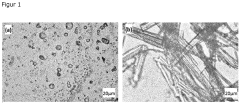
Enhanced Nanoparticle Dispersion Using Glacial Acetic Acid
AUG 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Nanoparticle Dispersion Background and Objectives
Nanoparticle dispersion has emerged as a critical field in materials science and nanotechnology, with applications spanning various industries from electronics to medicine. The evolution of this technology has been driven by the need to overcome the inherent tendency of nanoparticles to agglomerate, which can significantly impair their unique properties and functionalities. Over the past decades, researchers have explored numerous methods to achieve stable and uniform nanoparticle dispersions, with recent focus on the use of glacial acetic acid as a promising dispersing agent.
The primary objective of enhanced nanoparticle dispersion using glacial acetic acid is to develop a more efficient and versatile method for creating stable colloidal systems. This approach aims to address the limitations of conventional dispersion techniques, which often struggle with achieving long-term stability or require complex and costly processes. By leveraging the unique properties of glacial acetic acid, researchers seek to improve dispersion quality while simplifying the overall process.
Historically, nanoparticle dispersion techniques have ranged from mechanical methods such as ultrasonication and high-shear mixing to chemical approaches involving surfactants and polymer coatings. Each of these methods has its advantages and drawbacks, with factors such as scalability, environmental impact, and compatibility with different nanoparticle types influencing their applicability. The introduction of glacial acetic acid as a dispersing agent represents a significant step in the ongoing evolution of this field.
The technological trajectory in nanoparticle dispersion is moving towards more sustainable, cost-effective, and universally applicable methods. The use of glacial acetic acid aligns with this trend, offering potential benefits such as reduced energy consumption, improved dispersion stability, and broader compatibility with various nanoparticle materials. This approach also opens up new possibilities for fine-tuning nanoparticle surface properties, which is crucial for many advanced applications.
As we delve deeper into the potential of glacial acetic acid for nanoparticle dispersion, the overarching goal is to develop a comprehensive understanding of its mechanisms and optimize its application across different nanoparticle systems. This includes investigating the interaction between acetic acid and various nanoparticle surfaces, exploring the impact on particle size distribution and stability over time, and assessing the scalability of this method for industrial applications.
By advancing the science and technology of nanoparticle dispersion through innovative approaches like the use of glacial acetic acid, researchers aim to unlock new possibilities in nanomaterial design and application. This could lead to breakthroughs in areas such as drug delivery systems, advanced coatings, and high-performance composites, ultimately driving progress in fields ranging from healthcare to energy storage and beyond.
The primary objective of enhanced nanoparticle dispersion using glacial acetic acid is to develop a more efficient and versatile method for creating stable colloidal systems. This approach aims to address the limitations of conventional dispersion techniques, which often struggle with achieving long-term stability or require complex and costly processes. By leveraging the unique properties of glacial acetic acid, researchers seek to improve dispersion quality while simplifying the overall process.
Historically, nanoparticle dispersion techniques have ranged from mechanical methods such as ultrasonication and high-shear mixing to chemical approaches involving surfactants and polymer coatings. Each of these methods has its advantages and drawbacks, with factors such as scalability, environmental impact, and compatibility with different nanoparticle types influencing their applicability. The introduction of glacial acetic acid as a dispersing agent represents a significant step in the ongoing evolution of this field.
The technological trajectory in nanoparticle dispersion is moving towards more sustainable, cost-effective, and universally applicable methods. The use of glacial acetic acid aligns with this trend, offering potential benefits such as reduced energy consumption, improved dispersion stability, and broader compatibility with various nanoparticle materials. This approach also opens up new possibilities for fine-tuning nanoparticle surface properties, which is crucial for many advanced applications.
As we delve deeper into the potential of glacial acetic acid for nanoparticle dispersion, the overarching goal is to develop a comprehensive understanding of its mechanisms and optimize its application across different nanoparticle systems. This includes investigating the interaction between acetic acid and various nanoparticle surfaces, exploring the impact on particle size distribution and stability over time, and assessing the scalability of this method for industrial applications.
