Electron Uptake Mechanisms By Acetogenic Microorganisms
SEP 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Acetogenic Electron Uptake Background and Objectives
Acetogenic microorganisms have emerged as pivotal players in the global carbon cycle, demonstrating remarkable metabolic versatility through their ability to convert carbon dioxide into acetate and other valuable organic compounds. The historical trajectory of research in this field dates back to the early 20th century, with significant acceleration occurring in the 1980s when the Wood-Ljungdahl pathway was elucidated, revealing how these organisms can fix carbon dioxide using hydrogen as an electron donor. This biochemical pathway represents one of nature's most energy-efficient mechanisms for autotrophic growth.
Recent technological advances have expanded our understanding beyond traditional hydrogen-dependent metabolism to include direct electron uptake from solid surfaces, a process termed "microbial electrosynthesis." This discovery has fundamentally transformed our perception of acetogenic metabolism, suggesting these organisms possess sophisticated mechanisms for harvesting electrons from various sources, including electrodes, conductive minerals, and potentially through direct interspecies electron transfer in microbial communities.
The evolution of research in this field has progressed from initial observations of biofilm formation on cathodes to sophisticated investigations using advanced electrochemical, microscopic, and molecular techniques. Key milestones include the demonstration of acetate production from CO2 using electricity by Nevin et al. in 2010, followed by the identification of specific electron uptake mechanisms in model acetogens such as Sporomusa ovata and Clostridium ljungdahlii.
Current technological trends indicate growing interest in enhancing electron uptake efficiency through genetic engineering, electrode material optimization, and bioprocess design. The convergence of synthetic biology, materials science, and bioelectrochemical systems is creating unprecedented opportunities for harnessing acetogenic electron uptake for practical applications.
The primary objectives of investigating electron uptake mechanisms in acetogens encompass both fundamental scientific understanding and practical applications. From a scientific perspective, elucidating the molecular machinery involved in extracellular electron transfer will provide insights into how these ancient metabolic pathways evolved and function. This knowledge may reveal novel electron transport proteins, conductive appendages, or redox mediators that facilitate this remarkable capability.
From an applied standpoint, optimizing electron uptake efficiency represents a critical step toward developing economically viable microbial electrosynthesis technologies. These technologies hold promise for converting renewable electricity into storable chemical commodities, potentially enabling carbon-negative manufacturing processes and contributing to circular bioeconomy initiatives. The ultimate goal is to establish a robust technological platform that can efficiently capture electrons from renewable electricity sources and channel them into microbial metabolism for sustainable bioproduction.
Recent technological advances have expanded our understanding beyond traditional hydrogen-dependent metabolism to include direct electron uptake from solid surfaces, a process termed "microbial electrosynthesis." This discovery has fundamentally transformed our perception of acetogenic metabolism, suggesting these organisms possess sophisticated mechanisms for harvesting electrons from various sources, including electrodes, conductive minerals, and potentially through direct interspecies electron transfer in microbial communities.
The evolution of research in this field has progressed from initial observations of biofilm formation on cathodes to sophisticated investigations using advanced electrochemical, microscopic, and molecular techniques. Key milestones include the demonstration of acetate production from CO2 using electricity by Nevin et al. in 2010, followed by the identification of specific electron uptake mechanisms in model acetogens such as Sporomusa ovata and Clostridium ljungdahlii.
Current technological trends indicate growing interest in enhancing electron uptake efficiency through genetic engineering, electrode material optimization, and bioprocess design. The convergence of synthetic biology, materials science, and bioelectrochemical systems is creating unprecedented opportunities for harnessing acetogenic electron uptake for practical applications.
The primary objectives of investigating electron uptake mechanisms in acetogens encompass both fundamental scientific understanding and practical applications. From a scientific perspective, elucidating the molecular machinery involved in extracellular electron transfer will provide insights into how these ancient metabolic pathways evolved and function. This knowledge may reveal novel electron transport proteins, conductive appendages, or redox mediators that facilitate this remarkable capability.
