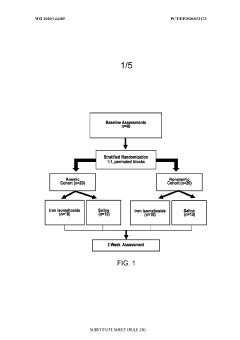
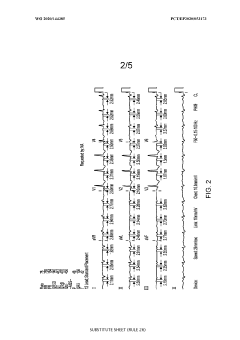
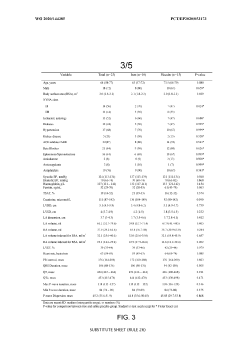
Effects of antiarrhythmic drugs on P wave morphology
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Antiarrhythmic Drug Background and Objectives
Antiarrhythmic drugs have been a cornerstone in the management of cardiac arrhythmias for decades. These medications aim to restore normal heart rhythm by modifying the electrical properties of cardiac tissue. The evolution of antiarrhythmic drugs has been marked by significant milestones, from the discovery of quinidine in the early 20th century to the development of more targeted and safer agents in recent years.
The primary objective of antiarrhythmic therapy is to suppress abnormal heart rhythms while minimizing adverse effects on normal cardiac function. This delicate balance has driven continuous research and development in the field. Over time, the classification of antiarrhythmic drugs has evolved, with the Vaughan Williams classification system providing a framework for understanding their mechanisms of action.
Recent technological advancements have allowed for more precise evaluation of drug effects on cardiac electrophysiology. One area of particular interest is the impact of antiarrhythmic drugs on P wave morphology. The P wave, representing atrial depolarization, serves as a crucial indicator of atrial electrical activity and can provide valuable insights into the efficacy and mechanisms of antiarrhythmic agents.
Understanding the effects of antiarrhythmic drugs on P wave morphology is essential for several reasons. Firstly, it can help in predicting the likelihood of successful rhythm control in atrial arrhythmias. Secondly, changes in P wave characteristics may serve as early markers of proarrhythmic effects, allowing for timely intervention and prevention of more serious arrhythmias.
The current technological landscape offers sophisticated tools for analyzing P wave morphology, including high-resolution electrocardiography and advanced signal processing techniques. These innovations enable researchers and clinicians to detect subtle changes in P wave duration, amplitude, and dispersion, providing a more nuanced understanding of drug effects on atrial electrophysiology.
As we look towards the future, the goals of antiarrhythmic drug research are multifaceted. There is a push towards developing more targeted therapies that can modulate specific ion channels or cellular pathways involved in arrhythmogenesis. Additionally, there is growing interest in personalized approaches to antiarrhythmic therapy, taking into account individual patient characteristics and genetic factors that may influence drug response.
The study of P wave morphology in the context of antiarrhythmic drug effects aligns with these broader objectives. By elucidating the precise impact of various agents on atrial electrical activity, researchers aim to develop more effective and safer antiarrhythmic strategies. This research has the potential to revolutionize arrhythmia management, leading to improved patient outcomes and a deeper understanding of cardiac electrophysiology.
The primary objective of antiarrhythmic therapy is to suppress abnormal heart rhythms while minimizing adverse effects on normal cardiac function. This delicate balance has driven continuous research and development in the field. Over time, the classification of antiarrhythmic drugs has evolved, with the Vaughan Williams classification system providing a framework for understanding their mechanisms of action.
Recent technological advancements have allowed for more precise evaluation of drug effects on cardiac electrophysiology. One area of particular interest is the impact of antiarrhythmic drugs on P wave morphology. The P wave, representing atrial depolarization, serves as a crucial indicator of atrial electrical activity and can provide valuable insights into the efficacy and mechanisms of antiarrhythmic agents.
