Examination of P wave during cardiac ventricular reconstruction
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
P Wave Analysis Goals
The examination of P waves during cardiac ventricular reconstruction is a critical aspect of cardiac electrophysiology, aiming to enhance our understanding of atrial activity and its relationship to ventricular function. This analysis seeks to provide valuable insights into the electrical conduction system of the heart, particularly focusing on the atrial depolarization phase.
The primary goal of P wave analysis in this context is to accurately identify and characterize the morphology, duration, and amplitude of P waves during the complex process of ventricular reconstruction. By doing so, researchers and clinicians can gain a more comprehensive understanding of atrial function and its potential impact on overall cardiac performance.
One key objective is to develop advanced signal processing techniques that can effectively isolate P waves from other cardiac electrical activities, especially during the challenging period of ventricular reconstruction. This involves the implementation of sophisticated filtering algorithms and noise reduction methods to enhance the signal-to-noise ratio of P waves.
Another important aim is to establish standardized protocols for P wave measurement and interpretation in the context of ventricular reconstruction. This standardization will facilitate more accurate comparisons across different patient populations and research studies, leading to more robust and reproducible findings in the field of cardiac electrophysiology.
Researchers also seek to investigate the potential correlations between P wave characteristics and specific ventricular reconstruction techniques or outcomes. This analysis may reveal important relationships between atrial electrical activity and the success or complications of ventricular reconstruction procedures, potentially informing clinical decision-making and treatment strategies.
Furthermore, the examination of P waves during ventricular reconstruction aims to explore the temporal dynamics of atrial-ventricular interactions. This includes studying how P wave morphology and timing may change throughout the reconstruction process and in the immediate post-procedural period, providing insights into the adaptive mechanisms of the heart.
Ultimately, the goals of P wave analysis in this context extend beyond basic research to clinical applications. By improving our understanding of atrial electrical activity during ventricular reconstruction, clinicians hope to develop more effective strategies for managing arrhythmias, optimizing cardiac resynchronization therapy, and predicting post-procedural outcomes.
The primary goal of P wave analysis in this context is to accurately identify and characterize the morphology, duration, and amplitude of P waves during the complex process of ventricular reconstruction. By doing so, researchers and clinicians can gain a more comprehensive understanding of atrial function and its potential impact on overall cardiac performance.
One key objective is to develop advanced signal processing techniques that can effectively isolate P waves from other cardiac electrical activities, especially during the challenging period of ventricular reconstruction. This involves the implementation of sophisticated filtering algorithms and noise reduction methods to enhance the signal-to-noise ratio of P waves.
Another important aim is to establish standardized protocols for P wave measurement and interpretation in the context of ventricular reconstruction. This standardization will facilitate more accurate comparisons across different patient populations and research studies, leading to more robust and reproducible findings in the field of cardiac electrophysiology.
Researchers also seek to investigate the potential correlations between P wave characteristics and specific ventricular reconstruction techniques or outcomes. This analysis may reveal important relationships between atrial electrical activity and the success or complications of ventricular reconstruction procedures, potentially informing clinical decision-making and treatment strategies.
Furthermore, the examination of P waves during ventricular reconstruction aims to explore the temporal dynamics of atrial-ventricular interactions. This includes studying how P wave morphology and timing may change throughout the reconstruction process and in the immediate post-procedural period, providing insights into the adaptive mechanisms of the heart.
Ultimately, the goals of P wave analysis in this context extend beyond basic research to clinical applications. By improving our understanding of atrial electrical activity during ventricular reconstruction, clinicians hope to develop more effective strategies for managing arrhythmias, optimizing cardiac resynchronization therapy, and predicting post-procedural outcomes.
Clinical Demand
The examination of P waves during cardiac ventricular reconstruction has become increasingly important in clinical cardiology. This advanced diagnostic technique addresses a critical need in the assessment and management of various cardiac conditions, particularly those affecting the heart's electrical conduction system and structural integrity.
Cardiologists and electrophysiologists face significant challenges in accurately diagnosing and treating complex arrhythmias and structural heart diseases. The P wave, representing atrial depolarization, provides crucial information about the heart's electrical activity and can indicate potential abnormalities in the atrial chambers. By examining the P wave during ventricular reconstruction, clinicians can gain valuable insights into the relationship between atrial electrical activity and ventricular structural changes.
