Conflict of Goethitization in Iron-Based MOFs for Catalysis
AUG 11, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
MOF Goethitization Background and Objectives
Metal-Organic Frameworks (MOFs) have emerged as a revolutionary class of porous materials with exceptional potential in catalysis, gas storage, and separation processes. Among these, iron-based MOFs have garnered significant attention due to their abundance, low toxicity, and versatile redox properties. However, a critical challenge in their application for catalysis is the phenomenon of goethitization, which refers to the transformation of iron-based MOFs into goethite (α-FeOOH) under certain conditions.
The evolution of iron-based MOFs and their catalytic applications can be traced back to the early 2000s when researchers began exploring their unique structural and chemical properties. As the field progressed, the potential of these materials in various catalytic reactions became increasingly apparent. However, the issue of goethitization emerged as a significant obstacle, particularly in aqueous and oxidative environments, limiting the long-term stability and efficiency of iron-based MOF catalysts.
The conflict of goethitization in iron-based MOFs for catalysis presents a complex interplay between desired catalytic activity and undesired structural transformation. This phenomenon is driven by the inherent reactivity of iron centers and their susceptibility to hydrolysis and oxidation. Understanding and mitigating this process is crucial for developing stable and efficient iron-based MOF catalysts for practical applications.
The primary objective of this technical research is to comprehensively examine the goethitization process in iron-based MOFs, with a focus on its impact on catalytic performance. This involves investigating the underlying mechanisms, identifying key factors influencing the transformation, and exploring strategies to enhance the stability of iron-based MOFs under catalytic conditions.
Furthermore, this research aims to elucidate the relationship between MOF structure, composition, and susceptibility to goethitization. By analyzing various iron-based MOF architectures and their behavior in different catalytic environments, we seek to establish design principles for developing more resistant MOF catalysts. This includes exploring the role of ligand design, metal node engineering, and post-synthetic modifications in mitigating goethitization.
Another crucial aspect of this study is to evaluate the potential benefits and drawbacks of controlled goethitization in certain catalytic applications. While generally considered detrimental, there may be instances where partial or controlled goethitization could enhance catalytic activity or selectivity. Understanding these nuances is essential for optimizing iron-based MOF catalysts for specific reactions and conditions.
Ultimately, this research aims to bridge the gap between fundamental understanding and practical application of iron-based MOFs in catalysis. By addressing the conflict of goethitization, we seek to unlock the full potential of these materials, paving the way for their widespread adoption in industrial catalytic processes and contributing to the development of more sustainable and efficient chemical technologies.
The evolution of iron-based MOFs and their catalytic applications can be traced back to the early 2000s when researchers began exploring their unique structural and chemical properties. As the field progressed, the potential of these materials in various catalytic reactions became increasingly apparent. However, the issue of goethitization emerged as a significant obstacle, particularly in aqueous and oxidative environments, limiting the long-term stability and efficiency of iron-based MOF catalysts.
The conflict of goethitization in iron-based MOFs for catalysis presents a complex interplay between desired catalytic activity and undesired structural transformation. This phenomenon is driven by the inherent reactivity of iron centers and their susceptibility to hydrolysis and oxidation. Understanding and mitigating this process is crucial for developing stable and efficient iron-based MOF catalysts for practical applications.
The primary objective of this technical research is to comprehensively examine the goethitization process in iron-based MOFs, with a focus on its impact on catalytic performance. This involves investigating the underlying mechanisms, identifying key factors influencing the transformation, and exploring strategies to enhance the stability of iron-based MOFs under catalytic conditions.
Furthermore, this research aims to elucidate the relationship between MOF structure, composition, and susceptibility to goethitization. By analyzing various iron-based MOF architectures and their behavior in different catalytic environments, we seek to establish design principles for developing more resistant MOF catalysts. This includes exploring the role of ligand design, metal node engineering, and post-synthetic modifications in mitigating goethitization.
Another crucial aspect of this study is to evaluate the potential benefits and drawbacks of controlled goethitization in certain catalytic applications. While generally considered detrimental, there may be instances where partial or controlled goethitization could enhance catalytic activity or selectivity. Understanding these nuances is essential for optimizing iron-based MOF catalysts for specific reactions and conditions.
Ultimately, this research aims to bridge the gap between fundamental understanding and practical application of iron-based MOFs in catalysis. By addressing the conflict of goethitization, we seek to unlock the full potential of these materials, paving the way for their widespread adoption in industrial catalytic processes and contributing to the development of more sustainable and efficient chemical technologies.
