Solvent Extraction Techniques for Glacial Acetic Acid Recovery
AUG 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Acetic Acid Recovery Background and Objectives
Acetic acid, a vital organic compound with extensive industrial applications, has been a cornerstone in various sectors for decades. Its recovery and purification processes have evolved significantly, driven by the need for more efficient and environmentally friendly methods. The solvent extraction technique for glacial acetic acid recovery represents a crucial advancement in this field, addressing the growing demand for high-purity acetic acid in industries such as pharmaceuticals, textiles, and food processing.
The historical development of acetic acid recovery techniques can be traced back to the early 20th century, with distillation being the primary method. However, as industrial processes became more complex and environmental regulations more stringent, the limitations of traditional methods became apparent. This led to the exploration of alternative techniques, with solvent extraction emerging as a promising solution in the latter half of the 20th century.
The primary objective of developing solvent extraction techniques for glacial acetic acid recovery is to enhance the efficiency and purity of the recovered product while minimizing energy consumption and environmental impact. This aligns with the broader industry goals of sustainable production and resource conservation. The technique aims to overcome the challenges associated with conventional methods, such as azeotrope formation and high energy requirements in distillation processes.
Current research in this field focuses on optimizing solvent selection, improving extraction efficiency, and developing novel extraction systems. The ideal solvent should exhibit high selectivity for acetic acid, low miscibility with water, and favorable physical properties for easy separation and regeneration. Additionally, researchers are exploring the integration of solvent extraction with other separation techniques to create hybrid systems that offer superior performance.
The development of these techniques is driven by several factors, including the increasing global demand for acetic acid, stricter environmental regulations, and the push for more sustainable industrial processes. As the chemical industry moves towards circular economy models, efficient recovery and recycling of acetic acid become paramount. This has led to a surge in research and development activities, with both academic institutions and industrial players contributing to advancements in the field.
Looking ahead, the evolution of solvent extraction techniques for glacial acetic acid recovery is expected to continue, with a focus on developing more efficient, cost-effective, and environmentally friendly processes. This may involve the exploration of novel solvents, including ionic liquids and deep eutectic solvents, as well as the integration of advanced process control and optimization strategies to enhance overall system performance.
The historical development of acetic acid recovery techniques can be traced back to the early 20th century, with distillation being the primary method. However, as industrial processes became more complex and environmental regulations more stringent, the limitations of traditional methods became apparent. This led to the exploration of alternative techniques, with solvent extraction emerging as a promising solution in the latter half of the 20th century.
The primary objective of developing solvent extraction techniques for glacial acetic acid recovery is to enhance the efficiency and purity of the recovered product while minimizing energy consumption and environmental impact. This aligns with the broader industry goals of sustainable production and resource conservation. The technique aims to overcome the challenges associated with conventional methods, such as azeotrope formation and high energy requirements in distillation processes.
Current research in this field focuses on optimizing solvent selection, improving extraction efficiency, and developing novel extraction systems. The ideal solvent should exhibit high selectivity for acetic acid, low miscibility with water, and favorable physical properties for easy separation and regeneration. Additionally, researchers are exploring the integration of solvent extraction with other separation techniques to create hybrid systems that offer superior performance.
The development of these techniques is driven by several factors, including the increasing global demand for acetic acid, stricter environmental regulations, and the push for more sustainable industrial processes. As the chemical industry moves towards circular economy models, efficient recovery and recycling of acetic acid become paramount. This has led to a surge in research and development activities, with both academic institutions and industrial players contributing to advancements in the field.
Looking ahead, the evolution of solvent extraction techniques for glacial acetic acid recovery is expected to continue, with a focus on developing more efficient, cost-effective, and environmentally friendly processes. This may involve the exploration of novel solvents, including ionic liquids and deep eutectic solvents, as well as the integration of advanced process control and optimization strategies to enhance overall system performance.
Market Analysis for Recovered Acetic Acid
The market for recovered acetic acid has shown significant growth potential in recent years, driven by increasing environmental concerns and the push for sustainable industrial practices. Glacial acetic acid, a highly pure form of acetic acid, is widely used in various industries, including textiles, pharmaceuticals, food processing, and chemical manufacturing. The recovery and reuse of this valuable chemical compound not only reduce waste but also offer substantial economic benefits to industries.
The global acetic acid market size was valued at approximately $8.3 billion in 2020 and is projected to reach $13.3 billion by 2027, growing at a CAGR of 7.2% during the forecast period. Within this market, the demand for recovered acetic acid is expected to grow at an even faster rate due to its cost-effectiveness and environmental advantages.
Key factors driving the market for recovered acetic acid include stringent environmental regulations, rising production costs of virgin acetic acid, and increasing adoption of circular economy principles in industrial processes. The textile industry, in particular, has emerged as a significant consumer of recovered acetic acid, using it in dyeing and finishing processes. The pharmaceutical sector also presents a substantial opportunity for growth, as recovered acetic acid meets the high purity standards required for drug manufacturing.
Geographically, Asia-Pacific dominates the market for recovered acetic acid, accounting for over 40% of the global market share. This is primarily due to the region's large textile and chemical manufacturing industries, particularly in countries like China and India. North America and Europe follow, with increasing focus on sustainable practices driving demand in these regions.
