Development of P wave-centred cardiac interfacing technologies
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
P Wave Tech Background
The development of P wave-centred cardiac interfacing technologies represents a significant advancement in the field of cardiology and biomedical engineering. This innovative approach focuses on the P wave, a crucial component of the cardiac cycle that signifies atrial depolarization. The P wave, typically the first deflection seen on an electrocardiogram (ECG), has long been recognized for its diagnostic value in identifying various cardiac arrhythmias and conduction abnormalities.
Historically, cardiac monitoring and interfacing technologies have primarily focused on the QRS complex and T wave, which represent ventricular activity. However, the growing understanding of atrial contributions to overall cardiac function has shifted attention towards the P wave. This paradigm shift has been driven by the increasing prevalence of atrial fibrillation and other atrial arrhythmias, which are often precursors to more severe cardiac conditions.
The evolution of P wave-centred technologies can be traced back to the early 2000s when researchers began to explore advanced signal processing techniques to enhance P wave detection and analysis. These efforts were initially hampered by the low amplitude and variable morphology of P waves, which made them challenging to isolate and interpret accurately.
Significant breakthroughs in sensor technology, particularly in the development of high-sensitivity electrodes and noise reduction algorithms, have paved the way for more reliable P wave detection. Concurrently, advancements in machine learning and artificial intelligence have enabled more sophisticated analysis of P wave characteristics, including duration, amplitude, and morphology.
The primary goal of P wave-centred cardiac interfacing technologies is to provide earlier and more accurate detection of atrial abnormalities. This includes improved diagnosis of conditions such as atrial fibrillation, atrial flutter, and interatrial conduction delays. Additionally, these technologies aim to enhance the monitoring of treatment efficacy for atrial arrhythmias and guide personalized therapeutic interventions.
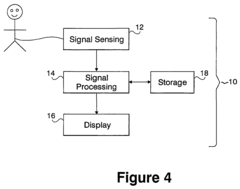
Current research in this field is focused on developing non-invasive, wearable devices that can continuously monitor P wave activity in real-time. These devices promise to revolutionize outpatient cardiac monitoring, enabling long-term data collection and analysis that was previously only possible in clinical settings. The integration of P wave-centred technologies with existing cardiac monitoring systems is expected to provide a more comprehensive view of cardiac function, potentially leading to earlier interventions and improved patient outcomes.
Historically, cardiac monitoring and interfacing technologies have primarily focused on the QRS complex and T wave, which represent ventricular activity. However, the growing understanding of atrial contributions to overall cardiac function has shifted attention towards the P wave. This paradigm shift has been driven by the increasing prevalence of atrial fibrillation and other atrial arrhythmias, which are often precursors to more severe cardiac conditions.
The evolution of P wave-centred technologies can be traced back to the early 2000s when researchers began to explore advanced signal processing techniques to enhance P wave detection and analysis. These efforts were initially hampered by the low amplitude and variable morphology of P waves, which made them challenging to isolate and interpret accurately.
Significant breakthroughs in sensor technology, particularly in the development of high-sensitivity electrodes and noise reduction algorithms, have paved the way for more reliable P wave detection. Concurrently, advancements in machine learning and artificial intelligence have enabled more sophisticated analysis of P wave characteristics, including duration, amplitude, and morphology.
The primary goal of P wave-centred cardiac interfacing technologies is to provide earlier and more accurate detection of atrial abnormalities. This includes improved diagnosis of conditions such as atrial fibrillation, atrial flutter, and interatrial conduction delays. Additionally, these technologies aim to enhance the monitoring of treatment efficacy for atrial arrhythmias and guide personalized therapeutic interventions.
Current research in this field is focused on developing non-invasive, wearable devices that can continuously monitor P wave activity in real-time. These devices promise to revolutionize outpatient cardiac monitoring, enabling long-term data collection and analysis that was previously only possible in clinical settings. The integration of P wave-centred technologies with existing cardiac monitoring systems is expected to provide a more comprehensive view of cardiac function, potentially leading to earlier interventions and improved patient outcomes.
Cardiac Monitoring Needs
Cardiac monitoring plays a crucial role in modern healthcare, providing essential insights into heart function and enabling early detection of cardiovascular issues. The development of P wave-centred cardiac interfacing technologies addresses a significant need in this field, offering potential improvements in accuracy, reliability, and patient outcomes.