From an applied standpoint, optimizing electron uptake efficiency represents a critical step toward developing economically viable microbial electrosynthesis technologies. These technologies hold promise for converting renewable electricity into storable chemical commodities, potentially enabling carbon-negative manufacturing processes and contributing to circular bioeconomy initiatives. The ultimate goal is to establish a robust technological platform that can efficiently capture electrons from renewable electricity sources and channel them into microbial metabolism for sustainable bioproduction.
Market Applications of Microbial Electrosynthesis
Microbial electrosynthesis (MES) represents a transformative technology at the intersection of microbiology, electrochemistry, and sustainable production. This process, leveraging acetogenic microorganisms' ability to uptake electrons from cathodes and convert CO2 into value-added compounds, has emerged as a promising platform for various market applications across multiple sectors.
In the chemical manufacturing industry, MES offers a revolutionary approach to producing commodity chemicals like acetate, ethanol, and butyrate using electricity as the primary energy input rather than fossil fuels. Companies like LanzaTech have already demonstrated commercial viability by scaling up gas fermentation technologies that share biological principles with MES. The global market for bio-based chemicals, estimated to grow significantly through 2030, presents a substantial opportunity for MES implementation.
The pharmaceutical sector represents another high-value application area. Acetogenic microorganisms can be engineered to produce pharmaceutical precursors and specialty chemicals that traditionally require complex synthesis routes. The precision of microbial production, coupled with the sustainability benefits of using renewable electricity, positions MES as an attractive alternative to conventional pharmaceutical manufacturing processes.
In the energy storage domain, MES enables the conversion of surplus renewable electricity into storable chemical compounds. This "power-to-gas" or "power-to-liquid" approach addresses intermittency challenges associated with renewable energy sources. The technology allows excess electricity generated during peak production periods to be stored as energy-dense compounds like methane or alcohols, which can later be utilized when demand exceeds supply.
The agricultural sector presents opportunities for MES in fertilizer production. Nitrogen-fixing acetogenic microorganisms could potentially use electrical energy to produce ammonia or other nitrogen compounds under ambient conditions, offering an alternative to the energy-intensive Haber-Bosch process currently dominating fertilizer manufacturing.
Carbon capture and utilization (CCU) represents perhaps the most significant market application. MES effectively transforms CO2 from industrial emissions or direct air capture into valuable products, creating economic incentives for carbon capture while reducing greenhouse gas concentrations. This dual benefit aligns perfectly with global decarbonization efforts and emerging carbon markets.
Food and beverage industries could benefit from MES through the production of food additives, flavorings, and nutritional supplements. Single-cell proteins produced via electrosynthesis could address growing protein demands while reducing agricultural land requirements.
The waste management sector presents opportunities for integrating MES with existing biogas or wastewater treatment facilities, where CO2-rich streams could be upgraded to higher-value products while simultaneously treating waste.
In the chemical manufacturing industry, MES offers a revolutionary approach to producing commodity chemicals like acetate, ethanol, and butyrate using electricity as the primary energy input rather than fossil fuels. Companies like LanzaTech have already demonstrated commercial viability by scaling up gas fermentation technologies that share biological principles with MES. The global market for bio-based chemicals, estimated to grow significantly through 2030, presents a substantial opportunity for MES implementation.
The pharmaceutical sector represents another high-value application area. Acetogenic microorganisms can be engineered to produce pharmaceutical precursors and specialty chemicals that traditionally require complex synthesis routes. The precision of microbial production, coupled with the sustainability benefits of using renewable electricity, positions MES as an attractive alternative to conventional pharmaceutical manufacturing processes.
In the energy storage domain, MES enables the conversion of surplus renewable electricity into storable chemical compounds. This "power-to-gas" or "power-to-liquid" approach addresses intermittency challenges associated with renewable energy sources. The technology allows excess electricity generated during peak production periods to be stored as energy-dense compounds like methane or alcohols, which can later be utilized when demand exceeds supply.
