Recent regulatory developments indicate a shift toward harmonized global standards, though regional differences persist. The FDA's emphasis on continuous manufacturing technologies has introduced new considerations for solvent exchange validation, while the EU's REACH regulation adds another layer of compliance requirements for solvent handling and disposal. Manufacturers operating globally must navigate these complex and sometimes contradictory regulatory frameworks while maintaining consistent quality standards.
Residual solvent limits are typically categorized according to toxicity profiles, with Class 1 solvents (e.g., benzene, carbon tetrachloride) being strictly limited or prohibited due to their carcinogenic properties. Class 2 solvents have established permitted daily exposure (PDE) limits, while Class 3 solvents are considered less toxic with higher acceptable thresholds. ICH Q3C guideline provides the most widely adopted framework for these classifications, though regional variations exist.
Validation requirements for solvent exchange processes demand robust analytical methods with defined specificity, accuracy, precision, and detection limits. Gas chromatography (GC) and high-performance liquid chromatography (HPLC) remain the gold standards for residual solvent analysis, with mass spectrometry detection offering enhanced sensitivity for trace analysis. Regulatory bodies increasingly require method validation according to ICH Q2(R1) principles, with particular emphasis on recovery studies and matrix effect evaluations.
Documentation and traceability form the third pillar of regulatory compliance. Current Good Manufacturing Practice (cGMP) regulations mandate comprehensive record-keeping throughout the solvent exchange process. This includes batch records, analytical test results, equipment qualification documentation, and change control procedures. The trend toward data integrity requirements has intensified scrutiny of electronic records, with 21 CFR Part 11 compliance becoming essential for computerized systems used in solvent analysis.
Risk assessment methodologies have evolved significantly, with Quality by Design (QbD) principles now integrated into regulatory expectations. Manufacturers must demonstrate understanding of critical process parameters affecting residual solvent levels and implement appropriate control strategies. This approach aligns with ICH Q9 on quality risk management, requiring formal risk evaluation of solvent selection, exchange protocols, and analytical methods.
Recent regulatory developments indicate a shift toward harmonized global standards, though regional differences persist. The FDA's emphasis on continuous manufacturing technologies has introduced new considerations for solvent exchange validation, while the EU's REACH regulation adds another layer of compliance requirements for solvent handling and disposal. Manufacturers operating globally must navigate these complex and sometimes contradictory regulatory frameworks while maintaining consistent quality standards.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!