Catalysis Market Demand Analysis
The catalysis market has been experiencing significant growth and transformation, driven by increasing demand across various industries. In the context of iron-based Metal-Organic Frameworks (MOFs) for catalysis, the market demand is particularly influenced by the need for more efficient and sustainable catalytic processes in chemical manufacturing, environmental remediation, and energy production.
The global catalysis market is projected to expand substantially in the coming years, with a notable focus on heterogeneous catalysts. Iron-based MOFs, as a subset of this market, are gaining traction due to their potential for high catalytic activity, selectivity, and recyclability. These materials offer promising applications in areas such as fine chemical synthesis, pollutant degradation, and fuel cell technology.
One of the key drivers for the demand of iron-based MOF catalysts is the growing emphasis on green chemistry and sustainable production methods. Industries are increasingly seeking catalysts that can operate under milder conditions, reduce waste generation, and improve overall process efficiency. Iron-based MOFs align well with these requirements, offering the potential for more environmentally friendly catalytic processes.
The automotive and transportation sectors represent a significant market for catalysts, particularly in emission control systems. As regulations become more stringent worldwide, there is a rising demand for advanced catalytic materials that can effectively reduce harmful emissions. Iron-based MOFs are being explored as potential alternatives or enhancements to traditional catalytic converters, driving research and development in this area.
In the pharmaceutical and fine chemicals industries, there is a constant need for selective and efficient catalysts for complex organic transformations. Iron-based MOFs are attracting attention in this sector due to their tunable properties and potential for high selectivity in catalytic reactions. This presents opportunities for market growth as researchers and companies explore the application of these materials in drug synthesis and specialty chemical production.
The energy sector, particularly in the realm of renewable energy and fuel cells, is another area driving demand for advanced catalytic materials. Iron-based MOFs are being investigated for their potential in electrocatalysis, photocatalysis, and energy storage applications. As the world transitions towards cleaner energy sources, the demand for efficient and cost-effective catalysts in these areas is expected to increase significantly.
However, the market demand for iron-based MOF catalysts is not without challenges. The issue of goethitization, which can affect the stability and performance of these materials, represents a significant hurdle that needs to be addressed. Overcoming this challenge is crucial for the widespread adoption of iron-based MOFs in industrial catalytic applications and will likely influence market dynamics in the coming years.
The global catalysis market is projected to expand substantially in the coming years, with a notable focus on heterogeneous catalysts. Iron-based MOFs, as a subset of this market, are gaining traction due to their potential for high catalytic activity, selectivity, and recyclability. These materials offer promising applications in areas such as fine chemical synthesis, pollutant degradation, and fuel cell technology.
One of the key drivers for the demand of iron-based MOF catalysts is the growing emphasis on green chemistry and sustainable production methods. Industries are increasingly seeking catalysts that can operate under milder conditions, reduce waste generation, and improve overall process efficiency. Iron-based MOFs align well with these requirements, offering the potential for more environmentally friendly catalytic processes.
The automotive and transportation sectors represent a significant market for catalysts, particularly in emission control systems. As regulations become more stringent worldwide, there is a rising demand for advanced catalytic materials that can effectively reduce harmful emissions. Iron-based MOFs are being explored as potential alternatives or enhancements to traditional catalytic converters, driving research and development in this area.
In the pharmaceutical and fine chemicals industries, there is a constant need for selective and efficient catalysts for complex organic transformations. Iron-based MOFs are attracting attention in this sector due to their tunable properties and potential for high selectivity in catalytic reactions. This presents opportunities for market growth as researchers and companies explore the application of these materials in drug synthesis and specialty chemical production.
The energy sector, particularly in the realm of renewable energy and fuel cells, is another area driving demand for advanced catalytic materials. Iron-based MOFs are being investigated for their potential in electrocatalysis, photocatalysis, and energy storage applications. As the world transitions towards cleaner energy sources, the demand for efficient and cost-effective catalysts in these areas is expected to increase significantly.
However, the market demand for iron-based MOF catalysts is not without challenges. The issue of goethitization, which can affect the stability and performance of these materials, represents a significant hurdle that needs to be addressed. Overcoming this challenge is crucial for the widespread adoption of iron-based MOFs in industrial catalytic applications and will likely influence market dynamics in the coming years.