Understanding the effects of antiarrhythmic drugs on P wave morphology is essential for several reasons. Firstly, it can help in predicting the likelihood of successful rhythm control in atrial arrhythmias. Secondly, changes in P wave characteristics may serve as early markers of proarrhythmic effects, allowing for timely intervention and prevention of more serious arrhythmias.
The current technological landscape offers sophisticated tools for analyzing P wave morphology, including high-resolution electrocardiography and advanced signal processing techniques. These innovations enable researchers and clinicians to detect subtle changes in P wave duration, amplitude, and dispersion, providing a more nuanced understanding of drug effects on atrial electrophysiology.
As we look towards the future, the goals of antiarrhythmic drug research are multifaceted. There is a push towards developing more targeted therapies that can modulate specific ion channels or cellular pathways involved in arrhythmogenesis. Additionally, there is growing interest in personalized approaches to antiarrhythmic therapy, taking into account individual patient characteristics and genetic factors that may influence drug response.
The study of P wave morphology in the context of antiarrhythmic drug effects aligns with these broader objectives. By elucidating the precise impact of various agents on atrial electrical activity, researchers aim to develop more effective and safer antiarrhythmic strategies. This research has the potential to revolutionize arrhythmia management, leading to improved patient outcomes and a deeper understanding of cardiac electrophysiology.
Clinical Need for P Wave Morphology Analysis
P wave morphology analysis has emerged as a critical tool in the field of cardiology, particularly in the diagnosis and management of atrial arrhythmias. The clinical need for this analysis stems from the increasing prevalence of atrial fibrillation (AF) and other atrial arrhythmias, which are associated with significant morbidity and mortality. P wave morphology provides valuable insights into atrial conduction and can serve as an early indicator of atrial remodeling and potential arrhythmogenic substrates.
The primary clinical application of P wave morphology analysis lies in its ability to detect subtle changes in atrial conduction that may precede the onset of AF. By identifying these early markers, clinicians can implement preventive strategies and interventions before the arrhythmia becomes established. This proactive approach has the potential to significantly reduce the burden of AF-related complications, such as stroke and heart failure.
Furthermore, P wave morphology analysis plays a crucial role in guiding antiarrhythmic drug therapy. Different antiarrhythmic medications can have varying effects on atrial conduction, which are reflected in changes to the P wave morphology. By carefully monitoring these changes, clinicians can assess the efficacy of drug treatments and make informed decisions about dose adjustments or alternative therapies.
In the context of ablation procedures for AF, P wave morphology analysis can aid in identifying the optimal targets for intervention. By mapping the atrial activation sequence and detecting areas of conduction delay or block, electrophysiologists can tailor their approach to each patient's specific atrial substrate, potentially improving procedural success rates and reducing the need for repeat interventions.
The clinical need for P wave morphology analysis extends beyond AF management. It has shown promise in predicting the risk of new-onset AF in various patient populations, including those with hypertension, heart failure, and post-cardiac surgery. This predictive capability allows for targeted monitoring and early intervention in high-risk individuals, potentially altering the natural history of the disease.
As the population ages and the prevalence of cardiovascular diseases increases, the demand for more sophisticated diagnostic and prognostic tools in arrhythmia management continues to grow. P wave morphology analysis addresses this need by providing a non-invasive, cost-effective method for assessing atrial function and predicting arrhythmic risk. Its integration into routine clinical practice has the potential to enhance patient care, improve outcomes, and optimize resource utilization in the management of atrial arrhythmias.
The primary clinical application of P wave morphology analysis lies in its ability to detect subtle changes in atrial conduction that may precede the onset of AF. By identifying these early markers, clinicians can implement preventive strategies and interventions before the arrhythmia becomes established. This proactive approach has the potential to significantly reduce the burden of AF-related complications, such as stroke and heart failure.