The agricultural sector presents opportunities for MES in fertilizer production. Nitrogen-fixing acetogenic microorganisms could potentially use electrical energy to produce ammonia or other nitrogen compounds under ambient conditions, offering an alternative to the energy-intensive Haber-Bosch process currently dominating fertilizer manufacturing.
Carbon capture and utilization (CCU) represents perhaps the most significant market application. MES effectively transforms CO2 from industrial emissions or direct air capture into valuable products, creating economic incentives for carbon capture while reducing greenhouse gas concentrations. This dual benefit aligns perfectly with global decarbonization efforts and emerging carbon markets.
Food and beverage industries could benefit from MES through the production of food additives, flavorings, and nutritional supplements. Single-cell proteins produced via electrosynthesis could address growing protein demands while reducing agricultural land requirements.
The waste management sector presents opportunities for integrating MES with existing biogas or wastewater treatment facilities, where CO2-rich streams could be upgraded to higher-value products while simultaneously treating waste.
Current Challenges in Electron Transfer to Acetogens
Despite significant advancements in microbial electrosynthesis using acetogens, several critical challenges persist in electron transfer mechanisms that limit practical applications and scalability. The primary obstacle remains the inherently low electron transfer rates between electrodes and acetogenic microorganisms. Unlike metal-reducing bacteria with evolved extracellular electron transfer systems, acetogens lack specialized conductive appendages, resulting in inefficient electron uptake that constrains production rates and economic viability.
Biofilm formation presents another significant challenge, as acetogens typically form thin, sparse biofilms on electrode surfaces compared to electroactive organisms like Geobacter. This limited colonization restricts the active biocatalyst density and consequently the overall system performance. The biofilm architecture and composition directly impact electron transfer efficiency, yet controlling these parameters remains difficult under industrial conditions.
Energy losses during electron transfer represent a substantial hurdle, with significant voltage losses occurring at the microbe-electrode interface. These overpotentials increase energy input requirements and reduce the overall energy efficiency of the process. The thermodynamic constraints of the Wood-Ljungdahl pathway further complicate matters, as acetogens must balance energy conservation with product formation under already energy-limited conditions.
Competing metabolic pathways pose additional challenges, as electrons directed toward acetogenic metabolism may be diverted to alternative pathways, reducing product specificity and yield. Many acetogens possess multiple electron uptake mechanisms that operate simultaneously, making it difficult to optimize electron flow toward desired products without comprehensive metabolic engineering approaches.
The limited understanding of direct electron uptake mechanisms in acetogens represents perhaps the most fundamental scientific challenge. While hydrogen-mediated electron transfer is relatively well-characterized, direct electron transfer pathways remain poorly understood. The molecular components involved in electrode-microbe interactions, including potential cytochromes, hydrogenases, or other redox-active proteins, require further elucidation to enable rational engineering of enhanced electron uptake.
Scale-up challenges further complicate industrial implementation, as laboratory-scale successes often fail to translate to larger systems due to mass transfer limitations, uneven current distribution, and pH gradients. The electrode materials themselves present a compromise between biocompatibility, conductivity, durability, and cost that has not been optimally resolved for acetogenic electron uptake applications.
Biofilm formation presents another significant challenge, as acetogens typically form thin, sparse biofilms on electrode surfaces compared to electroactive organisms like Geobacter. This limited colonization restricts the active biocatalyst density and consequently the overall system performance. The biofilm architecture and composition directly impact electron transfer efficiency, yet controlling these parameters remains difficult under industrial conditions.
Energy losses during electron transfer represent a substantial hurdle, with significant voltage losses occurring at the microbe-electrode interface. These overpotentials increase energy input requirements and reduce the overall energy efficiency of the process. The thermodynamic constraints of the Wood-Ljungdahl pathway further complicate matters, as acetogens must balance energy conservation with product formation under already energy-limited conditions.