By advancing the science and technology of nanoparticle dispersion through innovative approaches like the use of glacial acetic acid, researchers aim to unlock new possibilities in nanomaterial design and application. This could lead to breakthroughs in areas such as drug delivery systems, advanced coatings, and high-performance composites, ultimately driving progress in fields ranging from healthcare to energy storage and beyond.
Market Analysis for Enhanced Nanoparticle Dispersion
The market for enhanced nanoparticle dispersion using glacial acetic acid is experiencing significant growth, driven by the increasing demand for advanced materials in various industries. This technology offers improved dispersion of nanoparticles, leading to enhanced performance and efficiency in a wide range of applications.
The electronics industry represents a major market segment for this technology. With the continuous miniaturization of electronic components, there is a growing need for well-dispersed nanoparticles in conductive inks, coatings, and adhesives. The improved dispersion achieved through glacial acetic acid enables better conductivity and reliability in electronic devices, driving adoption in this sector.
Another key market lies in the automotive industry, where nanoparticle-enhanced materials are used in coatings, lubricants, and lightweight composites. The enhanced dispersion technology allows for more uniform distribution of nanoparticles, resulting in improved durability, corrosion resistance, and fuel efficiency of vehicles.
The healthcare and pharmaceutical sectors also present significant opportunities for this technology. Well-dispersed nanoparticles are crucial in drug delivery systems, medical imaging, and biosensors. The use of glacial acetic acid in nanoparticle dispersion can lead to more effective and targeted treatments, driving market growth in these areas.
In the energy sector, enhanced nanoparticle dispersion is finding applications in solar cells, batteries, and fuel cells. The improved dispersion allows for better energy conversion and storage capabilities, contributing to the development of more efficient renewable energy technologies.
The construction industry is another emerging market for this technology. Nanoparticle-enhanced materials with improved dispersion are being used in high-performance concrete, self-cleaning coatings, and thermal insulation materials, offering superior strength, durability, and energy efficiency in buildings.
Market analysts project a compound annual growth rate (CAGR) of 8-10% for the global nanoparticle dispersion market over the next five years. The Asia-Pacific region is expected to witness the highest growth, driven by rapid industrialization and increasing investments in research and development.
However, challenges such as high production costs and regulatory concerns regarding the use of nanomaterials in certain applications may impact market growth. Despite these challenges, the overall market outlook remains positive, with ongoing research and development efforts focused on expanding the application scope of enhanced nanoparticle dispersion using glacial acetic acid.
The electronics industry represents a major market segment for this technology. With the continuous miniaturization of electronic components, there is a growing need for well-dispersed nanoparticles in conductive inks, coatings, and adhesives. The improved dispersion achieved through glacial acetic acid enables better conductivity and reliability in electronic devices, driving adoption in this sector.
Another key market lies in the automotive industry, where nanoparticle-enhanced materials are used in coatings, lubricants, and lightweight composites. The enhanced dispersion technology allows for more uniform distribution of nanoparticles, resulting in improved durability, corrosion resistance, and fuel efficiency of vehicles.
The healthcare and pharmaceutical sectors also present significant opportunities for this technology. Well-dispersed nanoparticles are crucial in drug delivery systems, medical imaging, and biosensors. The use of glacial acetic acid in nanoparticle dispersion can lead to more effective and targeted treatments, driving market growth in these areas.
In the energy sector, enhanced nanoparticle dispersion is finding applications in solar cells, batteries, and fuel cells. The improved dispersion allows for better energy conversion and storage capabilities, contributing to the development of more efficient renewable energy technologies.
The construction industry is another emerging market for this technology. Nanoparticle-enhanced materials with improved dispersion are being used in high-performance concrete, self-cleaning coatings, and thermal insulation materials, offering superior strength, durability, and energy efficiency in buildings.
