How To Scale Chromatography From Lab To Pilot: Column, Resin, And Flow Considerations
AUG 22, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Chromatography Scale-up Background and Objectives
Chromatography has evolved significantly since its inception in the early 20th century, transforming from a simple analytical technique to a sophisticated purification method essential in biopharmaceutical manufacturing. The progression from laboratory-scale experiments to pilot-scale production represents a critical transition in biopharmaceutical development, particularly for protein-based therapeutics and vaccines. This scale-up process is not merely a matter of increasing dimensions but requires careful consideration of complex fluid dynamics, resin characteristics, and column geometry.
The historical development of chromatography scale-up methodologies has been driven by the biopharmaceutical industry's need for efficient, reproducible, and cost-effective purification processes. Early approaches in the 1980s and 1990s often relied on trial-and-error methods, resulting in significant resource expenditure and unpredictable outcomes. The advent of computational fluid dynamics and advanced modeling techniques in the early 2000s began to transform this landscape, enabling more systematic approaches to scale-up challenges.
Current technological trends in chromatography scale-up focus on establishing robust, predictive frameworks that account for the multifaceted interactions between process parameters. These include the development of scale-down models that accurately mimic larger systems, implementation of quality-by-design principles, and utilization of process analytical technologies for real-time monitoring and control. The industry is increasingly moving toward continuous processing paradigms, which present unique scale-up considerations compared to traditional batch operations.
The primary objective of chromatography scale-up is to maintain process performance and product quality attributes while increasing throughput. This involves preserving critical parameters such as resolution, yield, and purity across different scales. Secondary objectives include minimizing development time, reducing material consumption during process development, and ensuring operational robustness at larger scales.
Technical goals for successful scale-up encompass the establishment of scalable parameters (e.g., linear velocity, residence time), understanding of non-linear scaling effects, development of predictive models for process performance, and identification of scale-independent operating conditions. Additionally, there is growing emphasis on sustainability considerations, including resin lifetime optimization, buffer consumption reduction, and overall process intensification.
The achievement of these objectives requires a multidisciplinary approach, integrating principles from chemical engineering, material science, fluid dynamics, and analytical chemistry. As biopharmaceutical products become increasingly complex and regulatory requirements more stringent, the importance of systematic, science-based approaches to chromatography scale-up continues to grow, driving innovation in both theoretical frameworks and practical methodologies.
The historical development of chromatography scale-up methodologies has been driven by the biopharmaceutical industry's need for efficient, reproducible, and cost-effective purification processes. Early approaches in the 1980s and 1990s often relied on trial-and-error methods, resulting in significant resource expenditure and unpredictable outcomes. The advent of computational fluid dynamics and advanced modeling techniques in the early 2000s began to transform this landscape, enabling more systematic approaches to scale-up challenges.
Current technological trends in chromatography scale-up focus on establishing robust, predictive frameworks that account for the multifaceted interactions between process parameters. These include the development of scale-down models that accurately mimic larger systems, implementation of quality-by-design principles, and utilization of process analytical technologies for real-time monitoring and control. The industry is increasingly moving toward continuous processing paradigms, which present unique scale-up considerations compared to traditional batch operations.
The primary objective of chromatography scale-up is to maintain process performance and product quality attributes while increasing throughput. This involves preserving critical parameters such as resolution, yield, and purity across different scales. Secondary objectives include minimizing development time, reducing material consumption during process development, and ensuring operational robustness at larger scales.
Technical goals for successful scale-up encompass the establishment of scalable parameters (e.g., linear velocity, residence time), understanding of non-linear scaling effects, development of predictive models for process performance, and identification of scale-independent operating conditions. Additionally, there is growing emphasis on sustainability considerations, including resin lifetime optimization, buffer consumption reduction, and overall process intensification.
The achievement of these objectives requires a multidisciplinary approach, integrating principles from chemical engineering, material science, fluid dynamics, and analytical chemistry. As biopharmaceutical products become increasingly complex and regulatory requirements more stringent, the importance of systematic, science-based approaches to chromatography scale-up continues to grow, driving innovation in both theoretical frameworks and practical methodologies.
Industrial Demand Analysis for Chromatography Scale-up
The global chromatography market is experiencing robust growth, driven primarily by the biopharmaceutical industry's expanding pipeline and increasing adoption of biosimilars. Current market valuations place the chromatography systems and consumables sector at approximately 9 billion USD, with projections indicating a compound annual growth rate of 6-7% through 2028. This growth trajectory underscores the critical importance of efficient scale-up methodologies in chromatographic processes.
Biopharmaceutical manufacturing represents the largest demand segment, accounting for nearly 45% of the total chromatography market. Within this sector, monoclonal antibody production constitutes the most significant application area, followed by recombinant proteins and vaccines. The COVID-19 pandemic has further accelerated demand, particularly for process-scale chromatography solutions capable of rapid deployment and scale-up.
Contract manufacturing organizations (CMOs) and contract development and manufacturing organizations (CDMOs) have emerged as key market drivers, reporting increased client requests for scalable chromatography processes. These organizations require robust scale-up methodologies to accommodate diverse client projects while maintaining efficiency and cost-effectiveness. Industry surveys indicate that over 70% of CMOs consider chromatography scale-up capabilities as "highly important" to their competitive positioning.