Competing metabolic pathways pose additional challenges, as electrons directed toward acetogenic metabolism may be diverted to alternative pathways, reducing product specificity and yield. Many acetogens possess multiple electron uptake mechanisms that operate simultaneously, making it difficult to optimize electron flow toward desired products without comprehensive metabolic engineering approaches.
The limited understanding of direct electron uptake mechanisms in acetogens represents perhaps the most fundamental scientific challenge. While hydrogen-mediated electron transfer is relatively well-characterized, direct electron transfer pathways remain poorly understood. The molecular components involved in electrode-microbe interactions, including potential cytochromes, hydrogenases, or other redox-active proteins, require further elucidation to enable rational engineering of enhanced electron uptake.
Scale-up challenges further complicate industrial implementation, as laboratory-scale successes often fail to translate to larger systems due to mass transfer limitations, uneven current distribution, and pH gradients. The electrode materials themselves present a compromise between biocompatibility, conductivity, durability, and cost that has not been optimally resolved for acetogenic electron uptake applications.
Established Electron Uptake Pathways in Acetogens
01 Direct electron transfer mechanisms in acetogenic microorganisms
Acetogenic microorganisms can directly accept electrons through specialized membrane proteins and cytochromes that facilitate electron transfer from external sources. These direct electron uptake mechanisms involve conductive pili or nanowires that extend from the cell surface, allowing the microorganisms to capture electrons from solid surfaces or other cells. This direct electron transfer capability enables acetogens to convert carbon dioxide into acetate and other valuable compounds using electrons derived from various sources.- Direct electron transfer mechanisms in acetogenic microorganisms: Acetogenic microorganisms can directly accept electrons through specialized membrane proteins and cytochromes that facilitate electron transfer from external sources. These mechanisms involve conductive pili or nanowires that extend from the cell surface to capture electrons from solid surfaces or other microorganisms. This direct electron uptake enables acetogens to convert carbon dioxide into acetate and other valuable compounds without requiring dissolved electron carriers.
- Hydrogen-mediated electron uptake in acetogens: Acetogenic microorganisms can utilize hydrogen as an electron carrier in a process known as hydrogen-mediated electron uptake. In this mechanism, hydrogen is produced at cathode surfaces or by other microorganisms and then consumed by acetogens as an electron donor. This indirect electron transfer pathway allows acetogens to reduce carbon dioxide to acetate and other organic compounds through the Wood-Ljungdahl pathway, enabling efficient carbon fixation and energy conservation.
- Mediator-based electron transfer systems: Acetogenic microorganisms can utilize soluble redox mediators to facilitate electron uptake from various sources. These mediators, including flavins, quinones, and synthetic compounds, shuttle electrons between external electron donors and the microbial cell. The mediators can be either naturally produced by the microorganisms or artificially added to enhance electron transfer rates. This mechanism enables acetogens to access electrons from sources that may not be in direct contact with the cell surface.
- Genetic engineering of electron uptake pathways: Genetic modification techniques can be applied to enhance electron uptake mechanisms in acetogenic microorganisms. By introducing or overexpressing genes encoding for electron transfer proteins, cytochromes, or conductive appendages, the electron uptake capacity of acetogens can be significantly improved. These engineered strains demonstrate enhanced ability to capture electrons from various sources and convert them into valuable products through carbon dioxide fixation, offering promising applications in microbial electrosynthesis and biofuel production.
- Biofilm-based electron uptake enhancement: Acetogenic microorganisms can form biofilms on electrode surfaces to enhance electron uptake efficiency. Within these biofilms, cells develop specialized structures and extracellular polymeric substances that facilitate electron transfer between cells and from electrodes to cells. The three-dimensional architecture of biofilms creates a conducive environment for electron sharing and increases the surface area available for electron capture, resulting in improved electron uptake rates and higher product yields in bioelectrochemical systems.