Market analysts project a compound annual growth rate (CAGR) of 8-10% for the global nanoparticle dispersion market over the next five years. The Asia-Pacific region is expected to witness the highest growth, driven by rapid industrialization and increasing investments in research and development.
However, challenges such as high production costs and regulatory concerns regarding the use of nanomaterials in certain applications may impact market growth. Despite these challenges, the overall market outlook remains positive, with ongoing research and development efforts focused on expanding the application scope of enhanced nanoparticle dispersion using glacial acetic acid.
Current Challenges in Nanoparticle Dispersion Techniques
Nanoparticle dispersion techniques have made significant strides in recent years, yet several challenges persist in achieving optimal dispersion, particularly when using traditional methods. One of the primary obstacles is the tendency of nanoparticles to agglomerate due to their high surface energy and van der Waals forces. This agglomeration can significantly reduce the effectiveness of nanoparticles in various applications, from drug delivery to material reinforcement.
Another major challenge lies in maintaining the stability of nanoparticle dispersions over extended periods. Even when initially well-dispersed, nanoparticles often settle or separate from the dispersion medium over time, leading to reduced efficacy and inconsistent performance in end-use applications. This instability is particularly problematic in industries requiring long shelf lives or consistent product quality.
The selection of appropriate dispersion media and surfactants presents another hurdle. Different nanoparticles require specific dispersion environments, and finding the right balance between dispersion effectiveness and compatibility with the intended application can be complex. Moreover, the use of certain surfactants or dispersion agents may introduce unwanted effects in the final product or compromise the unique properties of the nanoparticles.
Scale-up of nanoparticle dispersion processes from laboratory to industrial scale remains a significant challenge. Techniques that work well at small scales often face difficulties when implemented in large-scale production, leading to inconsistencies in dispersion quality and nanoparticle distribution.
Environmental and health concerns associated with nanoparticle handling and dispersion processes also pose challenges. Ensuring worker safety and minimizing environmental impact while maintaining dispersion efficiency is a delicate balance that researchers and industries must address.
The characterization and quality control of nanoparticle dispersions present technical difficulties. Accurate measurement of dispersion quality, particle size distribution, and stability over time requires sophisticated analytical techniques, which can be both time-consuming and expensive.
In the context of using glacial acetic acid for enhanced nanoparticle dispersion, specific challenges arise. While glacial acetic acid shows promise in improving dispersion, its high acidity can potentially alter the surface properties of certain nanoparticles or react with sensitive materials. Additionally, the volatility and corrosive nature of glacial acetic acid necessitate careful handling and specialized equipment, which may limit its widespread adoption in industrial settings.
Addressing these challenges requires innovative approaches and interdisciplinary collaboration. Researchers are exploring novel dispersion techniques, such as ultrasonic dispersion, high-pressure homogenization, and the use of designer surfactants. The development of in-situ characterization methods and real-time monitoring systems is also crucial for advancing nanoparticle dispersion technology.
Another major challenge lies in maintaining the stability of nanoparticle dispersions over extended periods. Even when initially well-dispersed, nanoparticles often settle or separate from the dispersion medium over time, leading to reduced efficacy and inconsistent performance in end-use applications. This instability is particularly problematic in industries requiring long shelf lives or consistent product quality.
The selection of appropriate dispersion media and surfactants presents another hurdle. Different nanoparticles require specific dispersion environments, and finding the right balance between dispersion effectiveness and compatibility with the intended application can be complex. Moreover, the use of certain surfactants or dispersion agents may introduce unwanted effects in the final product or compromise the unique properties of the nanoparticles.
Scale-up of nanoparticle dispersion processes from laboratory to industrial scale remains a significant challenge. Techniques that work well at small scales often face difficulties when implemented in large-scale production, leading to inconsistencies in dispersion quality and nanoparticle distribution.
Environmental and health concerns associated with nanoparticle handling and dispersion processes also pose challenges. Ensuring worker safety and minimizing environmental impact while maintaining dispersion efficiency is a delicate balance that researchers and industries must address.