This technique has emerged as a response to the limitations of traditional electrocardiography (ECG) in providing comprehensive information about cardiac function, especially in patients with complex cardiac conditions. The demand for more precise diagnostic tools has grown as the prevalence of cardiovascular diseases continues to rise globally. According to recent epidemiological data, cardiovascular diseases remain the leading cause of death worldwide, accounting for approximately 31% of all deaths.
The clinical demand for P wave examination during cardiac ventricular reconstruction is driven by several factors. Firstly, it offers enhanced diagnostic accuracy in identifying atrial arrhythmias, such as atrial fibrillation and atrial flutter, which are often associated with structural heart diseases. Early detection and accurate characterization of these arrhythmias are crucial for timely intervention and improved patient outcomes.
Secondly, this technique provides valuable information for pre-operative planning in patients undergoing cardiac surgeries or interventional procedures. By assessing the P wave characteristics in relation to ventricular structure, surgeons and interventional cardiologists can better understand the potential impact of structural changes on atrial function and optimize their treatment strategies accordingly.
Furthermore, the examination of P waves during ventricular reconstruction has shown promise in risk stratification for sudden cardiac death and other adverse cardiac events. This capability addresses a significant clinical need for more accurate prognostic tools in cardiology, enabling healthcare providers to tailor treatment plans and follow-up strategies based on individual patient risk profiles.
The growing adoption of advanced cardiac imaging technologies, such as cardiac magnetic resonance imaging (MRI) and computed tomography (CT), has further fueled the demand for integrating P wave analysis with structural assessment. This integration allows for a more comprehensive evaluation of cardiac function and structure, leading to improved diagnostic accuracy and treatment planning.
As the field of personalized medicine continues to evolve, there is an increasing need for diagnostic techniques that can provide detailed, patient-specific information. The examination of P waves during cardiac ventricular reconstruction aligns with this trend, offering a tailored approach to cardiac assessment that considers both electrical and structural aspects of heart function.
Cardiologists and electrophysiologists face significant challenges in accurately diagnosing and treating complex arrhythmias and structural heart diseases. The P wave, representing atrial depolarization, provides crucial information about the heart's electrical activity and can indicate potential abnormalities in the atrial chambers. By examining the P wave during ventricular reconstruction, clinicians can gain valuable insights into the relationship between atrial electrical activity and ventricular structural changes.
This technique has emerged as a response to the limitations of traditional electrocardiography (ECG) in providing comprehensive information about cardiac function, especially in patients with complex cardiac conditions. The demand for more precise diagnostic tools has grown as the prevalence of cardiovascular diseases continues to rise globally. According to recent epidemiological data, cardiovascular diseases remain the leading cause of death worldwide, accounting for approximately 31% of all deaths.
The clinical demand for P wave examination during cardiac ventricular reconstruction is driven by several factors. Firstly, it offers enhanced diagnostic accuracy in identifying atrial arrhythmias, such as atrial fibrillation and atrial flutter, which are often associated with structural heart diseases. Early detection and accurate characterization of these arrhythmias are crucial for timely intervention and improved patient outcomes.
Secondly, this technique provides valuable information for pre-operative planning in patients undergoing cardiac surgeries or interventional procedures. By assessing the P wave characteristics in relation to ventricular structure, surgeons and interventional cardiologists can better understand the potential impact of structural changes on atrial function and optimize their treatment strategies accordingly.
Furthermore, the examination of P waves during ventricular reconstruction has shown promise in risk stratification for sudden cardiac death and other adverse cardiac events. This capability addresses a significant clinical need for more accurate prognostic tools in cardiology, enabling healthcare providers to tailor treatment plans and follow-up strategies based on individual patient risk profiles.
The growing adoption of advanced cardiac imaging technologies, such as cardiac magnetic resonance imaging (MRI) and computed tomography (CT), has further fueled the demand for integrating P wave analysis with structural assessment. This integration allows for a more comprehensive evaluation of cardiac function and structure, leading to improved diagnostic accuracy and treatment planning.
As the field of personalized medicine continues to evolve, there is an increasing need for diagnostic techniques that can provide detailed, patient-specific information. The examination of P waves during cardiac ventricular reconstruction aligns with this trend, offering a tailored approach to cardiac assessment that considers both electrical and structural aspects of heart function.
P Wave Detection Challenges
The detection of P waves during cardiac ventricular reconstruction presents several significant challenges that researchers and clinicians must address. One of the primary difficulties lies in the low amplitude of P waves compared to other cardiac signals, particularly the QRS complex. This low signal-to-noise ratio makes it challenging to accurately identify and isolate P waves from the overall electrocardiogram (ECG) signal.