02 Hydrogen-mediated electron uptake in acetogens
Acetogenic microorganisms can utilize hydrogen as an electron carrier in a process known as hydrogen-mediated electron uptake. In this mechanism, hydrogen serves as an intermediate electron carrier, where electrons from various sources are first used to produce hydrogen, which is then consumed by acetogens. This indirect electron transfer pathway involves hydrogenase enzymes that catalyze the oxidation of hydrogen, releasing electrons that can be used in the Wood-Ljungdahl pathway for carbon fixation and acetate production.Expand Specific Solutions03 Mediated electron transfer using redox shuttles
Acetogenic microorganisms can uptake electrons through redox-active molecules that act as electron shuttles between an electron source and the microbial cell. These mediators, including natural compounds like flavins, humic substances, and synthetic mediators such as neutral red or methyl viologen, can transfer electrons across the cell membrane. This mechanism allows acetogens to access electrons from sources they cannot directly contact, enhancing their metabolic versatility and enabling them to produce valuable compounds from carbon dioxide.Expand Specific Solutions04 Genetic engineering to enhance electron uptake capabilities
Genetic modification techniques can be applied to enhance the electron uptake mechanisms in acetogenic microorganisms. By introducing or overexpressing genes encoding for electron transfer proteins, cytochromes, or conductive pili, the electron uptake efficiency can be significantly improved. Additionally, engineering the cell surface properties or membrane composition can facilitate better electron transfer. These genetic modifications enable acetogens to more effectively capture electrons for the reduction of carbon dioxide to valuable products.Expand Specific Solutions05 Biofilm formation and interspecies electron transfer
Acetogenic microorganisms can form biofilms that enhance their electron uptake capabilities through interspecies electron transfer. Within these biofilms, different microbial species can exchange electrons directly or via electron shuttles, creating syntrophic relationships. The structured environment of biofilms provides optimal conditions for electron transfer by minimizing diffusion distances and creating microenvironments with favorable redox conditions. This cooperative electron sharing mechanism allows acetogens to access electrons from partner organisms and improve their metabolic efficiency.Expand Specific Solutions
Leading Research Groups in Microbial Electrosynthesis
The electron uptake mechanisms by acetogenic microorganisms represent an emerging field at the intersection of microbiology and renewable energy, currently in the early growth phase. The market is expanding rapidly with an estimated value of $300-500 million, driven by applications in carbon capture and biofuel production. Academic institutions dominate the research landscape, with the University of Massachusetts, Arizona State University, and Tianjin University leading fundamental investigations. Commercial development is advancing through companies like LanzaTech, Kiverdi, and Synata Bio, which are scaling microbial electrosynthesis technologies. The field is transitioning from laboratory research to pilot-scale demonstrations, with increasing industry-academic collaborations focused on optimizing electron transfer efficiency and developing economically viable bioprocesses for industrial implementation.
University of Massachusetts
Technical Solution: The University of Massachusetts has established itself as a leader in microbial electrochemistry research, with significant contributions to understanding electron uptake mechanisms in acetogenic microorganisms. UMass researchers, particularly through the Lovley Lab, have pioneered work on extracellular electron transfer in various microbial systems, including acetogens. Their research has identified direct electron transfer mechanisms in acetogenic bacteria, demonstrating that certain species can accept electrons directly from cathode surfaces without requiring hydrogen as an intermediate[10]. UMass has developed innovative bioelectrochemical systems specifically designed to study electron uptake by acetogens, incorporating advanced electrochemical techniques like cyclic voltammetry and chronoamperometry to quantify electron transfer rates. Their research has characterized the molecular components involved in electron uptake, including membrane-bound cytochromes, conductive pili structures, and electron shuttling compounds that facilitate electron movement between electrodes and microbial cells[11]. The university has also investigated the genetic basis of electron uptake capabilities in acetogens, identifying key genes that could be targeted for enhanced performance through genetic engineering. UMass researchers have demonstrated that acetogenic microorganisms can be used in microbial electrosynthesis systems to convert CO2 into acetate and other organic compounds using electrons derived directly from cathodes, with potential applications in renewable energy storage and carbon capture technologies[12]. Their work has also explored the integration of these systems with renewable electricity sources to create sustainable bioproduction platforms.