The characterization and quality control of nanoparticle dispersions present technical difficulties. Accurate measurement of dispersion quality, particle size distribution, and stability over time requires sophisticated analytical techniques, which can be both time-consuming and expensive.
In the context of using glacial acetic acid for enhanced nanoparticle dispersion, specific challenges arise. While glacial acetic acid shows promise in improving dispersion, its high acidity can potentially alter the surface properties of certain nanoparticles or react with sensitive materials. Additionally, the volatility and corrosive nature of glacial acetic acid necessitate careful handling and specialized equipment, which may limit its widespread adoption in industrial settings.
Addressing these challenges requires innovative approaches and interdisciplinary collaboration. Researchers are exploring novel dispersion techniques, such as ultrasonic dispersion, high-pressure homogenization, and the use of designer surfactants. The development of in-situ characterization methods and real-time monitoring systems is also crucial for advancing nanoparticle dispersion technology.
Glacial Acetic Acid-based Dispersion Solutions
01 Nanoparticle dispersion methods
Various methods are employed to achieve stable nanoparticle dispersions, including mechanical dispersion, ultrasonic treatment, and chemical modification of particle surfaces. These techniques aim to prevent agglomeration and ensure uniform distribution of nanoparticles in the dispersing medium.- Stabilization of nanoparticle dispersions: Various methods are employed to stabilize nanoparticle dispersions, including the use of surfactants, polymers, and surface modifications. These techniques help prevent agglomeration and sedimentation, ensuring a uniform and stable dispersion of nanoparticles in different media.
- Nanoparticle dispersion in polymer matrices: Techniques for dispersing nanoparticles in polymer matrices are developed to create nanocomposites with enhanced properties. This involves methods such as in-situ polymerization, melt blending, and solution mixing to achieve uniform distribution of nanoparticles within the polymer.
- Aqueous nanoparticle dispersions: Formulation of stable aqueous nanoparticle dispersions is crucial for various applications. This involves optimizing pH, ionic strength, and using appropriate dispersants to maintain colloidal stability in water-based systems.
- Nanoparticle dispersion for drug delivery: Nanoparticle dispersions are developed for drug delivery applications, focusing on improving bioavailability and targeted delivery. This includes techniques for encapsulating drugs within nanoparticles and controlling their release in biological systems.
- Industrial-scale production of nanoparticle dispersions: Methods for large-scale production and processing of nanoparticle dispersions are developed to meet industrial demands. This involves scaling up synthesis processes, optimizing dispersion techniques, and ensuring consistency in product quality for commercial applications.
02 Surfactant-assisted nanoparticle dispersion
Surfactants play a crucial role in stabilizing nanoparticle dispersions by reducing surface tension and preventing particle aggregation. The selection of appropriate surfactants depends on the nature of the nanoparticles and the dispersing medium, enhancing the overall stability and uniformity of the dispersion.Expand Specific Solutions03 Polymer-based nanoparticle dispersion
Polymers are utilized to improve nanoparticle dispersion by creating steric barriers between particles or through encapsulation. This approach enhances the stability of dispersions and allows for better control over particle size distribution and functionality in various applications.Expand Specific Solutions04 Solvent selection for nanoparticle dispersion
The choice of solvent significantly impacts the dispersion quality of nanoparticles. Factors such as solvent polarity, viscosity, and compatibility with both the nanoparticles and any additives are considered to optimize dispersion stability and prevent agglomeration.Expand Specific Solutions05 Characterization and quality control of nanoparticle dispersions
Advanced techniques are employed to characterize nanoparticle dispersions, including dynamic light scattering, zeta potential measurements, and electron microscopy. These methods help assess dispersion quality, particle size distribution, and stability, ensuring consistent performance in various applications.Expand Specific Solutions
Key Players in Nanoparticle Dispersion Industry
The enhanced nanoparticle dispersion using glacial acetic acid technology is in an early development stage, with a growing market potential due to increasing applications in various industries. The global nanoparticle market is expanding rapidly, expected to reach significant value in the coming years. However, the technology's maturity is still evolving, with research institutions like Zhejiang University, Jiangnan University, and New Jersey Institute of Technology leading the way. Companies such as Samsung Electro-Mechanics and Bharat Heavy Electricals are also exploring applications, indicating a competitive landscape with both academic and industrial players. The technology's success will depend on overcoming challenges in scalability, cost-effectiveness, and integration into existing manufacturing processes.