The primary market demand for advanced cardiac monitoring stems from the increasing prevalence of cardiovascular diseases worldwide. As populations age and lifestyle-related risk factors become more common, the need for sophisticated monitoring solutions continues to grow. Healthcare providers require tools that can provide comprehensive, real-time data on cardiac function, with a particular focus on early detection and prevention of arrhythmias and other cardiac abnormalities.
P wave-centred technologies offer a promising approach to meeting these needs. By focusing on the P wave, which represents atrial depolarization, these technologies can provide more detailed information about the heart's electrical activity, potentially enabling earlier and more accurate diagnosis of conditions such as atrial fibrillation and other supraventricular arrhythmias.
The market for cardiac monitoring devices is expected to expand significantly in the coming years, driven by technological advancements and increasing awareness of cardiovascular health. There is a growing demand for non-invasive, user-friendly monitoring solutions that can be used in both clinical and home settings. Wearable devices and remote monitoring systems are particularly sought after, as they allow for continuous monitoring without significantly disrupting patients' daily lives.
Healthcare providers are seeking monitoring technologies that can integrate seamlessly with existing electronic health record systems, facilitating data analysis and enabling more personalized patient care. There is also a strong interest in solutions that incorporate artificial intelligence and machine learning algorithms to improve diagnostic accuracy and provide predictive insights.
The development of P wave-centred cardiac interfacing technologies aligns well with the trend towards more precise and personalized medicine. By providing more detailed information about cardiac electrical activity, these technologies could enable healthcare providers to tailor treatment plans more effectively and monitor treatment efficacy more closely.
As the global burden of cardiovascular disease continues to rise, there is an increasing emphasis on preventive care and early intervention. P wave-centred technologies have the potential to play a significant role in this shift, by enabling more accurate risk assessment and earlier detection of developing cardiac issues.
The primary market demand for advanced cardiac monitoring stems from the increasing prevalence of cardiovascular diseases worldwide. As populations age and lifestyle-related risk factors become more common, the need for sophisticated monitoring solutions continues to grow. Healthcare providers require tools that can provide comprehensive, real-time data on cardiac function, with a particular focus on early detection and prevention of arrhythmias and other cardiac abnormalities.
P wave-centred technologies offer a promising approach to meeting these needs. By focusing on the P wave, which represents atrial depolarization, these technologies can provide more detailed information about the heart's electrical activity, potentially enabling earlier and more accurate diagnosis of conditions such as atrial fibrillation and other supraventricular arrhythmias.
The market for cardiac monitoring devices is expected to expand significantly in the coming years, driven by technological advancements and increasing awareness of cardiovascular health. There is a growing demand for non-invasive, user-friendly monitoring solutions that can be used in both clinical and home settings. Wearable devices and remote monitoring systems are particularly sought after, as they allow for continuous monitoring without significantly disrupting patients' daily lives.
Healthcare providers are seeking monitoring technologies that can integrate seamlessly with existing electronic health record systems, facilitating data analysis and enabling more personalized patient care. There is also a strong interest in solutions that incorporate artificial intelligence and machine learning algorithms to improve diagnostic accuracy and provide predictive insights.
The development of P wave-centred cardiac interfacing technologies aligns well with the trend towards more precise and personalized medicine. By providing more detailed information about cardiac electrical activity, these technologies could enable healthcare providers to tailor treatment plans more effectively and monitor treatment efficacy more closely.
As the global burden of cardiovascular disease continues to rise, there is an increasing emphasis on preventive care and early intervention. P wave-centred technologies have the potential to play a significant role in this shift, by enabling more accurate risk assessment and earlier detection of developing cardiac issues.
P Wave Detection Challenges
P wave detection in cardiac interfacing technologies presents several significant challenges that researchers and engineers must overcome to develop effective and reliable systems. One of the primary difficulties lies in the low amplitude of P waves compared to other cardiac signals, particularly the QRS complex. This low signal-to-noise ratio makes it challenging to accurately identify and isolate P waves from background noise and other cardiac activities.
The morphological variability of P waves across different individuals and even within the same person over time further complicates detection efforts. Factors such as age, heart rate, and underlying cardiac conditions can significantly alter P wave characteristics, necessitating adaptive detection algorithms capable of handling diverse waveform shapes and durations.
Interference from other physiological signals, such as muscle artifacts and respiratory movements, poses another substantial challenge. These non-cardiac signals can obscure or distort P waves, leading to false detections or missed events. Additionally, electromagnetic interference from nearby electronic devices and power lines can introduce noise that further compromises P wave detection accuracy.