Furthermore, P wave morphology analysis plays a crucial role in guiding antiarrhythmic drug therapy. Different antiarrhythmic medications can have varying effects on atrial conduction, which are reflected in changes to the P wave morphology. By carefully monitoring these changes, clinicians can assess the efficacy of drug treatments and make informed decisions about dose adjustments or alternative therapies.
In the context of ablation procedures for AF, P wave morphology analysis can aid in identifying the optimal targets for intervention. By mapping the atrial activation sequence and detecting areas of conduction delay or block, electrophysiologists can tailor their approach to each patient's specific atrial substrate, potentially improving procedural success rates and reducing the need for repeat interventions.
The clinical need for P wave morphology analysis extends beyond AF management. It has shown promise in predicting the risk of new-onset AF in various patient populations, including those with hypertension, heart failure, and post-cardiac surgery. This predictive capability allows for targeted monitoring and early intervention in high-risk individuals, potentially altering the natural history of the disease.
As the population ages and the prevalence of cardiovascular diseases increases, the demand for more sophisticated diagnostic and prognostic tools in arrhythmia management continues to grow. P wave morphology analysis addresses this need by providing a non-invasive, cost-effective method for assessing atrial function and predicting arrhythmic risk. Its integration into routine clinical practice has the potential to enhance patient care, improve outcomes, and optimize resource utilization in the management of atrial arrhythmias.
Current Challenges in P Wave Assessment
P wave assessment in electrocardiography (ECG) plays a crucial role in diagnosing and managing various cardiac arrhythmias. However, several challenges persist in accurately evaluating P wave morphology, particularly when assessing the effects of antiarrhythmic drugs. One of the primary obstacles is the low amplitude of P waves, which often makes them difficult to distinguish from background noise in standard ECG recordings. This issue is further compounded by the variability in P wave morphology among different individuals and even within the same patient over time.
The influence of antiarrhythmic drugs on P wave characteristics adds another layer of complexity to the assessment process. These medications can alter the electrical properties of atrial tissue, leading to changes in P wave duration, amplitude, and overall morphology. Consequently, interpreting these drug-induced changes and differentiating them from pathological alterations becomes a significant challenge for clinicians and researchers alike.
Another hurdle in P wave assessment is the lack of standardized measurement techniques and criteria for evaluating P wave morphology. Different studies and clinical practices may employ varying methods for measuring P wave parameters, such as onset and offset determination, which can lead to inconsistencies in results and interpretations. This lack of standardization hampers the comparison of findings across different studies and limits the generalizability of research outcomes.
The presence of artifacts and interference in ECG recordings further complicates P wave assessment. Motion artifacts, electromagnetic interference, and poor electrode contact can distort the P wave signal, making it challenging to obtain accurate measurements. These technical issues are particularly problematic when evaluating subtle changes in P wave morphology induced by antiarrhythmic drugs.
Moreover, the dynamic nature of cardiac electrical activity poses a challenge in capturing and analyzing P waves consistently. Respiratory variations, changes in autonomic tone, and positional changes can all influence P wave characteristics, making it difficult to isolate the specific effects of antiarrhythmic drugs on P wave morphology.
The limitations of current ECG recording technologies also contribute to the challenges in P wave assessment. Standard 12-lead ECG systems may not provide sufficient spatial resolution to capture subtle changes in P wave morphology, especially in specific regions of the atria. Advanced mapping techniques, such as body surface potential mapping, offer improved spatial resolution but are not widely available in clinical settings.
Lastly, the interpretation of P wave changes in the context of antiarrhythmic drug effects requires a comprehensive understanding of both cardiac electrophysiology and pharmacology. The complex interplay between drug mechanisms, individual patient factors, and underlying cardiac pathology makes it challenging to establish clear cause-and-effect relationships between antiarrhythmic medications and observed P wave alterations.
The influence of antiarrhythmic drugs on P wave characteristics adds another layer of complexity to the assessment process. These medications can alter the electrical properties of atrial tissue, leading to changes in P wave duration, amplitude, and overall morphology. Consequently, interpreting these drug-induced changes and differentiating them from pathological alterations becomes a significant challenge for clinicians and researchers alike.