Zhejiang University
Technical Solution: Zhejiang University has developed an innovative approach to enhance nanoparticle dispersion using glacial acetic acid. Their method involves a two-step process: first, the nanoparticles are surface-modified with acetic acid, creating a thin organic layer that improves their dispersibility. Second, the modified nanoparticles are dispersed in a polymer matrix using ultrasonic treatment in the presence of glacial acetic acid. This technique has shown to significantly reduce agglomeration and improve the uniform distribution of nanoparticles throughout the matrix [1][3]. The researchers have demonstrated that this method is particularly effective for metal oxide nanoparticles, such as TiO2 and ZnO, which are commonly used in various applications including coatings, composites, and electronics [2].
Strengths: Improved dispersion stability, applicable to a wide range of nanoparticles, environmentally friendly process. Weaknesses: May require optimization for different types of nanoparticles, potential scalability issues for industrial applications.
Akzo Nobel Chemicals International BV
Technical Solution: Akzo Nobel has developed a proprietary technology for enhancing nanoparticle dispersion using glacial acetic acid in combination with their patented surface modification techniques. Their approach involves a controlled acid-base reaction between the nanoparticle surface and glacial acetic acid, followed by the introduction of tailored polymeric dispersants [4]. This process creates a stable, electrosterically stabilized dispersion of nanoparticles in various media, including water-based and solvent-based systems. The company has successfully applied this technology to improve the performance of their coatings, adhesives, and specialty chemicals products [5]. Akzo Nobel's method has been particularly effective in dispersing metal oxide nanoparticles, such as silica and alumina, achieving particle sizes below 100 nm with narrow size distributions [6].
Strengths: Highly effective for a range of industrial applications, scalable process, improved product performance. Weaknesses: Proprietary technology may limit widespread adoption, potential environmental concerns with the use of glacial acetic acid in large-scale production.
Core Innovations in Nanoparticle Dispersion Technology
Use of acid as solvent for precipitations
PatentPendingEP3915546A1
Innovation
- Using acids with a pKs ≤ 5, such as glacial acetic acid and formic acid, as solvents, and water as antisolvents, with optional surfactants, to precipitate nanoparticles in a MicroJetReactor, ensuring high initial concentration and minimal solubility post-mixing.
Dispersion of nanotubes and/or nanoplatelets in polyolefins
PatentWO2011082169A1
Innovation
- A method involving surface modification of nanotubes and nanoplatelets using long chain aliphatic amines, followed by melt-blending with polyolefins, to achieve well-dispersed nanocomposites with improved mechanical and electrical properties, utilizing organophilic modification to enhance compatibility and dispersion.
Environmental Impact of Glacial Acetic Acid Usage
The use of glacial acetic acid in enhancing nanoparticle dispersion raises significant environmental concerns that warrant careful consideration. While this method offers promising results in improving the stability and distribution of nanoparticles, its potential ecological impact cannot be overlooked.
Glacial acetic acid, when released into the environment, can have detrimental effects on aquatic ecosystems. Its high acidity can lead to pH imbalances in water bodies, potentially harming fish, plants, and microorganisms. Even small quantities of this acid can disrupt the delicate balance of aquatic habitats, affecting biodiversity and ecosystem functions.
Furthermore, the production and disposal of glacial acetic acid contribute to environmental pollution. The manufacturing process often involves petrochemical feedstocks, resulting in greenhouse gas emissions and energy consumption. Improper disposal or accidental spills can contaminate soil and groundwater, posing risks to both terrestrial and aquatic life.