The time-varying nature of the cardiac signal itself presents yet another hurdle. Beat-to-beat variations in heart rate and subtle changes in the cardiac conduction system can affect the timing and morphology of P waves, requiring detection algorithms to be robust and adaptable to these dynamic changes.
In ambulatory monitoring scenarios, motion artifacts become a significant concern. Patient movement can introduce substantial noise and baseline wander, making it particularly challenging to maintain reliable P wave detection during daily activities or exercise.
The computational complexity of real-time P wave detection algorithms is another consideration, especially for wearable or implantable devices with limited processing power and battery life. Striking a balance between detection accuracy and computational efficiency is crucial for practical implementation in resource-constrained environments.
Furthermore, the presence of cardiac arrhythmias or conduction abnormalities can dramatically alter the appearance and timing of P waves, necessitating sophisticated algorithms capable of distinguishing between normal and pathological P wave patterns. This is particularly important in applications aimed at early detection or monitoring of atrial fibrillation and other arrhythmias.
Addressing these challenges requires a multifaceted approach, combining advanced signal processing techniques, machine learning algorithms, and innovative sensor technologies. Continuous research and development efforts are essential to push the boundaries of P wave detection capabilities, ultimately leading to more accurate and reliable cardiac interfacing technologies centered around this critical component of the cardiac cycle.
The morphological variability of P waves across different individuals and even within the same person over time further complicates detection efforts. Factors such as age, heart rate, and underlying cardiac conditions can significantly alter P wave characteristics, necessitating adaptive detection algorithms capable of handling diverse waveform shapes and durations.
Interference from other physiological signals, such as muscle artifacts and respiratory movements, poses another substantial challenge. These non-cardiac signals can obscure or distort P waves, leading to false detections or missed events. Additionally, electromagnetic interference from nearby electronic devices and power lines can introduce noise that further compromises P wave detection accuracy.
The time-varying nature of the cardiac signal itself presents yet another hurdle. Beat-to-beat variations in heart rate and subtle changes in the cardiac conduction system can affect the timing and morphology of P waves, requiring detection algorithms to be robust and adaptable to these dynamic changes.
In ambulatory monitoring scenarios, motion artifacts become a significant concern. Patient movement can introduce substantial noise and baseline wander, making it particularly challenging to maintain reliable P wave detection during daily activities or exercise.
The computational complexity of real-time P wave detection algorithms is another consideration, especially for wearable or implantable devices with limited processing power and battery life. Striking a balance between detection accuracy and computational efficiency is crucial for practical implementation in resource-constrained environments.
Furthermore, the presence of cardiac arrhythmias or conduction abnormalities can dramatically alter the appearance and timing of P waves, necessitating sophisticated algorithms capable of distinguishing between normal and pathological P wave patterns. This is particularly important in applications aimed at early detection or monitoring of atrial fibrillation and other arrhythmias.
Addressing these challenges requires a multifaceted approach, combining advanced signal processing techniques, machine learning algorithms, and innovative sensor technologies. Continuous research and development efforts are essential to push the boundaries of P wave detection capabilities, ultimately leading to more accurate and reliable cardiac interfacing technologies centered around this critical component of the cardiac cycle.
Current P Wave Solutions
01 P wave detection and analysis
Technologies focusing on the detection and analysis of P waves in cardiac signals. These systems use advanced algorithms to identify and characterize P waves, which represent atrial depolarization. This information is crucial for diagnosing various cardiac conditions and optimizing pacemaker function.- P wave detection and analysis: Technologies focused on detecting and analyzing P waves in cardiac signals. These systems use advanced algorithms to identify and characterize P waves, which represent atrial depolarization. This information is crucial for diagnosing various cardiac conditions and optimizing pacemaker function.
- Implantable cardiac devices with P wave sensing: Implantable cardiac devices, such as pacemakers and defibrillators, that incorporate P wave sensing capabilities. These devices use P wave information to adjust their pacing and therapy delivery, improving synchronization between atrial and ventricular contractions and overall cardiac performance.
- Non-invasive P wave monitoring systems: External devices and systems designed for non-invasive monitoring of P waves. These technologies include advanced ECG systems, wearable devices, and remote monitoring solutions that can detect and analyze P waves without the need for invasive procedures, enabling long-term cardiac monitoring and early detection of arrhythmias.
- P wave-based cardiac resynchronization therapy: Cardiac resynchronization therapy (CRT) systems that utilize P wave information to optimize timing of pacing pulses. These technologies aim to improve the coordination of atrial and ventricular contractions in patients with heart failure, potentially leading to better cardiac output and quality of life.