Regulatory considerations significantly influence market demand patterns. The FDA and EMA's continued emphasis on process analytical technology (PAT) and quality by design (QbD) principles has intensified the need for predictable, well-characterized scale-up approaches. Companies are increasingly seeking chromatography solutions that facilitate regulatory compliance through consistent performance across different scales.
Cost pressures represent another critical market factor. The biopharmaceutical industry faces growing pressure to reduce production costs, particularly for biosimilars and generic biologics. Efficient chromatography scale-up methodologies that minimize resin usage, reduce buffer consumption, and optimize process economics are experiencing heightened demand. Industry data suggests that chromatography operations can account for up to 30% of downstream processing costs in biopharmaceutical manufacturing.
Geographical analysis reveals that North America dominates the market for advanced chromatography scale-up technologies, followed by Europe and the Asia-Pacific region. However, the fastest growth is occurring in emerging markets, particularly China and India, where rapid biopharmaceutical sector expansion is creating new demand centers for scalable purification technologies.
The continuous processing paradigm is reshaping market requirements, with increasing interest in chromatography solutions compatible with continuous manufacturing approaches. This trend is particularly evident in newer facilities, where design considerations increasingly accommodate potential transitions to continuous or hybrid processing models.
Biopharmaceutical manufacturing represents the largest demand segment, accounting for nearly 45% of the total chromatography market. Within this sector, monoclonal antibody production constitutes the most significant application area, followed by recombinant proteins and vaccines. The COVID-19 pandemic has further accelerated demand, particularly for process-scale chromatography solutions capable of rapid deployment and scale-up.
Contract manufacturing organizations (CMOs) and contract development and manufacturing organizations (CDMOs) have emerged as key market drivers, reporting increased client requests for scalable chromatography processes. These organizations require robust scale-up methodologies to accommodate diverse client projects while maintaining efficiency and cost-effectiveness. Industry surveys indicate that over 70% of CMOs consider chromatography scale-up capabilities as "highly important" to their competitive positioning.
Regulatory considerations significantly influence market demand patterns. The FDA and EMA's continued emphasis on process analytical technology (PAT) and quality by design (QbD) principles has intensified the need for predictable, well-characterized scale-up approaches. Companies are increasingly seeking chromatography solutions that facilitate regulatory compliance through consistent performance across different scales.
Cost pressures represent another critical market factor. The biopharmaceutical industry faces growing pressure to reduce production costs, particularly for biosimilars and generic biologics. Efficient chromatography scale-up methodologies that minimize resin usage, reduce buffer consumption, and optimize process economics are experiencing heightened demand. Industry data suggests that chromatography operations can account for up to 30% of downstream processing costs in biopharmaceutical manufacturing.
Geographical analysis reveals that North America dominates the market for advanced chromatography scale-up technologies, followed by Europe and the Asia-Pacific region. However, the fastest growth is occurring in emerging markets, particularly China and India, where rapid biopharmaceutical sector expansion is creating new demand centers for scalable purification technologies.