Another hurdle in P wave assessment is the lack of standardized measurement techniques and criteria for evaluating P wave morphology. Different studies and clinical practices may employ varying methods for measuring P wave parameters, such as onset and offset determination, which can lead to inconsistencies in results and interpretations. This lack of standardization hampers the comparison of findings across different studies and limits the generalizability of research outcomes.
The presence of artifacts and interference in ECG recordings further complicates P wave assessment. Motion artifacts, electromagnetic interference, and poor electrode contact can distort the P wave signal, making it challenging to obtain accurate measurements. These technical issues are particularly problematic when evaluating subtle changes in P wave morphology induced by antiarrhythmic drugs.
Moreover, the dynamic nature of cardiac electrical activity poses a challenge in capturing and analyzing P waves consistently. Respiratory variations, changes in autonomic tone, and positional changes can all influence P wave characteristics, making it difficult to isolate the specific effects of antiarrhythmic drugs on P wave morphology.
The limitations of current ECG recording technologies also contribute to the challenges in P wave assessment. Standard 12-lead ECG systems may not provide sufficient spatial resolution to capture subtle changes in P wave morphology, especially in specific regions of the atria. Advanced mapping techniques, such as body surface potential mapping, offer improved spatial resolution but are not widely available in clinical settings.
Lastly, the interpretation of P wave changes in the context of antiarrhythmic drug effects requires a comprehensive understanding of both cardiac electrophysiology and pharmacology. The complex interplay between drug mechanisms, individual patient factors, and underlying cardiac pathology makes it challenging to establish clear cause-and-effect relationships between antiarrhythmic medications and observed P wave alterations.
Existing Methods for P Wave Morphology Evaluation
01 P wave morphology analysis for arrhythmia detection
Analysis of P wave morphology in electrocardiograms can be used to detect and classify various types of arrhythmias. This method involves examining the shape, duration, and amplitude of P waves to identify abnormalities indicative of atrial arrhythmias or other cardiac conduction disorders.- P wave morphology analysis for arrhythmia detection: Analysis of P wave morphology in electrocardiograms can be used to detect and classify various types of arrhythmias. This method involves examining the shape, duration, and amplitude of P waves to identify abnormalities indicative of atrial arrhythmias or other cardiac conduction disorders.
- Antiarrhythmic drug effects on P wave characteristics: Antiarrhythmic medications can alter P wave morphology, which can be used to assess drug efficacy and monitor treatment response. Changes in P wave duration, amplitude, or dispersion may indicate the drug's impact on atrial conduction and can help in optimizing therapy for individual patients.
- Novel antiarrhythmic drug formulations: Development of new antiarrhythmic drug formulations aims to improve efficacy and reduce side effects. These formulations may include controlled-release mechanisms, combination therapies, or novel delivery methods to enhance the drug's impact on cardiac electrophysiology, including P wave characteristics.
- P wave analysis for personalized antiarrhythmic therapy: Personalized antiarrhythmic therapy can be guided by analyzing P wave morphology changes in response to different medications. This approach allows for tailored treatment strategies based on individual patient responses, potentially improving outcomes and reducing adverse effects.
- Devices for monitoring P wave changes during antiarrhythmic treatment: Specialized devices and algorithms have been developed to monitor P wave morphology changes during antiarrhythmic treatment. These tools can provide real-time feedback on drug efficacy and help in early detection of potential proarrhythmic effects or treatment failure.