- Machine learning and AI for P wave analysis: Integration of machine learning and artificial intelligence algorithms in P wave analysis systems. These advanced technologies can improve the accuracy of P wave detection, classification of arrhythmias, and prediction of cardiac events based on P wave morphology and timing.
02 Implantable cardiac devices with P wave sensing
Implantable cardiac devices, such as pacemakers and defibrillators, that incorporate P wave sensing capabilities. These devices use P wave information to improve timing and coordination of cardiac pacing, enhancing overall heart function and patient outcomes.Expand Specific Solutions03 Non-invasive P wave monitoring systems
External devices and systems designed for non-invasive monitoring of P waves. These technologies include advanced ECG systems, wearable devices, and remote monitoring solutions that can detect and analyze P waves without the need for implanted sensors.Expand Specific Solutions04 P wave-based arrhythmia detection and treatment
Systems that use P wave characteristics to detect and classify cardiac arrhythmias. These technologies employ machine learning algorithms and real-time analysis to identify abnormal P wave patterns, enabling early intervention and personalized treatment strategies.Expand Specific Solutions05 Integration of P wave data in cardiac monitoring platforms
Comprehensive cardiac monitoring platforms that integrate P wave data with other cardiac parameters. These systems provide a holistic view of cardiac function, combining P wave analysis with heart rate variability, QRS complex analysis, and other metrics to improve diagnostic accuracy and treatment planning.Expand Specific Solutions
Key Players in ECG Tech
The development of P wave-centred cardiac interfacing technologies is in a growth phase, with increasing market size and technological advancements. The global cardiac monitoring market, which includes this technology, is expected to expand significantly in the coming years. Major players like Medtronic, Boston Scientific (through Cardiac Pacemakers, Inc.), and Abbott Laboratories (via Pacesetter, Inc.) are driving innovation in this field. Emerging companies such as Bardy Diagnostics and Biosense Webster are also contributing to technological progress. The technology's maturity is advancing, with established firms and startups alike focusing on improving P wave detection and analysis for more accurate cardiac diagnostics and interventions.
Medtronic, Inc.
Technical Solution: Medtronic has developed advanced P wave-centred cardiac interfacing technologies, focusing on improving atrial sensing and pacing. Their latest pacemakers utilize proprietary algorithms to detect and analyze P waves with high accuracy, enabling more physiological pacing. The company has introduced adaptive P wave sensing technology that automatically adjusts sensitivity based on signal amplitude and noise levels, reducing the risk of undersensing or oversensing[1]. Medtronic's devices also incorporate P wave morphology analysis to differentiate between normal sinus rhythm and atrial arrhythmias, enhancing diagnostic capabilities and therapy delivery[2]. Additionally, they have implemented P wave-triggered pacing modes that aim to preserve natural AV conduction and optimize cardiac resynchronization therapy[3].
Strengths: Industry leader with extensive R&D resources, large market share, and established clinical evidence. Weaknesses: Higher cost of devices, potential for software vulnerabilities in complex algorithms.
Cardiac Pacemakers, Inc.
Technical Solution: Cardiac Pacemakers, Inc., a subsidiary of Boston Scientific, has developed P wave-centred cardiac interfacing technologies that focus on improving atrial sensing accuracy and reducing inappropriate pacing. Their devices utilize advanced digital signal processing techniques to enhance P wave detection in noisy environments. The company has introduced a novel P wave confirmation algorithm that verifies detected P waves against expected timing and morphology criteria, reducing the risk of far-field R wave oversensing[10]. Additionally, they have implemented adaptive P wave blanking periods that adjust based on the patient's intrinsic rhythm, optimizing sensing performance while minimizing crosstalk between chambers[11]. Cardiac Pacemakers' technology also includes P wave trend analysis features that track changes in atrial activity over time, providing valuable diagnostic information for clinicians[12].
Strengths: Specialized focus on cardiac pacing, strong integration with Boston Scientific's broader portfolio. Weaknesses: Potential overlap with parent company's offerings, may face challenges in differentiating products in the market.
P Wave Detection Innovations
Cardiac acceleration based p wave window determination
PatentWO2024123517A1
Innovation
- The system determines a P wave window using cardiac acceleration information, specifically by correlating an S4 heart sound template with cardiac acceleration data and applying an electromechanical delay, allowing for precise detection of cardiac electrical features and reducing false positives.