The continuous processing paradigm is reshaping market requirements, with increasing interest in chromatography solutions compatible with continuous manufacturing approaches. This trend is particularly evident in newer facilities, where design considerations increasingly accommodate potential transitions to continuous or hybrid processing models.
Current Challenges in Lab-to-Pilot Chromatography Transfer
The transition from laboratory-scale chromatography to pilot-scale operations presents numerous technical challenges that significantly impact process efficiency and product quality. One of the primary obstacles is maintaining consistent separation performance across different scales. Parameters that work effectively at small volumes often behave unpredictably when scaled up, leading to reduced resolution, altered elution profiles, and diminished product purity.
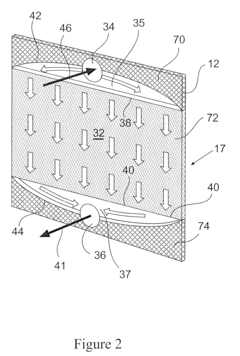
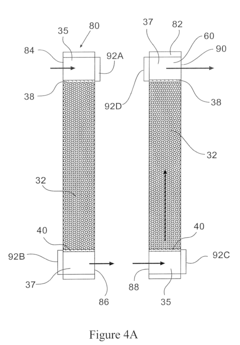
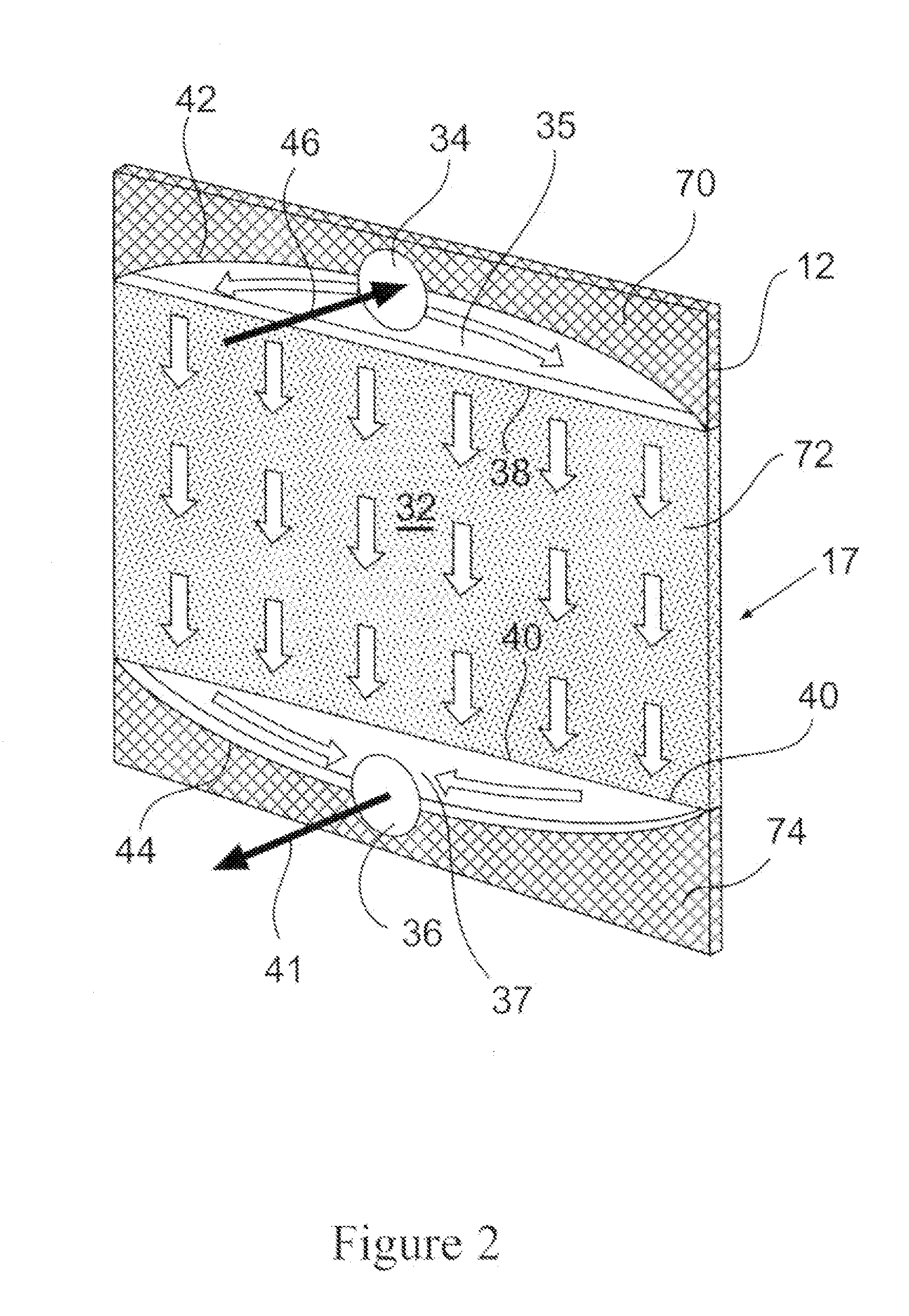
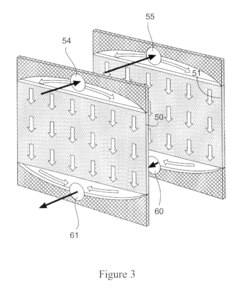
Column geometry considerations represent a critical scaling challenge. As diameter increases in larger columns, flow distribution becomes increasingly problematic, with potential for channeling, dead zones, and uneven resin bed utilization. These hydrodynamic issues can dramatically reduce separation efficiency and create inconsistent results between laboratory and pilot operations.
Resin performance variability between scales presents another significant hurdle. Batch-to-batch inconsistencies in resin properties become more pronounced at larger scales, where minor variations in particle size distribution, ligand density, or porosity can substantially impact separation outcomes. Additionally, resin compression behavior differs markedly between small and large columns, affecting bed stability and pressure drop characteristics.
Flow rate optimization becomes exponentially more complex during scale-up. While linear velocity should theoretically remain constant across scales, practical limitations in equipment capabilities and pressure handling often necessitate compromises. The relationship between flow rate, residence time, and separation efficiency becomes more tenuous at larger scales, requiring careful recalibration of operating parameters.
Pressure management emerges as a critical concern during scale-up. Larger columns generate significantly higher back pressure, potentially exceeding system limitations or causing bed compression issues. This often forces process developers to reduce flow rates, extending processing times and potentially affecting product quality through increased exposure to degradation conditions.
Sample loading capacity represents another scaling challenge. The relationship between sample volume, concentration, and column capacity is rarely linear across scales. Overloading phenomena that might be negligible in laboratory settings can become process-limiting factors at pilot scale, necessitating fundamental rethinking of loading strategies.
Temperature control becomes increasingly difficult with scale-up. Larger columns have less favorable surface-to-volume ratios, making heat dissipation more challenging. Temperature gradients within columns can develop, leading to inconsistent separation performance across the bed and potential product quality issues.
Equipment limitations further complicate scale-up efforts. Pilot-scale chromatography systems often have different detector sensitivities, pump capabilities, and control systems compared to laboratory equipment. These differences can introduce unexpected variables that complicate direct translation of methods between scales.
Column geometry considerations represent a critical scaling challenge. As diameter increases in larger columns, flow distribution becomes increasingly problematic, with potential for channeling, dead zones, and uneven resin bed utilization. These hydrodynamic issues can dramatically reduce separation efficiency and create inconsistent results between laboratory and pilot operations.