Furthermore, the morphology of P waves can vary considerably between individuals and even within the same patient under different physiological conditions. This variability complicates the development of universal detection algorithms and necessitates adaptive approaches that can account for patient-specific characteristics.
Another major challenge is the presence of various artifacts and interference sources that can obscure or distort P waves. These include muscle artifacts, baseline wander, power line interference, and motion artifacts. During cardiac ventricular reconstruction procedures, additional electromagnetic interference from medical equipment can further complicate P wave detection.
The temporal relationship between P waves and other cardiac events also poses difficulties. In some cases, P waves may be partially or completely obscured by T waves or other ECG components, particularly in patients with abnormal cardiac rhythms or conduction disorders. This overlap can make it challenging to accurately determine the onset and offset of P waves.
Moreover, the dynamic nature of cardiac activity during ventricular reconstruction procedures adds another layer of complexity. Changes in heart rate, rhythm, and conduction patterns can occur rapidly, requiring real-time adaptation of detection algorithms to maintain accuracy.
The computational demands of P wave detection algorithms present an additional challenge, especially in the context of real-time monitoring during cardiac procedures. Balancing the need for high accuracy with the requirement for low latency and efficient processing is crucial for practical implementation in clinical settings.
Lastly, the validation and standardization of P wave detection methods remain ongoing challenges. The lack of a universally accepted gold standard for P wave annotation makes it difficult to compare and evaluate different detection algorithms objectively. This limitation hinders the development and adoption of robust, clinically validated techniques for P wave detection during cardiac ventricular reconstruction.
Furthermore, the morphology of P waves can vary considerably between individuals and even within the same patient under different physiological conditions. This variability complicates the development of universal detection algorithms and necessitates adaptive approaches that can account for patient-specific characteristics.
Another major challenge is the presence of various artifacts and interference sources that can obscure or distort P waves. These include muscle artifacts, baseline wander, power line interference, and motion artifacts. During cardiac ventricular reconstruction procedures, additional electromagnetic interference from medical equipment can further complicate P wave detection.
The temporal relationship between P waves and other cardiac events also poses difficulties. In some cases, P waves may be partially or completely obscured by T waves or other ECG components, particularly in patients with abnormal cardiac rhythms or conduction disorders. This overlap can make it challenging to accurately determine the onset and offset of P waves.
Moreover, the dynamic nature of cardiac activity during ventricular reconstruction procedures adds another layer of complexity. Changes in heart rate, rhythm, and conduction patterns can occur rapidly, requiring real-time adaptation of detection algorithms to maintain accuracy.
The computational demands of P wave detection algorithms present an additional challenge, especially in the context of real-time monitoring during cardiac procedures. Balancing the need for high accuracy with the requirement for low latency and efficient processing is crucial for practical implementation in clinical settings.
Lastly, the validation and standardization of P wave detection methods remain ongoing challenges. The lack of a universally accepted gold standard for P wave annotation makes it difficult to compare and evaluate different detection algorithms objectively. This limitation hinders the development and adoption of robust, clinically validated techniques for P wave detection during cardiac ventricular reconstruction.
Current P Wave Analysis Methods
01 P wave detection and analysis in ECG signals
Methods and systems for detecting and analyzing P waves in electrocardiogram (ECG) signals. This includes techniques for identifying P wave morphology, measuring P wave duration, and assessing P wave variability. These approaches can be used for diagnosing various cardiac conditions and evaluating atrial function.- P wave detection and analysis in ECG signals: Methods and systems for detecting and analyzing P waves in electrocardiogram (ECG) signals. These techniques involve signal processing algorithms to identify, isolate, and characterize P waves, which represent atrial depolarization. The analysis of P waves can provide valuable information about cardiac function and help in diagnosing various heart conditions.
- P wave-based cardiac monitoring and diagnosis: Devices and methods for monitoring cardiac activity and diagnosing heart conditions based on P wave characteristics. These systems analyze P wave morphology, duration, and timing to detect abnormalities such as atrial fibrillation, atrial flutter, and other arrhythmias. The technology can be used in both clinical settings and wearable devices for continuous monitoring.
- P wave signal processing in communication systems: Techniques for processing P waves in communication systems, particularly in wireless and optical communications. These methods involve modulation, demodulation, and filtering of P waves to improve signal quality, reduce interference, and enhance data transmission efficiency. The technology is applicable in various fields, including telecommunications and data networking.