02 Antiarrhythmic drug effects on P wave characteristics
Antiarrhythmic medications can significantly alter P wave morphology. These drugs may affect the duration, amplitude, or overall shape of P waves, which can be used as indicators of drug efficacy or potential side effects. Monitoring these changes can help in assessing treatment response and optimizing drug therapy.Expand Specific Solutions03 Novel antiarrhythmic drug formulations
Development of new antiarrhythmic drug formulations aims to improve efficacy and reduce side effects. These formulations may include modified release mechanisms, combination therapies, or novel delivery systems to enhance the drug's impact on cardiac electrophysiology, including P wave characteristics.Expand Specific Solutions04 Personalized antiarrhythmic therapy based on P wave analysis
Tailoring antiarrhythmic treatment to individual patients based on their specific P wave morphology patterns can improve therapeutic outcomes. This approach involves analyzing a patient's unique P wave characteristics to select the most appropriate antiarrhythmic drug and dosage regimen.Expand Specific Solutions05 Continuous monitoring of P wave changes during antiarrhythmic therapy
Implementing continuous or frequent monitoring of P wave morphology changes during antiarrhythmic drug therapy can provide real-time feedback on treatment efficacy. This approach allows for timely adjustments to medication regimens and early detection of potential adverse effects or arrhythmia recurrence.Expand Specific Solutions
Key Players in Antiarrhythmic Drug Research
The competitive landscape for research on the effects of antiarrhythmic drugs on P wave morphology is in a mature stage, with a significant market size due to the prevalence of cardiac arrhythmias. The technology has reached a high level of maturity, with established players like Janssen Pharmaceutica, Novartis, and Merck & Co. leading the field. Smaller companies such as Bardy Diagnostics and AnaBios are also contributing innovative approaches. Academic institutions like King's College London and The Johns Hopkins University are actively involved in advancing the research. The market is characterized by a mix of pharmaceutical giants, specialized cardiac device manufacturers, and emerging biotech firms, indicating a diverse and competitive environment.
Janssen Pharmaceutica NV
Technical Solution: Janssen Pharmaceutica has developed a novel approach to assessing the effects of antiarrhythmic drugs on P wave morphology using in silico modeling and simulation. Their technology combines detailed biophysical models of atrial electrophysiology with pharmacokinetic/pharmacodynamic (PK/PD) models of drug action to predict how different compounds will affect P wave characteristics[10]. Janssen's platform can simulate the effects of various drug doses and combinations on P wave parameters, allowing for rapid screening and optimization of potential antiarrhythmic therapies. The company has also integrated machine learning algorithms to improve the accuracy of their predictions based on real-world clinical data[11]. This approach enables more efficient drug development and personalized treatment selection for patients with atrial arrhythmias.
Strengths: Innovative in silico modeling approach, ability to rapidly screen multiple drug candidates, potential for personalized therapy optimization. Weaknesses: Reliance on computational models that may not fully capture real-world complexity, need for extensive validation against clinical data.
Medtronic, Inc.
Technical Solution: Medtronic has developed advanced implantable cardiac monitors and pacemakers that can detect and analyze P wave morphology changes in real-time. Their devices use proprietary algorithms to process intracardiac electrograms and identify subtle alterations in P wave characteristics that may indicate the onset or progression of atrial arrhythmias[1]. The company's latest pacemakers incorporate machine learning models trained on large datasets of P wave morphologies to improve the accuracy of arrhythmia detection and classification[2]. Medtronic's technology can also track the effects of antiarrhythmic drugs on P wave parameters over time, providing valuable data to clinicians for optimizing treatment strategies[3].
Strengths: Extensive experience in cardiac device development, large installed base of devices generating real-world data, advanced signal processing capabilities. Weaknesses: Reliance on invasive implantable devices, potential for over-detection of benign P wave changes.
Core Innovations in P Wave Analysis
N- or c- terminally modified small peptides
PatentWO2007007060A2
Innovation
- Development of N- or C-terminally modified small peptides with specific terminal groups, such as alkylcarbonyl or hydrophobic aromatic groups, that reduce inhibition of cytochrome P 450 oxidase and improve pharmacokinetic properties, including reduced ability to cross the blood-brain barrier.
Use of an iron agent for the treatment of atrial fibrillation
PatentWO2020144385A1
Innovation
- The use of iron agents, such as ferrous sulphate, ferrous fumarate, and iron polysaccharide, administered via oral, intramuscular, or intravenous routes, to reduce P-wave dispersion/duration and improve atrial electrophysiology, thereby preventing or treating atrial fibrillation.