Resin performance variability between scales presents another significant hurdle. Batch-to-batch inconsistencies in resin properties become more pronounced at larger scales, where minor variations in particle size distribution, ligand density, or porosity can substantially impact separation outcomes. Additionally, resin compression behavior differs markedly between small and large columns, affecting bed stability and pressure drop characteristics.
Flow rate optimization becomes exponentially more complex during scale-up. While linear velocity should theoretically remain constant across scales, practical limitations in equipment capabilities and pressure handling often necessitate compromises. The relationship between flow rate, residence time, and separation efficiency becomes more tenuous at larger scales, requiring careful recalibration of operating parameters.
Pressure management emerges as a critical concern during scale-up. Larger columns generate significantly higher back pressure, potentially exceeding system limitations or causing bed compression issues. This often forces process developers to reduce flow rates, extending processing times and potentially affecting product quality through increased exposure to degradation conditions.
Sample loading capacity represents another scaling challenge. The relationship between sample volume, concentration, and column capacity is rarely linear across scales. Overloading phenomena that might be negligible in laboratory settings can become process-limiting factors at pilot scale, necessitating fundamental rethinking of loading strategies.
Temperature control becomes increasingly difficult with scale-up. Larger columns have less favorable surface-to-volume ratios, making heat dissipation more challenging. Temperature gradients within columns can develop, leading to inconsistent separation performance across the bed and potential product quality issues.
Equipment limitations further complicate scale-up efforts. Pilot-scale chromatography systems often have different detector sensitivities, pump capabilities, and control systems compared to laboratory equipment. These differences can introduce unexpected variables that complicate direct translation of methods between scales.
Current Column and Resin Scale-up Methodologies
01 Column design and scaling parameters
Column design is critical for chromatography scaling, with key parameters including diameter, height, and aspect ratio. Proper scaling requires maintaining bed height while increasing diameter proportionally to the desired throughput. The column design must account for pressure drop, flow distribution, and wall effects. Advanced column designs incorporate features for improved flow distribution and reduced channeling, which are essential for maintaining separation efficiency during scale-up.- Column design and scaling parameters: Column design is critical for chromatography scaling, involving considerations of diameter, height, and material. Proper scaling requires maintaining geometric similarity between laboratory and production columns. Key parameters include column dimensions, bed height, and aspect ratio. Optimizing these parameters ensures efficient separation while minimizing pressure drop and maximizing resolution during scale-up operations.
- Resin selection and characteristics: Selection of appropriate chromatography resins is essential for successful scale-up. Considerations include particle size, pore structure, ligand density, and mechanical stability. Different resins offer varying binding capacities, selectivity, and flow properties. The resin characteristics must be evaluated based on the target molecule properties and separation requirements to achieve optimal purification performance at larger scales.
- Flow rate optimization and pressure considerations: Flow parameters significantly impact chromatography performance during scale-up. Linear velocity must be maintained while increasing column diameter. Pressure drop across the column increases with bed height and flow rate, requiring careful optimization. Proper flow distribution systems help prevent channeling and ensure uniform flow through the packed bed, which is critical for maintaining separation efficiency at larger scales.
- Monitoring and control systems for scale-up: Advanced monitoring and control systems are essential for successful chromatography scale-up. These include in-line sensors for pH, conductivity, UV absorbance, and pressure. Real-time monitoring enables process adjustments and ensures consistency between runs. Automated control systems help maintain critical parameters within specified ranges, improving reproducibility and product quality during large-scale operations.
- Validation and transfer methods for scale-up: Validation protocols are crucial when scaling chromatography processes from laboratory to production scale. This includes establishing scale-independent parameters, performing small-scale models, and conducting intermediate-scale runs. Transfer methods must account for differences in equipment geometry and flow dynamics. Systematic approaches to validation ensure that separation performance, yield, and product quality are maintained throughout the scale-up process.
02 Resin selection and characteristics
Selecting appropriate chromatography resins is fundamental for successful scale-up. Resin characteristics such as particle size, pore structure, ligand density, and mechanical stability significantly impact separation performance. Smaller particle sizes generally provide better resolution but cause higher backpressure, requiring a balance for large-scale operations. Resins must maintain consistent performance across scales while handling increased flow rates and pressure conditions encountered in production-scale chromatography.Expand Specific Solutions03 Flow rate optimization and linear velocity
Optimizing flow parameters is essential when scaling chromatography processes. Linear velocity should be maintained across scales to ensure comparable separation performance. The relationship between flow rate, column dimensions, and separation efficiency must be carefully balanced. Higher flow rates increase productivity but may compromise resolution and create uneven flow distribution. Advanced flow control systems can help maintain consistent performance during scale-up by adjusting for pressure fluctuations and temperature effects.Expand Specific Solutions04 Pressure-flow relationships and system constraints
Understanding pressure-flow relationships is critical for successful chromatography scaling. As column dimensions increase, system pressure constraints become more significant. Bed compression, channeling, and wall effects can occur at higher pressures, affecting separation efficiency. Proper column packing techniques and hardware selection help mitigate these issues. System design must account for maximum operating pressure limits of resins and equipment while maintaining desired flow rates for optimal separation.Expand Specific Solutions05 Monitoring and control systems for scale-up
Advanced monitoring and control systems are essential for successful chromatography scale-up. These systems track critical parameters such as pressure, flow rate, pH, conductivity, and UV absorbance in real-time. Automated control systems help maintain consistent conditions throughout the separation process, ensuring reproducibility between batches and scales. Process analytical technology (PAT) tools enable continuous monitoring and adjustment of operating parameters, facilitating robust scale-up and troubleshooting of separation issues.Expand Specific Solutions
Key Industry Players in Chromatography Technology
Chromatography scale-up from lab to pilot represents a critical phase in biopharmaceutical development, currently positioned at a mature yet evolving stage. The market is estimated at $1.5-2 billion annually with steady growth as biologics manufacturing expands globally. Leading players demonstrate varying levels of technical maturity: Agilent Technologies, Cytiva (formerly GE Healthcare), and Waters Technology offer comprehensive scale-up solutions with advanced predictive modeling capabilities; EMD Millipore and Pall Corporation provide specialized resin and column technologies; while Thermo Fisher Scientific (through acquisitions like Dionex) focuses on analytical tools supporting the scale-up process. Emerging competition from Asian manufacturers like Nanjing Haina is increasing, though established Western companies maintain technological advantages in process automation and digital integration of chromatography workflows.