Iron-Based MOFs: Current State and Challenges
Iron-based Metal-Organic Frameworks (MOFs) have emerged as promising materials for catalysis due to their high surface area, tunable pore structure, and abundant active sites. However, the field faces significant challenges that hinder their widespread application and commercialization.
One of the primary challenges is the stability of iron-based MOFs under catalytic conditions. Many iron-based MOFs suffer from structural degradation when exposed to moisture, high temperatures, or certain chemical environments. This instability often leads to a loss of catalytic activity and selectivity over time, limiting their long-term performance and reusability.
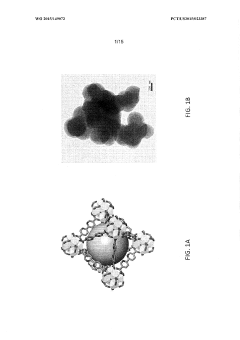
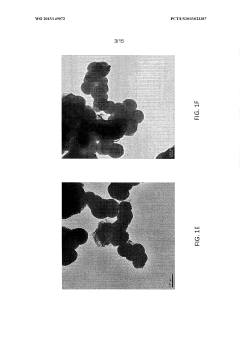
The phenomenon of goethitization presents a particular challenge for iron-based MOFs in catalysis. Goethitization refers to the transformation of iron species within the MOF structure into goethite (α-FeO(OH)), an iron oxyhydroxide mineral. This process can occur under various conditions, including exposure to water or oxidizing environments, and results in the collapse of the MOF structure and loss of its unique properties.
Another significant challenge is the control of iron oxidation states within the MOF framework. The catalytic activity of iron-based MOFs often depends on the specific oxidation state of the iron centers. However, maintaining the desired oxidation state throughout the catalytic process can be difficult, as iron can easily undergo redox changes under reaction conditions.
The synthesis of iron-based MOFs with high crystallinity and uniform pore distribution remains challenging. Achieving precise control over the MOF structure, especially when incorporating iron as the metal node, often requires careful optimization of synthesis conditions. This challenge becomes more pronounced when attempting to scale up production for industrial applications.
Furthermore, the rational design of iron-based MOFs for specific catalytic applications is hindered by the complex interplay between the MOF structure, iron centers, and catalytic substrates. Predicting and tailoring the catalytic performance of these materials often requires extensive experimental work and computational modeling.
Despite these challenges, research in iron-based MOFs for catalysis continues to advance. Scientists are exploring various strategies to enhance the stability of these materials, such as post-synthetic modifications, incorporation of stabilizing ligands, and development of core-shell structures. Additionally, efforts are being made to better understand the mechanisms of goethitization and develop methods to mitigate its effects.
One of the primary challenges is the stability of iron-based MOFs under catalytic conditions. Many iron-based MOFs suffer from structural degradation when exposed to moisture, high temperatures, or certain chemical environments. This instability often leads to a loss of catalytic activity and selectivity over time, limiting their long-term performance and reusability.
The phenomenon of goethitization presents a particular challenge for iron-based MOFs in catalysis. Goethitization refers to the transformation of iron species within the MOF structure into goethite (α-FeO(OH)), an iron oxyhydroxide mineral. This process can occur under various conditions, including exposure to water or oxidizing environments, and results in the collapse of the MOF structure and loss of its unique properties.
Another significant challenge is the control of iron oxidation states within the MOF framework. The catalytic activity of iron-based MOFs often depends on the specific oxidation state of the iron centers. However, maintaining the desired oxidation state throughout the catalytic process can be difficult, as iron can easily undergo redox changes under reaction conditions.
The synthesis of iron-based MOFs with high crystallinity and uniform pore distribution remains challenging. Achieving precise control over the MOF structure, especially when incorporating iron as the metal node, often requires careful optimization of synthesis conditions. This challenge becomes more pronounced when attempting to scale up production for industrial applications.
Furthermore, the rational design of iron-based MOFs for specific catalytic applications is hindered by the complex interplay between the MOF structure, iron centers, and catalytic substrates. Predicting and tailoring the catalytic performance of these materials often requires extensive experimental work and computational modeling.
Despite these challenges, research in iron-based MOFs for catalysis continues to advance. Scientists are exploring various strategies to enhance the stability of these materials, such as post-synthetic modifications, incorporation of stabilizing ligands, and development of core-shell structures. Additionally, efforts are being made to better understand the mechanisms of goethitization and develop methods to mitigate its effects.