Systems for processing electrocardiac signals having superimposed complexes
PatentInactiveUS7272437B2
Innovation
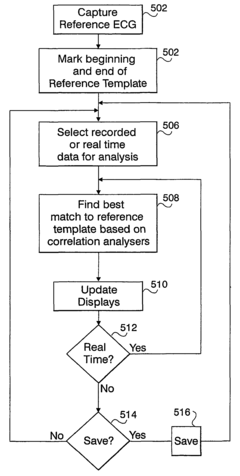
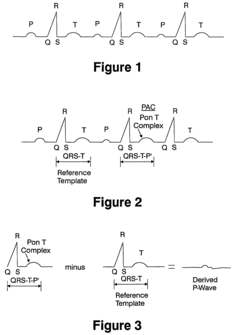
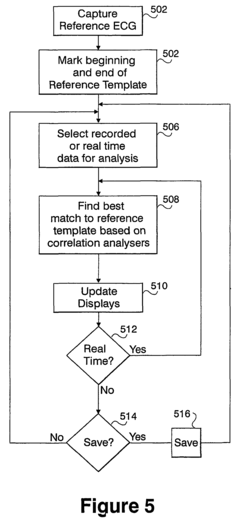
- A computerized system for processing ECG signals that objectively derives and compares P-wave signals by using correlation analysis and template matching, allowing for real-time pace mapping and monitoring, even when P-waves overlap with T-waves, and quantitatively measures the quality of T-wave subtraction results to account for variations caused by respiration or movement.
Regulatory Framework
The regulatory framework surrounding P wave-centred cardiac interfacing technologies is complex and multifaceted, encompassing various aspects of medical device regulation, patient safety, and data protection. In the United States, the Food and Drug Administration (FDA) plays a crucial role in overseeing the development and approval of such technologies. The FDA classifies cardiac interfacing devices as Class II or Class III medical devices, depending on their intended use and risk profile.
For P wave-centred technologies, manufacturers must navigate the premarket notification (510(k)) or premarket approval (PMA) pathways. The 510(k) process is typically used for devices that are substantially equivalent to existing approved devices, while novel technologies often require the more rigorous PMA process. Clinical trials are usually necessary to demonstrate safety and efficacy, with specific requirements varying based on the device's classification and intended use.
In the European Union, the Medical Device Regulation (MDR) governs the development and marketing of cardiac interfacing technologies. The MDR imposes stringent requirements for clinical evidence, post-market surveillance, and risk management. Manufacturers must obtain CE marking to indicate compliance with EU health, safety, and environmental protection standards before their devices can be marketed in the European Economic Area.
Data protection regulations, such as the General Data Protection Regulation (GDPR) in the EU and the Health Insurance Portability and Accountability Act (HIPAA) in the US, also significantly impact the development and implementation of P wave-centred technologies. These regulations mandate strict protocols for handling patient data, including requirements for data encryption, storage, and transmission.
Internationally, the International Medical Device Regulators Forum (IMDRF) provides guidance on harmonizing medical device regulations across different countries. Their recommendations often influence national regulatory frameworks, promoting global consistency in the approach to cardiac interfacing technologies.
As the field of P wave-centred cardiac interfacing continues to evolve, regulatory bodies are adapting their frameworks to address emerging challenges. This includes considerations for artificial intelligence and machine learning algorithms integrated into these technologies, as well as cybersecurity requirements to protect against potential vulnerabilities in connected devices.
For P wave-centred technologies, manufacturers must navigate the premarket notification (510(k)) or premarket approval (PMA) pathways. The 510(k) process is typically used for devices that are substantially equivalent to existing approved devices, while novel technologies often require the more rigorous PMA process. Clinical trials are usually necessary to demonstrate safety and efficacy, with specific requirements varying based on the device's classification and intended use.
In the European Union, the Medical Device Regulation (MDR) governs the development and marketing of cardiac interfacing technologies. The MDR imposes stringent requirements for clinical evidence, post-market surveillance, and risk management. Manufacturers must obtain CE marking to indicate compliance with EU health, safety, and environmental protection standards before their devices can be marketed in the European Economic Area.
Data protection regulations, such as the General Data Protection Regulation (GDPR) in the EU and the Health Insurance Portability and Accountability Act (HIPAA) in the US, also significantly impact the development and implementation of P wave-centred technologies. These regulations mandate strict protocols for handling patient data, including requirements for data encryption, storage, and transmission.