Strengths: World-leading expertise in microbial electrochemistry and extracellular electron transfer; sophisticated bioelectrochemical testing platforms; strong track record of fundamental discoveries in electron transfer mechanisms. Weaknesses: Focus primarily on fundamental research rather than commercial applications; limited demonstration at scales beyond laboratory systems; research spread across multiple microbial systems beyond just acetogens.
The Regents of the University of California
Technical Solution: The University of California system has conducted extensive research on electron uptake mechanisms in acetogenic microorganisms across several campuses. UC Berkeley and UC Davis researchers have pioneered work on microbial electrosynthesis systems that enable acetogens to directly capture electrons from cathodes for CO2 reduction. Their research has characterized the molecular machinery involved in extracellular electron transfer, including specialized membrane-bound cytochromes, conductive pili (microbial nanowires), and electron shuttling compounds[8]. UC researchers have developed advanced bioelectrochemical reactors with precisely controlled electrode potentials to study the energetics and kinetics of electron uptake by acetogenic microbes. Their work has demonstrated that certain acetogenic species can form robust biofilms on cathode surfaces, facilitating direct electron transfer without soluble mediators. The UC system has also investigated the genetic basis for electron uptake capabilities, identifying key genes involved in extracellular electron transfer and the Wood-Ljungdahl pathway that can be targeted for strain improvement[9]. Their research groups have explored the use of various electrode materials, including carbon-based materials and metal oxides, to enhance electron transfer rates to acetogenic microorganisms. Additionally, UC researchers have investigated the integration of photovoltaic systems with microbial electrosynthesis to create solar-powered CO2 conversion platforms using acetogens as biocatalysts.
Strengths: Comprehensive research spanning fundamental mechanisms to applied systems; strong interdisciplinary collaboration between microbiologists, electrochemists, and materials scientists; access to advanced analytical facilities for detailed characterization of electron transfer processes. Weaknesses: Research distributed across multiple campuses may lead to fragmented approaches; primarily focused on laboratory-scale demonstrations rather than industrial implementation; competing research priorities may limit focused development of specific technologies.
Key Molecular Components of Acetogenic Electron Capture
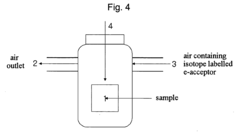
Method of detecting microorgan ISMS using labelled electron acceptors
PatentInactiveUS20040241848A1
Innovation
- A method involving loading a contaminant sample into a sealed chamber, replacing the air with isotopically labelled electron acceptors, and comparing metabolite content through isotopic analysis to differentiate between samples with and without microorganisms, using isotopically labelled electron acceptors like 18O2, allowing for the detection of microorganisms by analyzing the incorporation of labelled metabolites.
Method for enhancing activity to regenerate electron acceptor for oxidoreductase in microorganism capable of producing said oxidoreductase, and use of microorganism prepared by said method
PatentInactiveUS20020068337A1
Innovation
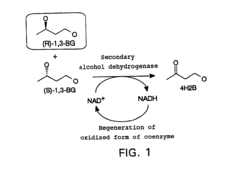
- Culturing microorganisms, such as Escherichia coli, in a medium with a low concentration of dissolved oxygen to enhance the activity of electron acceptor regeneration, specifically maintaining the dissolved oxygen at 50% or less saturation during enzyme expression, which increases the microorganisms' ability to regenerate oxidized coenzymes like NAD+.
Bioelectrochemical System Design Considerations
The design of bioelectrochemical systems (BES) for acetogenic microorganisms requires careful consideration of multiple factors to optimize electron uptake efficiency. The electrode material selection significantly impacts system performance, with carbon-based materials like graphite, carbon cloth, and carbon felt demonstrating superior biocompatibility and conductivity. These materials provide extensive surface area for microbial colonization while facilitating efficient electron transfer between the electrode and microorganisms.