- P wave-based seismic exploration and analysis: Methods and systems for using P waves in seismic exploration and analysis. These techniques involve generating, detecting, and analyzing P waves to study subsurface structures and properties. The technology is particularly useful in oil and gas exploration, geological surveys, and earthquake monitoring.
- P wave applications in medical imaging: Utilization of P waves in medical imaging technologies, such as ultrasound and echocardiography. These methods involve generating and analyzing P waves to create detailed images of internal body structures, particularly the heart and blood vessels. The technology enables non-invasive diagnosis and monitoring of various cardiac conditions.
02 P wave signal processing in wireless communication
Techniques for processing P wave signals in wireless communication systems. This involves methods for modulating, demodulating, and filtering P waves to improve signal quality and reduce interference. These approaches can enhance the performance and reliability of wireless communication networks.Expand Specific Solutions03 P wave analysis for seismic exploration
Methods for analyzing P waves in seismic exploration. This includes techniques for generating, detecting, and interpreting P waves to gather information about subsurface structures. These approaches can be used in oil and gas exploration, as well as in studying geological formations.Expand Specific Solutions04 P wave-based biometric authentication
Systems and methods for using P wave characteristics in biometric authentication. This involves analyzing unique features of an individual's P waves for identity verification. These approaches can be applied in security systems and access control applications.Expand Specific Solutions05 P wave analysis in speech processing
Techniques for analyzing P waves in speech signals for various applications. This includes methods for speech recognition, speaker identification, and voice quality assessment based on P wave characteristics. These approaches can improve the accuracy and efficiency of speech processing systems.Expand Specific Solutions
Key ECG Device Manufacturers
The examination of P waves during cardiac ventricular reconstruction is in a developing stage, with a growing market driven by increasing cardiovascular diseases. The technology's maturity is advancing, with key players like Medtronic, Boston Scientific, and Philips leading innovation. Smaller companies such as Bardy Diagnostics and Edan Instruments are also contributing to the field. The market is characterized by a mix of established medical device manufacturers and emerging specialized firms, indicating a competitive landscape with potential for further growth and technological advancements in cardiac monitoring and diagnostics.
Medtronic, Inc.
Technical Solution: Medtronic has developed advanced algorithms for P wave examination during cardiac ventricular reconstruction. Their technology utilizes high-resolution electrocardiogram (ECG) signals to isolate and analyze P waves with precision. The system employs adaptive filtering techniques to remove noise and artifacts, enhancing the clarity of P wave morphology[1]. Medtronic's approach incorporates machine learning algorithms to detect subtle changes in P wave characteristics, which can indicate atrial remodeling or impending arrhythmias[3]. Their devices also feature real-time P wave analysis during cardiac procedures, providing immediate feedback to clinicians for optimized lead placement and therapy delivery[5].
Strengths: Highly accurate P wave detection and analysis, integration with existing cardiac devices, and real-time feedback capabilities. Weaknesses: May require specialized training for clinicians to interpret complex data outputs, and potential for false positives in noisy environments.
Boston Scientific Scimed, Inc.
Technical Solution: Boston Scientific has innovated in P wave examination during cardiac ventricular reconstruction with their RHYTHMIA HDx™ Mapping System. This technology utilizes a combination of high-density electroanatomical mapping and advanced signal processing algorithms to precisely examine P waves[2]. The system can create detailed 3D maps of cardiac electrical activity, including P wave propagation patterns across the atria. Boston Scientific's approach incorporates automatic annotation of P waves, allowing for rapid identification of areas of interest during procedures[4]. Their technology also features adaptive gain control, which ensures consistent P wave signal quality across different regions of the heart, even in the presence of varying tissue contact[6].
Strengths: High-resolution 3D mapping capabilities, automated P wave annotation, and adaptive signal processing for consistent quality. Weaknesses: Requires specialized catheters and equipment, potentially increasing procedural complexity and cost.
P Wave Morphology Research
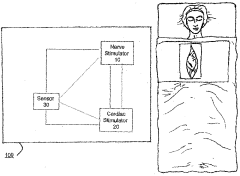
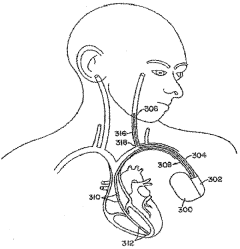
Method and system for vagal nerve stimulation with multi-site cardiac pacing
PatentWO2005053788A1
Innovation
- The method involves combining vagal nerve stimulation with multi-site cardiac pacing to modulate inflammatory responses, using an external or implantable pulse generator system that synchronizes the right and left heart chambers, and includes sensors for monitoring and adjusting therapy to optimize cardiac output and reduce inflammatory mediators.