Current Solutions for Goethitization Mitigation
01 Synthesis of iron-based MOFs for goethitization
Iron-based Metal-Organic Frameworks (MOFs) are synthesized using various methods to create precursors for goethitization. These MOFs serve as templates or starting materials for the formation of goethite (α-FeOOH) structures. The synthesis process often involves controlling factors such as temperature, pH, and reaction time to optimize the MOF structure for subsequent goethitization.- Synthesis of iron-based MOFs for goethitization: Iron-based Metal-Organic Frameworks (MOFs) are synthesized using various methods to create structures suitable for goethitization. These MOFs serve as precursors or templates for the formation of goethite (α-FeOOH) nanostructures. The synthesis process often involves controlling factors such as temperature, pH, and reaction time to achieve desired morphologies and properties.
- Goethitization process of iron-based MOFs: The goethitization process involves the transformation of iron-based MOFs into goethite structures. This typically occurs through controlled hydrolysis or oxidation of the iron centers in the MOF framework. The process may be influenced by factors such as humidity, temperature, and the presence of specific ions or additives. The resulting goethite structures often retain some characteristics of the original MOF, such as high surface area or porosity.
- Applications of goethitized iron-based MOFs: Goethitized iron-based MOFs find applications in various fields due to their unique properties. These materials are used in environmental remediation, catalysis, energy storage, and sensing applications. The high surface area, controlled porosity, and iron oxide content make them particularly suitable for adsorption of pollutants, catalytic reactions, and electrochemical processes.
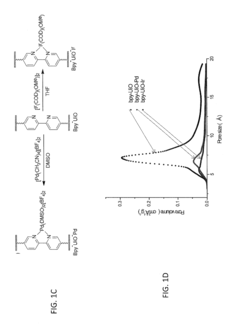
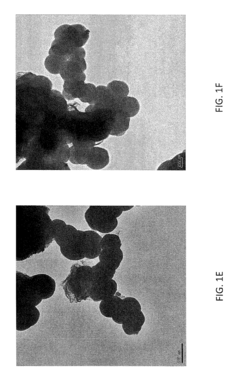
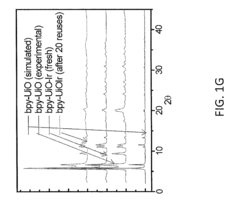
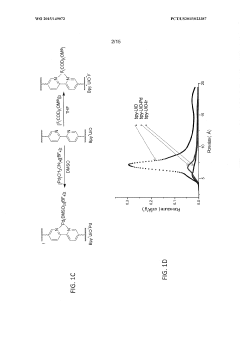
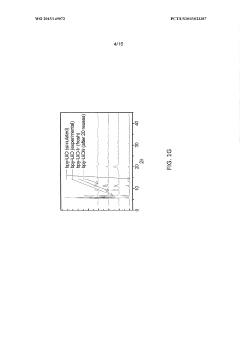
- Characterization techniques for goethitized MOFs: Various analytical techniques are employed to characterize the structure, composition, and properties of goethitized iron-based MOFs. These include X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), Fourier-transform infrared spectroscopy (FTIR), and Brunauer-Emmett-Teller (BET) surface area analysis. These techniques help in understanding the transformation process and the resulting material properties.
- Optimization of goethitization conditions: Research focuses on optimizing the conditions for goethitization of iron-based MOFs to achieve desired properties. This includes studying the effects of temperature, pH, reaction time, and the presence of additives on the goethitization process. The goal is to control the morphology, particle size, and crystallinity of the resulting goethite structures, which in turn influence their performance in various applications.