Reactor configuration represents another critical design element. Three-electrode systems offer precise control over electrode potential, enabling researchers to investigate specific electron uptake mechanisms under controlled conditions. Two-electrode systems, while simpler, provide less control but may be more suitable for scaled-up applications. The reactor geometry must accommodate sufficient electrode surface area while ensuring proper mass transfer of substrates and products.
Operational parameters substantially influence electron uptake efficiency in acetogenic bioelectrochemical systems. The applied potential must be carefully optimized to provide sufficient driving force for electron transfer without causing cellular damage or unwanted side reactions. Typically, potentials between -400 mV and -800 mV vs. SHE have shown favorable results for acetogenic electron uptake. Temperature control is equally important, as most acetogens operate optimally between 30-60°C depending on the specific strain.
Medium composition requires careful formulation to support acetogenic growth while facilitating electron transfer. The inclusion of trace metals, particularly iron, nickel, and tungsten, is essential as these elements are cofactors in key enzymes involved in the Wood-Ljungdahl pathway and electron transfer mechanisms. Buffer systems must maintain pH stability, typically between 5.5-7.5, to support both microbial growth and electrochemical reactions.
Monitoring and control systems represent a vital component of BES design. Real-time measurement of parameters such as electrode potential, current density, pH, and gas production enables process optimization and mechanistic insights. Advanced systems may incorporate in-situ spectroscopic techniques to monitor biofilm formation and metabolic activity without disrupting the electrochemical environment.
Scale-up considerations must address challenges in maintaining uniform current distribution across larger electrode surfaces and managing increased ohmic resistance. Modular designs with multiple smaller units operating in parallel often prove more effective than single large-scale systems. Additionally, membrane selection becomes increasingly important at larger scales to prevent crossover of metabolites while minimizing internal resistance.
Reactor configuration represents another critical design element. Three-electrode systems offer precise control over electrode potential, enabling researchers to investigate specific electron uptake mechanisms under controlled conditions. Two-electrode systems, while simpler, provide less control but may be more suitable for scaled-up applications. The reactor geometry must accommodate sufficient electrode surface area while ensuring proper mass transfer of substrates and products.
Operational parameters substantially influence electron uptake efficiency in acetogenic bioelectrochemical systems. The applied potential must be carefully optimized to provide sufficient driving force for electron transfer without causing cellular damage or unwanted side reactions. Typically, potentials between -400 mV and -800 mV vs. SHE have shown favorable results for acetogenic electron uptake. Temperature control is equally important, as most acetogens operate optimally between 30-60°C depending on the specific strain.
Medium composition requires careful formulation to support acetogenic growth while facilitating electron transfer. The inclusion of trace metals, particularly iron, nickel, and tungsten, is essential as these elements are cofactors in key enzymes involved in the Wood-Ljungdahl pathway and electron transfer mechanisms. Buffer systems must maintain pH stability, typically between 5.5-7.5, to support both microbial growth and electrochemical reactions.
Monitoring and control systems represent a vital component of BES design. Real-time measurement of parameters such as electrode potential, current density, pH, and gas production enables process optimization and mechanistic insights. Advanced systems may incorporate in-situ spectroscopic techniques to monitor biofilm formation and metabolic activity without disrupting the electrochemical environment.
Scale-up considerations must address challenges in maintaining uniform current distribution across larger electrode surfaces and managing increased ohmic resistance. Modular designs with multiple smaller units operating in parallel often prove more effective than single large-scale systems. Additionally, membrane selection becomes increasingly important at larger scales to prevent crossover of metabolites while minimizing internal resistance.