The persistence of glacial acetic acid in the environment is another concern. While it is biodegradable, high concentrations can overwhelm natural decomposition processes, leading to prolonged environmental presence. This persistence may result in chronic exposure for various organisms, potentially causing long-term ecological damage.
Additionally, the use of glacial acetic acid in nanoparticle dispersion may lead to the release of nanoparticles themselves into the environment. The environmental fate and behavior of these dispersed nanoparticles are not fully understood, raising concerns about their potential accumulation in food chains and impact on various organisms.
To mitigate these environmental risks, several strategies should be considered. Implementing closed-loop systems in manufacturing processes can minimize the release of glacial acetic acid. Developing more environmentally friendly alternatives or optimizing the use of glacial acetic acid to reduce the required quantities are also crucial steps.
Moreover, proper waste management and disposal protocols are essential to prevent environmental contamination. This includes treating acetic acid-containing waste before disposal and ensuring secure storage and transportation to avoid accidental releases.
Research into the long-term environmental effects of both glacial acetic acid and the dispersed nanoparticles is vital. This will help in developing more sustainable practices and inform regulatory decisions regarding the use of this technology.
In conclusion, while the enhanced nanoparticle dispersion using glacial acetic acid offers technological benefits, its environmental impact necessitates a cautious and responsible approach. Balancing technological advancement with environmental stewardship is crucial for the sustainable development of this promising field.
Glacial acetic acid, when released into the environment, can have detrimental effects on aquatic ecosystems. Its high acidity can lead to pH imbalances in water bodies, potentially harming fish, plants, and microorganisms. Even small quantities of this acid can disrupt the delicate balance of aquatic habitats, affecting biodiversity and ecosystem functions.
Furthermore, the production and disposal of glacial acetic acid contribute to environmental pollution. The manufacturing process often involves petrochemical feedstocks, resulting in greenhouse gas emissions and energy consumption. Improper disposal or accidental spills can contaminate soil and groundwater, posing risks to both terrestrial and aquatic life.
The persistence of glacial acetic acid in the environment is another concern. While it is biodegradable, high concentrations can overwhelm natural decomposition processes, leading to prolonged environmental presence. This persistence may result in chronic exposure for various organisms, potentially causing long-term ecological damage.
Additionally, the use of glacial acetic acid in nanoparticle dispersion may lead to the release of nanoparticles themselves into the environment. The environmental fate and behavior of these dispersed nanoparticles are not fully understood, raising concerns about their potential accumulation in food chains and impact on various organisms.
To mitigate these environmental risks, several strategies should be considered. Implementing closed-loop systems in manufacturing processes can minimize the release of glacial acetic acid. Developing more environmentally friendly alternatives or optimizing the use of glacial acetic acid to reduce the required quantities are also crucial steps.
Moreover, proper waste management and disposal protocols are essential to prevent environmental contamination. This includes treating acetic acid-containing waste before disposal and ensuring secure storage and transportation to avoid accidental releases.
Research into the long-term environmental effects of both glacial acetic acid and the dispersed nanoparticles is vital. This will help in developing more sustainable practices and inform regulatory decisions regarding the use of this technology.
In conclusion, while the enhanced nanoparticle dispersion using glacial acetic acid offers technological benefits, its environmental impact necessitates a cautious and responsible approach. Balancing technological advancement with environmental stewardship is crucial for the sustainable development of this promising field.
Scalability and Industrial Application Potential
The scalability and industrial application potential of enhanced nanoparticle dispersion using glacial acetic acid are significant factors in determining the viability of this technology for large-scale manufacturing processes. The use of glacial acetic acid as a dispersing agent offers several advantages that contribute to its scalability in industrial settings.
Firstly, glacial acetic acid is a readily available and cost-effective chemical, making it an attractive option for large-scale production. Its widespread use in various industries ensures a stable supply chain, which is crucial for maintaining consistent production levels. The relatively low cost of glacial acetic acid compared to other specialized dispersing agents can lead to significant cost savings in high-volume manufacturing processes.