Agilent Technologies, Inc.
Technical Solution: Agilent Technologies approaches chromatography scale-up through their Intelligent System Emulation Technology (ISET), which enables seamless method transfer between different instrument platforms and scales. Their methodology focuses on maintaining critical method parameters while accounting for system-specific differences in dwell volume, mixing behavior, and detector response. Agilent's technology incorporates sophisticated software algorithms that automatically adjust gradient profiles and injection parameters to ensure consistent chromatographic performance across scales. Their InfinityLab LC series features scalable flow rates from analytical to preparative ranges while maintaining pressure capabilities, allowing direct method transfer without compromising resolution. Agilent has developed specialized column technology with identical stationary phase chemistry across different dimensions, ensuring consistent selectivity during scale-up. Their approach emphasizes the importance of controlling extra-column volume contributions, utilizing low-dispersion flow paths that maintain separation efficiency at both laboratory and pilot scales. Additionally, Agilent's technology includes predictive modeling tools that simulate how changes in column dimensions and flow rates will affect separation performance, enabling rational design of scale-up experiments with minimal trial-and-error.
Strengths: Superior method transfer capabilities between different instrument platforms; advanced software algorithms for automatic parameter adjustment; comprehensive column portfolio with consistent stationary phase chemistry across scales. Weaknesses: Higher initial investment compared to basic systems; some advanced features may be underutilized in simpler applications; proprietary software may create dependency on Agilent's ecosystem.
EMD Millipore Corp.
Technical Solution: EMD Millipore's chromatography scale-up technology centers on their "Constant Performance" approach, which maintains critical separation parameters across different scales. Their methodology focuses on preserving the number of theoretical plates while increasing column diameter, ensuring consistent resolution and peak capacity. EMD Millipore has developed specialized computational tools that calculate appropriate flow rates and gradient conditions when transitioning between scales, maintaining constant residence time and separation selectivity. Their resin technology features uniform particle size distribution with minimal batch-to-batch variation, which is crucial for predictable scale-up performance. The company's column technology incorporates advanced flow distribution systems at column inlets and outlets, minimizing the formation of preferential flow paths that can compromise separation efficiency at larger scales. EMD Millipore's approach also includes standardized packing protocols that ensure consistent bed density and homogeneity across different column dimensions. Additionally, they've developed specialized hardware for pilot-scale operations that can accurately reproduce laboratory conditions while accommodating the increased flow rates and pressure requirements of larger systems.
Strengths: Comprehensive computational tools for parameter calculation; highly consistent resin manufacturing ensuring reproducibility; advanced flow distribution technology minimizing scale-up issues. Weaknesses: Higher cost compared to some alternatives; some solutions optimized primarily for biopharmaceutical applications; complex systems may require specialized training.