Cardiac Imaging Integration
Cardiac imaging integration plays a crucial role in the examination of P waves during cardiac ventricular reconstruction. This integration combines various imaging modalities to provide a comprehensive view of cardiac structure and function, enhancing the accuracy and reliability of P wave analysis.
The integration of multiple imaging techniques, such as echocardiography, cardiac magnetic resonance imaging (MRI), and computed tomography (CT), allows for a more detailed assessment of cardiac anatomy and electrophysiology. Each modality offers unique advantages in visualizing different aspects of cardiac function, and their combination provides a synergistic effect in P wave examination.
Echocardiography, particularly transesophageal echocardiography (TEE), offers real-time imaging of cardiac structures and blood flow. It is especially useful in assessing atrial size and function, which are closely related to P wave morphology. The high temporal resolution of echocardiography makes it valuable for capturing the rapid electrical events associated with P waves.
Cardiac MRI provides excellent soft tissue contrast and allows for detailed assessment of cardiac anatomy and function. It is particularly useful in evaluating atrial fibrosis and remodeling, which can significantly impact P wave characteristics. Advanced MRI techniques, such as late gadolinium enhancement (LGE) and T1 mapping, can provide insights into tissue characterization and fibrosis quantification.
CT imaging offers high spatial resolution and is valuable for assessing cardiac anatomy, including the atria and pulmonary veins. It can provide detailed information about atrial size, shape, and wall thickness, which are important factors in P wave analysis. CT also allows for the creation of three-dimensional models of cardiac structures, aiding in the visualization of complex anatomical relationships.
The integration of these imaging modalities enables a more comprehensive analysis of P waves in the context of cardiac ventricular reconstruction. By combining anatomical and functional information from different imaging techniques, clinicians can better understand the relationship between structural changes in the atria and ventricles and their impact on P wave characteristics.
Advanced image processing and analysis techniques, such as image registration and fusion, play a crucial role in cardiac imaging integration. These methods allow for the alignment and combination of images from different modalities, creating a unified view of cardiac structure and function. This integrated approach enhances the ability to correlate P wave abnormalities with specific anatomical or functional changes in the heart.
Furthermore, the integration of cardiac imaging with electrocardiographic data provides a powerful tool for investigating the electro-anatomical correlates of P wave abnormalities. This combined approach allows for a more precise localization of areas of abnormal conduction or ectopic foci within the atria, which may be contributing to P wave changes during ventricular reconstruction.
The integration of multiple imaging techniques, such as echocardiography, cardiac magnetic resonance imaging (MRI), and computed tomography (CT), allows for a more detailed assessment of cardiac anatomy and electrophysiology. Each modality offers unique advantages in visualizing different aspects of cardiac function, and their combination provides a synergistic effect in P wave examination.
Echocardiography, particularly transesophageal echocardiography (TEE), offers real-time imaging of cardiac structures and blood flow. It is especially useful in assessing atrial size and function, which are closely related to P wave morphology. The high temporal resolution of echocardiography makes it valuable for capturing the rapid electrical events associated with P waves.
Cardiac MRI provides excellent soft tissue contrast and allows for detailed assessment of cardiac anatomy and function. It is particularly useful in evaluating atrial fibrosis and remodeling, which can significantly impact P wave characteristics. Advanced MRI techniques, such as late gadolinium enhancement (LGE) and T1 mapping, can provide insights into tissue characterization and fibrosis quantification.
CT imaging offers high spatial resolution and is valuable for assessing cardiac anatomy, including the atria and pulmonary veins. It can provide detailed information about atrial size, shape, and wall thickness, which are important factors in P wave analysis. CT also allows for the creation of three-dimensional models of cardiac structures, aiding in the visualization of complex anatomical relationships.
The integration of these imaging modalities enables a more comprehensive analysis of P waves in the context of cardiac ventricular reconstruction. By combining anatomical and functional information from different imaging techniques, clinicians can better understand the relationship between structural changes in the atria and ventricles and their impact on P wave characteristics.