The simplicity of the dispersion process using glacial acetic acid is another factor that enhances its scalability. The method typically involves straightforward mixing and sonication procedures, which can be easily adapted to larger batch sizes or continuous production systems. This simplicity reduces the need for complex equipment or highly specialized training, making it easier to implement and scale up in diverse industrial settings.
Furthermore, the effectiveness of glacial acetic acid in dispersing nanoparticles across a wide range of materials opens up numerous industrial applications. Its versatility allows for potential use in sectors such as electronics, coatings, composites, and energy storage. For instance, in the electronics industry, well-dispersed nanoparticles can enhance the performance of conductive inks and printed electronics. In the coatings sector, improved dispersion can lead to more uniform and durable protective layers.
The environmental aspects of using glacial acetic acid also contribute to its industrial application potential. As a biodegradable substance, it poses fewer long-term environmental risks compared to some synthetic dispersing agents. This aligns with growing industry trends towards more sustainable manufacturing processes and can be a selling point for environmentally conscious consumers.
However, challenges in scaling up this technology must be addressed. These include optimizing the ratio of glacial acetic acid to nanoparticles for larger volumes, ensuring uniform dispersion in industrial-scale batches, and developing efficient methods for recovering and recycling the acetic acid after the dispersion process. Additionally, safety considerations for handling large quantities of glacial acetic acid in industrial settings need to be carefully managed.
In conclusion, the scalability and industrial application potential of enhanced nanoparticle dispersion using glacial acetic acid are promising. Its cost-effectiveness, process simplicity, versatility, and environmental friendliness make it an attractive option for various industries. With further research and development focused on overcoming scaling challenges, this technology could significantly impact the production of nanoparticle-enhanced materials across multiple sectors.
Firstly, glacial acetic acid is a readily available and cost-effective chemical, making it an attractive option for large-scale production. Its widespread use in various industries ensures a stable supply chain, which is crucial for maintaining consistent production levels. The relatively low cost of glacial acetic acid compared to other specialized dispersing agents can lead to significant cost savings in high-volume manufacturing processes.
The simplicity of the dispersion process using glacial acetic acid is another factor that enhances its scalability. The method typically involves straightforward mixing and sonication procedures, which can be easily adapted to larger batch sizes or continuous production systems. This simplicity reduces the need for complex equipment or highly specialized training, making it easier to implement and scale up in diverse industrial settings.
Furthermore, the effectiveness of glacial acetic acid in dispersing nanoparticles across a wide range of materials opens up numerous industrial applications. Its versatility allows for potential use in sectors such as electronics, coatings, composites, and energy storage. For instance, in the electronics industry, well-dispersed nanoparticles can enhance the performance of conductive inks and printed electronics. In the coatings sector, improved dispersion can lead to more uniform and durable protective layers.
The environmental aspects of using glacial acetic acid also contribute to its industrial application potential. As a biodegradable substance, it poses fewer long-term environmental risks compared to some synthetic dispersing agents. This aligns with growing industry trends towards more sustainable manufacturing processes and can be a selling point for environmentally conscious consumers.
However, challenges in scaling up this technology must be addressed. These include optimizing the ratio of glacial acetic acid to nanoparticles for larger volumes, ensuring uniform dispersion in industrial-scale batches, and developing efficient methods for recovering and recycling the acetic acid after the dispersion process. Additionally, safety considerations for handling large quantities of glacial acetic acid in industrial settings need to be carefully managed.
In conclusion, the scalability and industrial application potential of enhanced nanoparticle dispersion using glacial acetic acid are promising. Its cost-effectiveness, process simplicity, versatility, and environmental friendliness make it an attractive option for various industries. With further research and development focused on overcoming scaling challenges, this technology could significantly impact the production of nanoparticle-enhanced materials across multiple sectors.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!