02 Goethitization process of iron-based MOFs
The goethitization process involves the transformation of iron-based MOFs into goethite structures. This typically includes hydrolysis, oxidation, and/or thermal treatment steps. The process may be carried out under controlled conditions to maintain the desired morphology and properties of the resulting goethite material. Factors such as temperature, pressure, and the presence of specific ions or additives can influence the goethitization process.Expand Specific Solutions03 Applications of goethitized iron-based MOFs
Goethitized iron-based MOFs find applications in various fields due to their unique properties. These materials can be used in environmental remediation, catalysis, energy storage, and sensing applications. The high surface area, tunable pore structure, and iron oxide content make them particularly suitable for adsorption of pollutants, catalytic reactions, and electrochemical processes.Expand Specific Solutions04 Characterization techniques for goethitized MOFs
Various characterization techniques are employed to analyze the properties and structure of goethitized iron-based MOFs. These may include X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), Fourier-transform infrared spectroscopy (FTIR), and Brunauer-Emmett-Teller (BET) surface area analysis. These techniques help in understanding the crystalline structure, morphology, surface properties, and pore characteristics of the goethitized materials.Expand Specific Solutions05 Modification and functionalization of goethitized MOFs
Goethitized iron-based MOFs can be further modified or functionalized to enhance their properties or tailor them for specific applications. This may involve doping with other metals, surface modification with organic ligands, or incorporation of additional functional groups. Such modifications can improve the material's catalytic activity, adsorption capacity, or selectivity for target molecules.Expand Specific Solutions
Key Players in MOF Catalysis Research
The field of iron-based MOFs for catalysis is in a dynamic growth phase, with significant market potential due to their versatile applications in various industries. The technology is advancing rapidly, but still faces challenges in scalability and stability. Key players like Northwestern University, SABIC, and BASF are driving innovation, focusing on enhancing goethitization control and catalytic performance. Emerging research from institutions such as Korea Research Institute of Chemical Technology and China Petroleum & Chemical Corp. is contributing to the field's expansion. As the technology matures, collaboration between academia and industry is intensifying, aiming to overcome current limitations and unlock new applications in sustainable chemistry and energy sectors.
Northwestern University
Technical Solution: Northwestern University has developed innovative approaches to address the conflict of goethitization in iron-based MOFs for catalysis. Their research focuses on stabilizing the iron centers within the MOF structure to prevent the formation of goethite (α-FeOOH) during catalytic reactions. They have implemented a strategy of incorporating secondary metal ions, such as aluminum or chromium, into the MOF framework to create bimetallic nodes[1]. This approach helps to maintain the structural integrity of the MOF and preserves its catalytic activity. Additionally, they have explored the use of organic linkers with strong coordination abilities to enhance the stability of iron sites[3]. Their work has demonstrated improved catalytic performance in various reactions, including oxidation and hydrogenation processes, with extended catalyst lifetime and reduced deactivation due to goethitization[5].
Strengths: Enhanced stability of iron centers, improved catalytic longevity, and versatility in various reactions. Weaknesses: Potential complexity in synthesis and higher cost due to the use of secondary metals.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed a novel approach to mitigate the conflict of goethitization in iron-based MOFs for catalysis, particularly focusing on applications in the petrochemical industry. Their strategy involves the use of a protective coating technique, where the iron-based MOF particles are encapsulated within a thin layer of porous silica or alumina[2]. This coating acts as a barrier against moisture and oxygen, which are primary factors in goethitization. The company has also explored the incorporation of hydrophobic functional groups on the organic linkers of the MOF to repel water molecules[4]. In addition, Sinopec has developed a regeneration process that can restore the catalytic activity of partially goethitized MOFs, extending their operational lifespan in industrial settings[6]. Their research has shown promising results in maintaining catalytic activity for extended periods in harsh reaction conditions typical of petrochemical processes.
Strengths: Effective protection against goethitization, applicability in industrial settings, and innovative regeneration techniques. Weaknesses: Potential mass transfer limitations due to protective coating and possible reduction in overall catalytic activity.
Core Innovations in MOF Stability Enhancement
Metal-organic frameworks containing nitrogen-donor ligands for efficient catalytic organic transformations
PatentActiveUS20170182486A1
Innovation
- The development of crystalline and porous metal-organic frameworks (MOFs) using nitrogen donor-based bridging ligands such as bipyridines, phenanthrolines, and salicylaldimines, which are complexed with metal sources like Zr, Hf, Zn, and Cu to create catalytically active sites, allowing for the catalysis of organic transformations like borylation, hydrogenation, and hydroboration.
Metal-organic frameworks containing nitrogen-donor ligands for efficient catalytic organic transformations
PatentWO2015149072A1
Innovation
- The development of metal-organic frameworks (MOFs) containing nitrogen donor-based bridging ligands, such as bipyridines, phenanthrolines, and salicylaldimines, which are used to create crystalline and porous MOFs by contacting nitrogen donor ligands with metal sources, allowing for post-synthetic metalation with catalysts like Ir, Pd, and Co to enhance catalytic activity and selectivity.