Regulatory Framework for Antiarrhythmic Drugs
The regulatory framework for antiarrhythmic drugs is a complex and evolving system designed to ensure the safety and efficacy of medications used to treat cardiac arrhythmias. This framework is primarily overseen by regulatory bodies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), which play crucial roles in the approval and monitoring of these drugs.
Antiarrhythmic drugs are classified into different categories based on their mechanisms of action, with each category subject to specific regulatory requirements. The Vaughan Williams classification system, which divides these drugs into classes I through IV, is often used as a reference point in regulatory discussions and clinical guidelines.
The approval process for antiarrhythmic drugs involves rigorous clinical trials to demonstrate their safety and efficacy. These trials typically include assessments of the drugs' effects on various cardiac parameters, including P wave morphology. Regulatory agencies require comprehensive data on the pharmacokinetics, pharmacodynamics, and potential side effects of these medications before granting market authorization.
Post-marketing surveillance is a critical component of the regulatory framework. Manufacturers are required to conduct ongoing safety monitoring and report adverse events to regulatory authorities. This process helps identify any long-term effects or rare side effects that may not have been apparent during initial clinical trials.
The regulatory landscape for antiarrhythmic drugs has evolved significantly in recent years, with an increased focus on personalized medicine and the use of biomarkers to predict drug response. Regulatory agencies now encourage the development of companion diagnostics that can help identify patients most likely to benefit from specific antiarrhythmic treatments while minimizing the risk of adverse effects.
International harmonization efforts, such as the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), have aimed to standardize regulatory requirements across different regions. These initiatives facilitate global drug development and ensure consistent safety standards worldwide.
Regulatory bodies also provide guidance on the design of clinical trials for antiarrhythmic drugs, including recommendations for endpoint selection and patient population characteristics. The assessment of P wave morphology changes has become an important consideration in these trials, reflecting the growing understanding of its significance in predicting treatment outcomes and potential proarrhythmic effects.
Antiarrhythmic drugs are classified into different categories based on their mechanisms of action, with each category subject to specific regulatory requirements. The Vaughan Williams classification system, which divides these drugs into classes I through IV, is often used as a reference point in regulatory discussions and clinical guidelines.
The approval process for antiarrhythmic drugs involves rigorous clinical trials to demonstrate their safety and efficacy. These trials typically include assessments of the drugs' effects on various cardiac parameters, including P wave morphology. Regulatory agencies require comprehensive data on the pharmacokinetics, pharmacodynamics, and potential side effects of these medications before granting market authorization.
Post-marketing surveillance is a critical component of the regulatory framework. Manufacturers are required to conduct ongoing safety monitoring and report adverse events to regulatory authorities. This process helps identify any long-term effects or rare side effects that may not have been apparent during initial clinical trials.
The regulatory landscape for antiarrhythmic drugs has evolved significantly in recent years, with an increased focus on personalized medicine and the use of biomarkers to predict drug response. Regulatory agencies now encourage the development of companion diagnostics that can help identify patients most likely to benefit from specific antiarrhythmic treatments while minimizing the risk of adverse effects.
International harmonization efforts, such as the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), have aimed to standardize regulatory requirements across different regions. These initiatives facilitate global drug development and ensure consistent safety standards worldwide.
Regulatory bodies also provide guidance on the design of clinical trials for antiarrhythmic drugs, including recommendations for endpoint selection and patient population characteristics. The assessment of P wave morphology changes has become an important consideration in these trials, reflecting the growing understanding of its significance in predicting treatment outcomes and potential proarrhythmic effects.
Pharmacokinetics of Antiarrhythmic Agents
The pharmacokinetics of antiarrhythmic agents play a crucial role in understanding their effects on P wave morphology. These drugs are designed to manage and prevent cardiac arrhythmias by modulating the electrical activity of the heart. The absorption, distribution, metabolism, and excretion (ADME) processes of these agents significantly influence their efficacy and safety profiles.