Critical Parameters for Successful Scale-up Operations
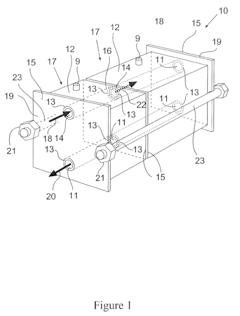
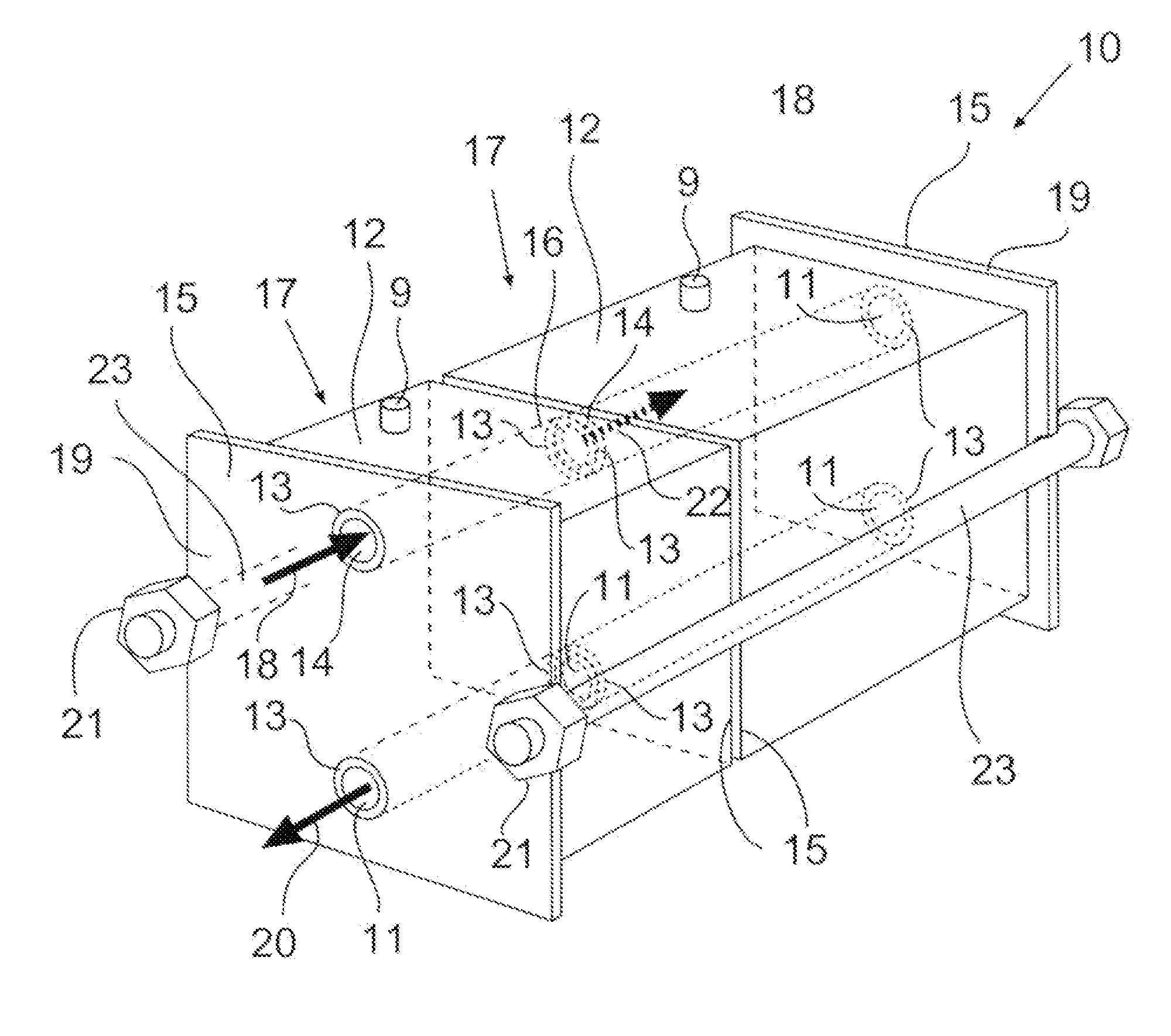
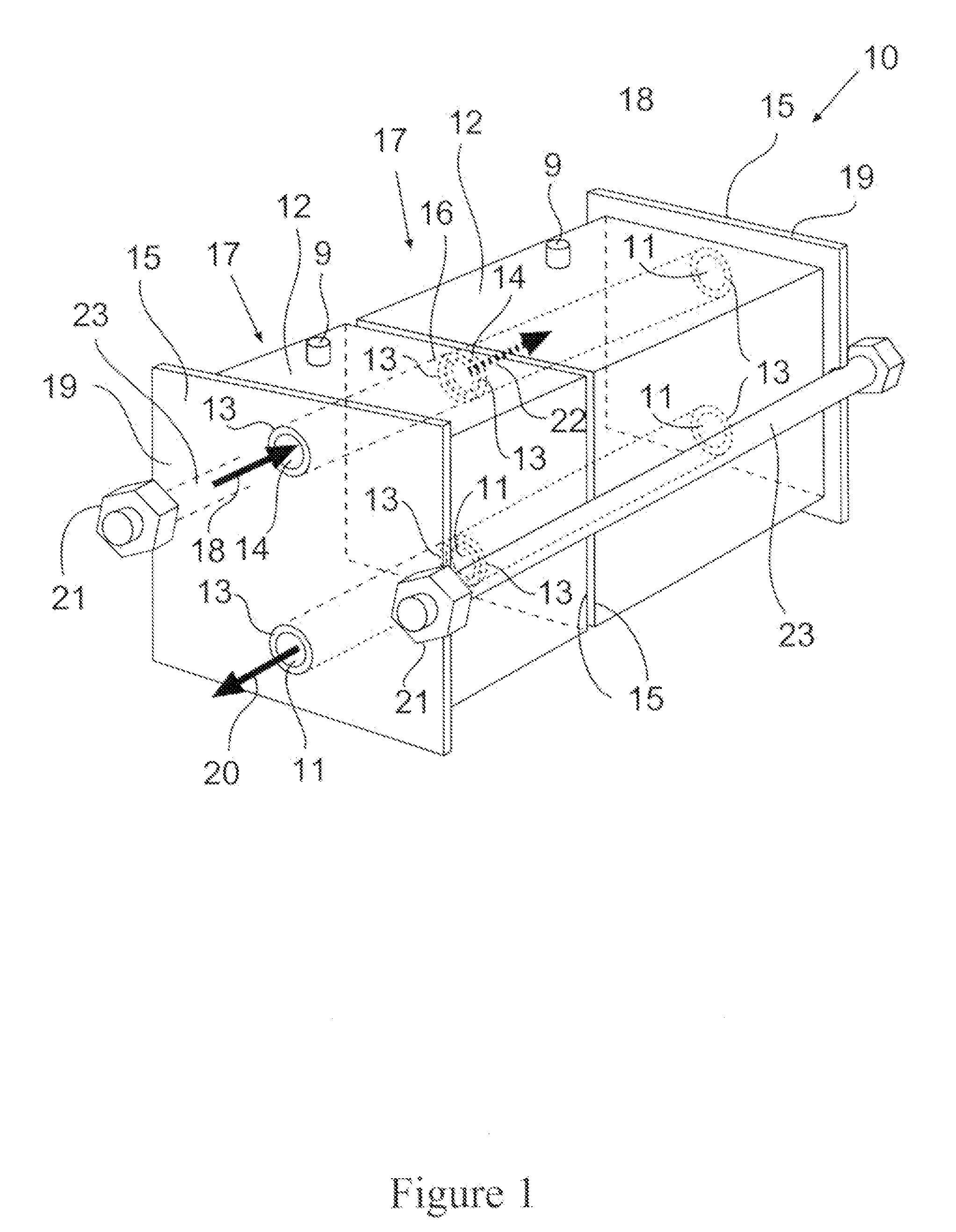
Chromatographic module and chromatographic apparatus
PatentInactiveEP2138839A2
Innovation
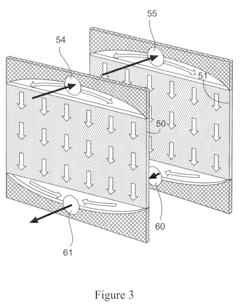
- A modular chromatographic apparatus composed of identical modules that can be connected in parallel or series to achieve linear scalability, allowing for a wide range of effective volumes and flexible operation, with each module having a consistent wall effect and identical packing, enabling efficient fluid flow and resin utilization.
Chromatography apparatus
PatentInactiveUS20120118807A1
Innovation
- A modular chromatography apparatus with identical modules that can be connected in parallel or series, maintaining consistent wall effects and resin volume, allowing for linear scalability and flexible capacity adjustments.
Regulatory Compliance in Scaled-up Chromatography Processes
Regulatory compliance represents a critical aspect of scaling chromatography processes from laboratory to pilot scale. As biopharmaceutical manufacturing advances, regulatory bodies including the FDA, EMA, and ICH have established increasingly stringent requirements governing the scale-up procedures. These regulations primarily focus on ensuring product quality, consistency, and patient safety throughout the transition process.
The Quality by Design (QbD) framework has become fundamental to regulatory compliance in chromatography scale-up. This approach requires thorough understanding of critical process parameters (CPPs) and critical quality attributes (CQAs) that must be maintained across different scales. When scaling columns, resins, and flow rates, manufacturers must demonstrate that these parameters remain within established design spaces to satisfy regulatory expectations.
Process validation constitutes another essential regulatory requirement during chromatography scale-up. This typically involves three stages: process design, process qualification, and continued process verification. Each stage requires comprehensive documentation demonstrating that the scaled-up chromatography process consistently produces material meeting predetermined quality specifications.