Metabolic Engineering Strategies for Improved Electron Utilization
Metabolic engineering offers significant potential for enhancing electron utilization in acetogenic microorganisms, thereby improving their capacity for converting CO2 into valuable biochemicals. Current strategies focus on modifying key metabolic pathways to optimize electron flow and energy conservation mechanisms within these organisms.
The Wood-Ljungdahl pathway represents a primary target for engineering efforts, as it serves as the central carbon fixation route in acetogens. Modifications to enzymes within this pathway, particularly carbon monoxide dehydrogenase and acetyl-CoA synthase, have demonstrated improved electron capture efficiency. Researchers have successfully employed directed evolution and rational protein engineering to enhance the catalytic properties of these enzymes, resulting in increased rates of electron uptake from various sources.
Redox balancing strategies constitute another critical approach, focusing on manipulating the NAD(P)H/NAD(P)+ and ferredoxin ratios within the cell. By introducing heterologous electron-bifurcating enzymes or modifying native ones, electron distribution can be optimized to favor desired product formation while maintaining cellular redox homeostasis. This approach has proven particularly effective when combined with modifications to membrane-bound electron transport complexes.
Engineering of extracellular electron transfer mechanisms represents a frontier area with substantial promise. Enhancing the expression of outer membrane cytochromes, conductive pili, and electron shuttles can significantly improve direct electron uptake from electrodes or other external sources. Recent studies have successfully transferred components from model electroactive organisms like Geobacter and Shewanella into acetogenic platforms, creating hybrid systems with enhanced electron uptake capabilities.
Synthetic biology approaches are increasingly being applied to create artificial electron uptake pathways. These include the introduction of light-harvesting complexes to capture photonic energy, artificial electron conduits to facilitate electron transfer across membranes, and novel electron storage mechanisms. Such synthetic pathways can bypass natural limitations and create more efficient routes for electron utilization.
Genome-scale metabolic modeling has emerged as an essential tool for predicting the effects of genetic modifications on electron flow. These computational approaches enable researchers to identify non-intuitive targets for metabolic engineering and optimize strain design before experimental implementation. Integration of transcriptomic and proteomic data with these models has further improved their predictive power for electron utilization optimization strategies.
The Wood-Ljungdahl pathway represents a primary target for engineering efforts, as it serves as the central carbon fixation route in acetogens. Modifications to enzymes within this pathway, particularly carbon monoxide dehydrogenase and acetyl-CoA synthase, have demonstrated improved electron capture efficiency. Researchers have successfully employed directed evolution and rational protein engineering to enhance the catalytic properties of these enzymes, resulting in increased rates of electron uptake from various sources.
Redox balancing strategies constitute another critical approach, focusing on manipulating the NAD(P)H/NAD(P)+ and ferredoxin ratios within the cell. By introducing heterologous electron-bifurcating enzymes or modifying native ones, electron distribution can be optimized to favor desired product formation while maintaining cellular redox homeostasis. This approach has proven particularly effective when combined with modifications to membrane-bound electron transport complexes.
Engineering of extracellular electron transfer mechanisms represents a frontier area with substantial promise. Enhancing the expression of outer membrane cytochromes, conductive pili, and electron shuttles can significantly improve direct electron uptake from electrodes or other external sources. Recent studies have successfully transferred components from model electroactive organisms like Geobacter and Shewanella into acetogenic platforms, creating hybrid systems with enhanced electron uptake capabilities.
Synthetic biology approaches are increasingly being applied to create artificial electron uptake pathways. These include the introduction of light-harvesting complexes to capture photonic energy, artificial electron conduits to facilitate electron transfer across membranes, and novel electron storage mechanisms. Such synthetic pathways can bypass natural limitations and create more efficient routes for electron utilization.
Genome-scale metabolic modeling has emerged as an essential tool for predicting the effects of genetic modifications on electron flow. These computational approaches enable researchers to identify non-intuitive targets for metabolic engineering and optimize strain design before experimental implementation. Integration of transcriptomic and proteomic data with these models has further improved their predictive power for electron utilization optimization strategies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!