Antiarrhythmic drugs are typically classified into four main categories based on their mechanisms of action: sodium channel blockers, beta-blockers, potassium channel blockers, and calcium channel blockers. Each class exhibits distinct pharmacokinetic properties that impact their therapeutic effects and potential side effects.
Absorption of antiarrhythmic agents varies depending on the drug and route of administration. Oral bioavailability can range from relatively low (e.g., propafenone) to nearly complete (e.g., amiodarone). Factors such as first-pass metabolism and gastrointestinal pH can significantly affect absorption rates and overall bioavailability.
Distribution patterns of antiarrhythmic drugs are influenced by their lipophilicity, protein binding, and tissue affinity. Highly lipophilic drugs like amiodarone have extensive tissue distribution and long half-lives, while more hydrophilic agents such as sotalol primarily remain in the extracellular fluid.
Metabolism of these drugs primarily occurs in the liver through various enzymatic pathways, predominantly involving cytochrome P450 enzymes. Genetic polymorphisms in these enzymes can lead to inter-individual variability in drug metabolism, affecting both efficacy and toxicity profiles.
Excretion routes for antiarrhythmic agents include renal and hepatic pathways. Drugs with significant renal excretion, like sotalol, require dose adjustments in patients with impaired kidney function to prevent accumulation and potential toxicity.
The pharmacokinetic profiles of antiarrhythmic drugs directly influence their effects on P wave morphology. Drugs with longer half-lives and steady-state concentrations, such as amiodarone, tend to produce more consistent changes in P wave characteristics. Conversely, agents with shorter half-lives may exhibit more variable effects on P wave morphology, potentially requiring more frequent dosing to maintain therapeutic levels.
Understanding the pharmacokinetics of antiarrhythmic agents is essential for optimizing drug selection, dosing regimens, and monitoring strategies. This knowledge enables clinicians to predict and manage drug interactions, adjust doses based on patient-specific factors, and anticipate potential adverse effects related to altered P wave morphology and other cardiac parameters.
Antiarrhythmic drugs are typically classified into four main categories based on their mechanisms of action: sodium channel blockers, beta-blockers, potassium channel blockers, and calcium channel blockers. Each class exhibits distinct pharmacokinetic properties that impact their therapeutic effects and potential side effects.
Absorption of antiarrhythmic agents varies depending on the drug and route of administration. Oral bioavailability can range from relatively low (e.g., propafenone) to nearly complete (e.g., amiodarone). Factors such as first-pass metabolism and gastrointestinal pH can significantly affect absorption rates and overall bioavailability.
Distribution patterns of antiarrhythmic drugs are influenced by their lipophilicity, protein binding, and tissue affinity. Highly lipophilic drugs like amiodarone have extensive tissue distribution and long half-lives, while more hydrophilic agents such as sotalol primarily remain in the extracellular fluid.
Metabolism of these drugs primarily occurs in the liver through various enzymatic pathways, predominantly involving cytochrome P450 enzymes. Genetic polymorphisms in these enzymes can lead to inter-individual variability in drug metabolism, affecting both efficacy and toxicity profiles.
Excretion routes for antiarrhythmic agents include renal and hepatic pathways. Drugs with significant renal excretion, like sotalol, require dose adjustments in patients with impaired kidney function to prevent accumulation and potential toxicity.
The pharmacokinetic profiles of antiarrhythmic drugs directly influence their effects on P wave morphology. Drugs with longer half-lives and steady-state concentrations, such as amiodarone, tend to produce more consistent changes in P wave characteristics. Conversely, agents with shorter half-lives may exhibit more variable effects on P wave morphology, potentially requiring more frequent dosing to maintain therapeutic levels.
Understanding the pharmacokinetics of antiarrhythmic agents is essential for optimizing drug selection, dosing regimens, and monitoring strategies. This knowledge enables clinicians to predict and manage drug interactions, adjust doses based on patient-specific factors, and anticipate potential adverse effects related to altered P wave morphology and other cardiac parameters.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!