Material compatibility and extractables/leachables testing gain heightened importance during scale-up. Regulatory agencies require thorough assessment of potential interactions between process fluids and chromatography components at larger scales, where contact times and surface areas increase significantly. This includes evaluation of resin stability and potential leaching of compounds from columns under scaled-up conditions.
Documentation requirements expand substantially during scale-up transitions. Manufacturers must maintain detailed records of equipment qualification, method transfer protocols, analytical method validation, and process parameter justifications. Change management documentation becomes particularly crucial when modifying column dimensions, resin volumes, or flow parameters.
Cleaning validation presents unique regulatory challenges in scaled-up chromatography. Larger columns and increased resin volumes necessitate demonstration that cleaning procedures effectively remove product residues, process impurities, and cleaning agents. This validation must account for the geometric differences between laboratory and pilot-scale equipment.
Risk assessment methodologies have become increasingly important in regulatory submissions for chromatography scale-up. Manufacturers must identify potential failure modes associated with changes in column dimensions, resin characteristics, and flow dynamics, then implement appropriate control strategies to mitigate these risks.
Regulatory expectations also extend to analytical comparability studies demonstrating that products manufactured at different scales maintain equivalent quality profiles. This includes comprehensive characterization of product-related impurities, process-related impurities, and biological activity across laboratory and pilot scales.
The Quality by Design (QbD) framework has become fundamental to regulatory compliance in chromatography scale-up. This approach requires thorough understanding of critical process parameters (CPPs) and critical quality attributes (CQAs) that must be maintained across different scales. When scaling columns, resins, and flow rates, manufacturers must demonstrate that these parameters remain within established design spaces to satisfy regulatory expectations.
Process validation constitutes another essential regulatory requirement during chromatography scale-up. This typically involves three stages: process design, process qualification, and continued process verification. Each stage requires comprehensive documentation demonstrating that the scaled-up chromatography process consistently produces material meeting predetermined quality specifications.