Environmental Impact of MOF Catalysis
The environmental impact of Metal-Organic Framework (MOF) catalysis, particularly in the context of iron-based MOFs and the conflict of goethitization, is a critical consideration in the development and application of these materials. MOFs have gained significant attention in catalysis due to their high surface area, tunable pore size, and diverse functionalities. However, their environmental implications must be carefully evaluated.
Iron-based MOFs, while promising for various catalytic applications, face challenges related to goethitization, which can affect their environmental footprint. Goethitization, the transformation of iron-containing compounds into goethite (α-FeO(OH)), can occur under certain conditions, potentially altering the catalytic properties and stability of these MOFs. This process may lead to changes in the material's structure and performance, impacting its overall environmental profile.
The use of iron-based MOFs in catalysis can contribute to more sustainable chemical processes by enabling reactions under milder conditions and with improved selectivity. This can result in reduced energy consumption and decreased production of unwanted by-products, thereby minimizing the environmental impact of chemical manufacturing. Additionally, the high catalytic efficiency of MOFs may lead to reduced catalyst loading, potentially decreasing the overall material consumption and associated environmental burdens.
However, the synthesis and disposal of MOFs also present environmental considerations. The production of MOFs often involves the use of organic solvents and metal precursors, which may have their own environmental implications. Efforts to develop more environmentally friendly synthesis methods, such as mechanochemical approaches or the use of bio-based linkers, are ongoing to address these concerns.
The stability and recyclability of iron-based MOFs in the presence of goethitization are crucial factors in assessing their long-term environmental impact. If goethitization leads to rapid degradation of the MOF structure, it may necessitate more frequent catalyst replacement, potentially increasing waste generation and resource consumption. Conversely, if the goethitization process can be controlled or even harnessed to enhance catalytic activity, it could lead to more durable and efficient catalysts with a reduced environmental footprint.
The potential leaching of iron or organic components from MOFs during catalytic processes is another environmental consideration. While iron is generally considered less toxic compared to many other metals used in catalysis, its release into the environment should still be minimized. The organic linkers used in MOFs may also have varying degrees of environmental persistence and toxicity, necessitating careful selection and evaluation of these components.
In conclusion, the environmental impact of MOF catalysis, particularly in the context of iron-based MOFs and goethitization, is a complex issue that requires comprehensive assessment. While these materials offer promising benefits in terms of improved catalytic efficiency and potential for greener chemical processes, their entire life cycle, from synthesis to disposal, must be considered to fully understand and optimize their environmental performance.
Iron-based MOFs, while promising for various catalytic applications, face challenges related to goethitization, which can affect their environmental footprint. Goethitization, the transformation of iron-containing compounds into goethite (α-FeO(OH)), can occur under certain conditions, potentially altering the catalytic properties and stability of these MOFs. This process may lead to changes in the material's structure and performance, impacting its overall environmental profile.
The use of iron-based MOFs in catalysis can contribute to more sustainable chemical processes by enabling reactions under milder conditions and with improved selectivity. This can result in reduced energy consumption and decreased production of unwanted by-products, thereby minimizing the environmental impact of chemical manufacturing. Additionally, the high catalytic efficiency of MOFs may lead to reduced catalyst loading, potentially decreasing the overall material consumption and associated environmental burdens.
However, the synthesis and disposal of MOFs also present environmental considerations. The production of MOFs often involves the use of organic solvents and metal precursors, which may have their own environmental implications. Efforts to develop more environmentally friendly synthesis methods, such as mechanochemical approaches or the use of bio-based linkers, are ongoing to address these concerns.
The stability and recyclability of iron-based MOFs in the presence of goethitization are crucial factors in assessing their long-term environmental impact. If goethitization leads to rapid degradation of the MOF structure, it may necessitate more frequent catalyst replacement, potentially increasing waste generation and resource consumption. Conversely, if the goethitization process can be controlled or even harnessed to enhance catalytic activity, it could lead to more durable and efficient catalysts with a reduced environmental footprint.
The potential leaching of iron or organic components from MOFs during catalytic processes is another environmental consideration. While iron is generally considered less toxic compared to many other metals used in catalysis, its release into the environment should still be minimized. The organic linkers used in MOFs may also have varying degrees of environmental persistence and toxicity, necessitating careful selection and evaluation of these components.