Internationally, the International Medical Device Regulators Forum (IMDRF) provides guidance on harmonizing medical device regulations across different countries. Their recommendations often influence national regulatory frameworks, promoting global consistency in the approach to cardiac interfacing technologies.
As the field of P wave-centred cardiac interfacing continues to evolve, regulatory bodies are adapting their frameworks to address emerging challenges. This includes considerations for artificial intelligence and machine learning algorithms integrated into these technologies, as well as cybersecurity requirements to protect against potential vulnerabilities in connected devices.
Clinical Validation Methods
Clinical validation is a critical step in the development of P wave-centred cardiac interfacing technologies. This process involves rigorous testing and evaluation to ensure the safety, efficacy, and reliability of these innovative devices in real-world clinical settings. The validation methods typically encompass a multi-phase approach, starting with preliminary bench testing and progressing to human trials.
Initially, in vitro studies are conducted to assess the basic functionality and performance of the P wave detection and analysis algorithms. These studies often utilize simulated cardiac signals and pre-recorded ECG datasets to evaluate the accuracy and sensitivity of the technology under controlled conditions. The results from these preliminary tests inform necessary refinements and optimizations before advancing to more complex validation stages.
Following in vitro testing, ex vivo studies may be performed using explanted animal hearts or human cardiac tissue samples. These experiments allow for a more realistic assessment of the technology's ability to detect and interpret P waves in the presence of biological variability and potential interfering signals. Such studies provide valuable insights into the robustness of the cardiac interfacing system in a quasi-physiological environment.
The next phase involves in vivo animal studies, typically conducted on large animal models such as pigs or sheep. These studies evaluate the technology's performance in living subjects, assessing factors such as signal quality, long-term stability, and potential tissue responses to implanted components. Animal studies also provide an opportunity to refine surgical techniques and optimize device placement for optimal P wave detection.
Human clinical trials represent the pinnacle of the validation process. These trials are typically conducted in phases, beginning with small-scale feasibility studies and progressing to larger, randomized controlled trials. Early-phase studies focus on safety and initial efficacy, while later phases assess long-term outcomes and compare the new technology to existing standard-of-care treatments. Throughout these trials, comprehensive data collection and analysis are performed to evaluate the technology's performance across diverse patient populations and clinical scenarios.
Importantly, clinical validation methods for P wave-centred cardiac interfacing technologies must adhere to stringent regulatory guidelines, such as those set forth by the FDA or EMA. This includes the development of detailed clinical protocols, obtaining ethical approvals, and implementing robust data management and reporting systems. Post-market surveillance is also a crucial component of the validation process, allowing for continuous monitoring of device performance and safety in real-world clinical practice.
Initially, in vitro studies are conducted to assess the basic functionality and performance of the P wave detection and analysis algorithms. These studies often utilize simulated cardiac signals and pre-recorded ECG datasets to evaluate the accuracy and sensitivity of the technology under controlled conditions. The results from these preliminary tests inform necessary refinements and optimizations before advancing to more complex validation stages.
Following in vitro testing, ex vivo studies may be performed using explanted animal hearts or human cardiac tissue samples. These experiments allow for a more realistic assessment of the technology's ability to detect and interpret P waves in the presence of biological variability and potential interfering signals. Such studies provide valuable insights into the robustness of the cardiac interfacing system in a quasi-physiological environment.
The next phase involves in vivo animal studies, typically conducted on large animal models such as pigs or sheep. These studies evaluate the technology's performance in living subjects, assessing factors such as signal quality, long-term stability, and potential tissue responses to implanted components. Animal studies also provide an opportunity to refine surgical techniques and optimize device placement for optimal P wave detection.
Human clinical trials represent the pinnacle of the validation process. These trials are typically conducted in phases, beginning with small-scale feasibility studies and progressing to larger, randomized controlled trials. Early-phase studies focus on safety and initial efficacy, while later phases assess long-term outcomes and compare the new technology to existing standard-of-care treatments. Throughout these trials, comprehensive data collection and analysis are performed to evaluate the technology's performance across diverse patient populations and clinical scenarios.
Importantly, clinical validation methods for P wave-centred cardiac interfacing technologies must adhere to stringent regulatory guidelines, such as those set forth by the FDA or EMA. This includes the development of detailed clinical protocols, obtaining ethical approvals, and implementing robust data management and reporting systems. Post-market surveillance is also a crucial component of the validation process, allowing for continuous monitoring of device performance and safety in real-world clinical practice.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!