Material compatibility and extractables/leachables testing gain heightened importance during scale-up. Regulatory agencies require thorough assessment of potential interactions between process fluids and chromatography components at larger scales, where contact times and surface areas increase significantly. This includes evaluation of resin stability and potential leaching of compounds from columns under scaled-up conditions.
Documentation requirements expand substantially during scale-up transitions. Manufacturers must maintain detailed records of equipment qualification, method transfer protocols, analytical method validation, and process parameter justifications. Change management documentation becomes particularly crucial when modifying column dimensions, resin volumes, or flow parameters.
Cleaning validation presents unique regulatory challenges in scaled-up chromatography. Larger columns and increased resin volumes necessitate demonstration that cleaning procedures effectively remove product residues, process impurities, and cleaning agents. This validation must account for the geometric differences between laboratory and pilot-scale equipment.
Risk assessment methodologies have become increasingly important in regulatory submissions for chromatography scale-up. Manufacturers must identify potential failure modes associated with changes in column dimensions, resin characteristics, and flow dynamics, then implement appropriate control strategies to mitigate these risks.
Regulatory expectations also extend to analytical comparability studies demonstrating that products manufactured at different scales maintain equivalent quality profiles. This includes comprehensive characterization of product-related impurities, process-related impurities, and biological activity across laboratory and pilot scales.
Economic Considerations for Chromatography Scale-up
The economic impact of scaling chromatography processes from laboratory to pilot scale represents a critical consideration for biopharmaceutical companies. Initial capital expenditure for pilot-scale chromatography equipment typically ranges from $100,000 to $500,000, depending on the level of automation and monitoring capabilities required. This investment must be carefully weighed against the projected timeline for return on investment and the strategic value of the developed process.
Resin costs constitute a significant portion of operational expenses, with prices varying from $1,000 to $10,000 per liter depending on the type and specificity. When scaling up, the economic efficiency of resin utilization becomes increasingly important, as underutilized resin capacity directly impacts cost-effectiveness. Companies must evaluate resin lifetime across multiple cycles and potential regeneration protocols to maximize economic value.
Buffer consumption increases substantially during scale-up, with pilot operations potentially requiring hundreds of liters per run. Implementation of buffer management strategies, including inline dilution systems and buffer recycling technologies, can reduce costs by 20-40% while simultaneously addressing environmental sustainability concerns. These considerations become particularly relevant when projecting costs for eventual commercial-scale operations.
Process development time represents another crucial economic factor. Extended development cycles directly impact time-to-market and competitive positioning. Implementing high-throughput screening methodologies and design of experiments (DoE) approaches during early development stages can significantly reduce the number of pilot-scale runs required, potentially saving weeks to months of development time and associated costs.
Labor requirements shift dramatically during scale-up, transitioning from scientist-intensive laboratory work to operations requiring engineering expertise. This transition necessitates careful workforce planning and potentially additional training investments. Automation systems, while increasing initial capital costs, can reduce long-term labor expenses and improve process consistency.
Facility considerations, including clean room requirements, utilities consumption, and waste management, contribute substantially to the overall economic equation. Flexible manufacturing approaches, such as single-use technologies, may offer economic advantages for certain applications by reducing cleaning validation requirements and changeover times between campaigns.
Risk assessment must incorporate economic dimensions, particularly regarding batch failures at larger scales. A single failed pilot batch may represent a loss of $10,000-$50,000 in materials alone, not accounting for opportunity costs and timeline impacts. Implementing robust process analytical technology (PAT) and appropriate scale-down models can mitigate these risks through improved process understanding and control strategy development.
Resin costs constitute a significant portion of operational expenses, with prices varying from $1,000 to $10,000 per liter depending on the type and specificity. When scaling up, the economic efficiency of resin utilization becomes increasingly important, as underutilized resin capacity directly impacts cost-effectiveness. Companies must evaluate resin lifetime across multiple cycles and potential regeneration protocols to maximize economic value.
Buffer consumption increases substantially during scale-up, with pilot operations potentially requiring hundreds of liters per run. Implementation of buffer management strategies, including inline dilution systems and buffer recycling technologies, can reduce costs by 20-40% while simultaneously addressing environmental sustainability concerns. These considerations become particularly relevant when projecting costs for eventual commercial-scale operations.
Process development time represents another crucial economic factor. Extended development cycles directly impact time-to-market and competitive positioning. Implementing high-throughput screening methodologies and design of experiments (DoE) approaches during early development stages can significantly reduce the number of pilot-scale runs required, potentially saving weeks to months of development time and associated costs.
Labor requirements shift dramatically during scale-up, transitioning from scientist-intensive laboratory work to operations requiring engineering expertise. This transition necessitates careful workforce planning and potentially additional training investments. Automation systems, while increasing initial capital costs, can reduce long-term labor expenses and improve process consistency.
Facility considerations, including clean room requirements, utilities consumption, and waste management, contribute substantially to the overall economic equation. Flexible manufacturing approaches, such as single-use technologies, may offer economic advantages for certain applications by reducing cleaning validation requirements and changeover times between campaigns.
Risk assessment must incorporate economic dimensions, particularly regarding batch failures at larger scales. A single failed pilot batch may represent a loss of $10,000-$50,000 in materials alone, not accounting for opportunity costs and timeline impacts. Implementing robust process analytical technology (PAT) and appropriate scale-down models can mitigate these risks through improved process understanding and control strategy development.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!