Advanced image processing and analysis techniques, such as image registration and fusion, play a crucial role in cardiac imaging integration. These methods allow for the alignment and combination of images from different modalities, creating a unified view of cardiac structure and function. This integrated approach enhances the ability to correlate P wave abnormalities with specific anatomical or functional changes in the heart.
Furthermore, the integration of cardiac imaging with electrocardiographic data provides a powerful tool for investigating the electro-anatomical correlates of P wave abnormalities. This combined approach allows for a more precise localization of areas of abnormal conduction or ectopic foci within the atria, which may be contributing to P wave changes during ventricular reconstruction.
AI in ECG Interpretation
Artificial Intelligence (AI) has revolutionized the field of ECG interpretation, offering unprecedented accuracy and efficiency in analyzing cardiac electrical activity. In the context of examining P waves during cardiac ventricular reconstruction, AI algorithms have demonstrated remarkable capabilities in detecting subtle abnormalities and patterns that may elude human observers.
Machine learning models, particularly deep neural networks, have been trained on vast datasets of ECG recordings to recognize and classify P wave morphologies associated with various cardiac conditions. These AI systems can rapidly process large volumes of ECG data, providing real-time insights into atrial activity and potential abnormalities during ventricular reconstruction procedures.
One of the key advantages of AI in P wave examination is its ability to detect and quantify minute changes in P wave amplitude, duration, and morphology. This level of precision is crucial for identifying early signs of atrial remodeling or conduction abnormalities that may impact ventricular reconstruction outcomes. AI algorithms can also correlate P wave characteristics with other ECG features and clinical data, offering a more comprehensive assessment of cardiac function.
Recent advancements in AI-driven ECG interpretation have focused on developing explainable models that provide clinicians with insights into the decision-making process. These interpretable AI systems not only flag potential abnormalities but also highlight specific P wave features that contribute to their assessments, enhancing the collaborative potential between AI and human experts.
The integration of AI in ECG analysis has also enabled the development of predictive models for assessing the risk of post-operative arrhythmias or other complications following ventricular reconstruction. By analyzing pre-operative P wave characteristics and their evolution during the procedure, these models can provide valuable prognostic information to guide clinical decision-making.
Furthermore, AI-powered ECG interpretation systems have shown promise in continuous monitoring scenarios, allowing for real-time tracking of P wave changes throughout the ventricular reconstruction process. This capability enables early detection of acute alterations in atrial electrical activity, potentially allowing for timely interventions to prevent adverse outcomes.
As AI technology continues to evolve, there is growing potential for personalized ECG interpretation models tailored to individual patient characteristics and specific cardiac procedures. These customized algorithms could offer even greater accuracy and clinical relevance in the examination of P waves during ventricular reconstruction, further enhancing patient care and procedural outcomes.
Machine learning models, particularly deep neural networks, have been trained on vast datasets of ECG recordings to recognize and classify P wave morphologies associated with various cardiac conditions. These AI systems can rapidly process large volumes of ECG data, providing real-time insights into atrial activity and potential abnormalities during ventricular reconstruction procedures.
One of the key advantages of AI in P wave examination is its ability to detect and quantify minute changes in P wave amplitude, duration, and morphology. This level of precision is crucial for identifying early signs of atrial remodeling or conduction abnormalities that may impact ventricular reconstruction outcomes. AI algorithms can also correlate P wave characteristics with other ECG features and clinical data, offering a more comprehensive assessment of cardiac function.
Recent advancements in AI-driven ECG interpretation have focused on developing explainable models that provide clinicians with insights into the decision-making process. These interpretable AI systems not only flag potential abnormalities but also highlight specific P wave features that contribute to their assessments, enhancing the collaborative potential between AI and human experts.
The integration of AI in ECG analysis has also enabled the development of predictive models for assessing the risk of post-operative arrhythmias or other complications following ventricular reconstruction. By analyzing pre-operative P wave characteristics and their evolution during the procedure, these models can provide valuable prognostic information to guide clinical decision-making.
Furthermore, AI-powered ECG interpretation systems have shown promise in continuous monitoring scenarios, allowing for real-time tracking of P wave changes throughout the ventricular reconstruction process. This capability enables early detection of acute alterations in atrial electrical activity, potentially allowing for timely interventions to prevent adverse outcomes.
As AI technology continues to evolve, there is growing potential for personalized ECG interpretation models tailored to individual patient characteristics and specific cardiac procedures. These customized algorithms could offer even greater accuracy and clinical relevance in the examination of P waves during ventricular reconstruction, further enhancing patient care and procedural outcomes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!