In conclusion, the environmental impact of MOF catalysis, particularly in the context of iron-based MOFs and goethitization, is a complex issue that requires comprehensive assessment. While these materials offer promising benefits in terms of improved catalytic efficiency and potential for greener chemical processes, their entire life cycle, from synthesis to disposal, must be considered to fully understand and optimize their environmental performance.
Scalability and Industrial Application Prospects
The scalability and industrial application prospects of iron-based MOFs for catalysis are promising, despite the challenges posed by goethitization. The potential for large-scale production and implementation in various industrial processes makes these materials attractive for commercial applications.
One of the key advantages of iron-based MOFs is their relatively low cost and abundance of raw materials. Iron is one of the most abundant elements on Earth, making it an economically viable option for large-scale production. This factor significantly contributes to the scalability of iron-based MOFs, as it reduces the overall production costs and ensures a stable supply chain.
The synthesis methods for iron-based MOFs have been extensively studied and optimized, allowing for potential scale-up in industrial settings. Techniques such as continuous flow synthesis and mechanochemical methods have shown promise in producing large quantities of MOFs with consistent quality. These advancements in synthesis techniques pave the way for industrial-scale production of iron-based MOFs for catalytic applications.
In terms of industrial applications, iron-based MOFs show potential in various sectors. The petrochemical industry could benefit from these materials in processes such as hydrogenation, oxidation, and isomerization reactions. Environmental applications, including water treatment and air purification, represent another significant area where iron-based MOFs could be implemented on a large scale.
The automotive industry is another sector that could potentially utilize iron-based MOFs in catalytic converters, leveraging their high surface area and tunable properties to enhance emission control systems. Additionally, the pharmaceutical industry may find applications for these materials in drug synthesis and purification processes.
However, the conflict of goethitization presents a challenge to the widespread industrial adoption of iron-based MOFs. The transformation of iron species into goethite under certain conditions can lead to reduced catalytic activity and stability. To address this issue, researchers are exploring various strategies, such as the incorporation of stabilizing agents or the development of core-shell structures to protect the iron centers.
As research progresses in mitigating the goethitization problem, the industrial prospects for iron-based MOFs in catalysis continue to expand. The development of more robust and stable iron-based MOFs could open up new opportunities in sectors such as energy storage, gas separation, and sustainable chemical production.
In conclusion, the scalability and industrial application prospects of iron-based MOFs for catalysis are promising, with potential impacts across multiple industries. Ongoing research to overcome the goethitization challenge will be crucial in realizing the full potential of these materials in large-scale industrial applications.
One of the key advantages of iron-based MOFs is their relatively low cost and abundance of raw materials. Iron is one of the most abundant elements on Earth, making it an economically viable option for large-scale production. This factor significantly contributes to the scalability of iron-based MOFs, as it reduces the overall production costs and ensures a stable supply chain.
The synthesis methods for iron-based MOFs have been extensively studied and optimized, allowing for potential scale-up in industrial settings. Techniques such as continuous flow synthesis and mechanochemical methods have shown promise in producing large quantities of MOFs with consistent quality. These advancements in synthesis techniques pave the way for industrial-scale production of iron-based MOFs for catalytic applications.
In terms of industrial applications, iron-based MOFs show potential in various sectors. The petrochemical industry could benefit from these materials in processes such as hydrogenation, oxidation, and isomerization reactions. Environmental applications, including water treatment and air purification, represent another significant area where iron-based MOFs could be implemented on a large scale.
The automotive industry is another sector that could potentially utilize iron-based MOFs in catalytic converters, leveraging their high surface area and tunable properties to enhance emission control systems. Additionally, the pharmaceutical industry may find applications for these materials in drug synthesis and purification processes.
However, the conflict of goethitization presents a challenge to the widespread industrial adoption of iron-based MOFs. The transformation of iron species into goethite under certain conditions can lead to reduced catalytic activity and stability. To address this issue, researchers are exploring various strategies, such as the incorporation of stabilizing agents or the development of core-shell structures to protect the iron centers.
As research progresses in mitigating the goethitization problem, the industrial prospects for iron-based MOFs in catalysis continue to expand. The development of more robust and stable iron-based MOFs could open up new opportunities in sectors such as energy storage, gas separation, and sustainable chemical production.
In conclusion, the scalability and industrial application prospects of iron-based MOFs for catalysis are promising, with potential impacts across multiple industries. Ongoing research to overcome the goethitization challenge will be crucial in realizing the full potential of these materials in large-scale industrial applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!