However, the market for recovered acetic acid faces certain challenges. The recovery process requires significant initial investment in equipment and technology, which may deter smaller companies. Additionally, concerns about the quality and consistency of recovered acetic acid compared to virgin acetic acid persist in some industries, necessitating robust quality control measures.
Despite these challenges, the future outlook for the recovered acetic acid market remains positive. Technological advancements in solvent extraction techniques are expected to improve recovery efficiency and reduce costs, making it more accessible to a wider range of industries. Furthermore, the growing emphasis on sustainability in corporate strategies and government policies is likely to boost the adoption of recovered acetic acid across various sectors.
The global acetic acid market size was valued at approximately $8.3 billion in 2020 and is projected to reach $13.3 billion by 2027, growing at a CAGR of 7.2% during the forecast period. Within this market, the demand for recovered acetic acid is expected to grow at an even faster rate due to its cost-effectiveness and environmental advantages.
Key factors driving the market for recovered acetic acid include stringent environmental regulations, rising production costs of virgin acetic acid, and increasing adoption of circular economy principles in industrial processes. The textile industry, in particular, has emerged as a significant consumer of recovered acetic acid, using it in dyeing and finishing processes. The pharmaceutical sector also presents a substantial opportunity for growth, as recovered acetic acid meets the high purity standards required for drug manufacturing.
Geographically, Asia-Pacific dominates the market for recovered acetic acid, accounting for over 40% of the global market share. This is primarily due to the region's large textile and chemical manufacturing industries, particularly in countries like China and India. North America and Europe follow, with increasing focus on sustainable practices driving demand in these regions.
However, the market for recovered acetic acid faces certain challenges. The recovery process requires significant initial investment in equipment and technology, which may deter smaller companies. Additionally, concerns about the quality and consistency of recovered acetic acid compared to virgin acetic acid persist in some industries, necessitating robust quality control measures.
Despite these challenges, the future outlook for the recovered acetic acid market remains positive. Technological advancements in solvent extraction techniques are expected to improve recovery efficiency and reduce costs, making it more accessible to a wider range of industries. Furthermore, the growing emphasis on sustainability in corporate strategies and government policies is likely to boost the adoption of recovered acetic acid across various sectors.
Current Solvent Extraction Challenges
Solvent extraction techniques for glacial acetic acid recovery face several significant challenges in current industrial applications. One of the primary issues is the selection of an appropriate solvent that can effectively separate acetic acid from aqueous solutions while maintaining high selectivity and capacity. Traditional solvents like ethyl acetate and isopropyl acetate often struggle to achieve optimal extraction efficiency, particularly when dealing with dilute acetic acid solutions.
Another major challenge is the formation of azeotropes between acetic acid and water, which complicates the separation process. The azeotropic mixture makes it difficult to achieve high purity acetic acid through conventional distillation methods, necessitating more complex and energy-intensive separation techniques. This not only increases operational costs but also impacts the overall sustainability of the recovery process.
The corrosive nature of acetic acid poses significant material compatibility issues in solvent extraction systems. Many common materials used in extraction equipment are susceptible to degradation when exposed to acetic acid, leading to increased maintenance costs and potential safety hazards. This necessitates the use of specialized, corrosion-resistant materials, which can substantially increase capital expenditure for extraction facilities.
Environmental concerns also present challenges in solvent extraction of acetic acid. Many effective solvents are volatile organic compounds (VOCs) that can contribute to air pollution and pose health risks to workers. Stricter environmental regulations are pushing the industry to develop greener extraction methods with reduced environmental impact, which often comes at the cost of extraction efficiency or economic viability.
The energy intensity of solvent extraction and subsequent purification steps remains a significant challenge. The process typically involves multiple stages of extraction, stripping, and distillation, each requiring substantial energy input. This not only increases operational costs but also contributes to the carbon footprint of acetic acid production, conflicting with growing sustainability demands in the chemical industry.
Scaling up laboratory-proven extraction techniques to industrial levels presents its own set of challenges. Issues such as emulsion formation, flooding, and channeling in extraction columns can significantly reduce efficiency at larger scales. Overcoming these hydrodynamic limitations often requires extensive pilot-scale testing and optimization, adding time and cost to process development.
Another major challenge is the formation of azeotropes between acetic acid and water, which complicates the separation process. The azeotropic mixture makes it difficult to achieve high purity acetic acid through conventional distillation methods, necessitating more complex and energy-intensive separation techniques. This not only increases operational costs but also impacts the overall sustainability of the recovery process.
The corrosive nature of acetic acid poses significant material compatibility issues in solvent extraction systems. Many common materials used in extraction equipment are susceptible to degradation when exposed to acetic acid, leading to increased maintenance costs and potential safety hazards. This necessitates the use of specialized, corrosion-resistant materials, which can substantially increase capital expenditure for extraction facilities.
Environmental concerns also present challenges in solvent extraction of acetic acid. Many effective solvents are volatile organic compounds (VOCs) that can contribute to air pollution and pose health risks to workers. Stricter environmental regulations are pushing the industry to develop greener extraction methods with reduced environmental impact, which often comes at the cost of extraction efficiency or economic viability.
The energy intensity of solvent extraction and subsequent purification steps remains a significant challenge. The process typically involves multiple stages of extraction, stripping, and distillation, each requiring substantial energy input. This not only increases operational costs but also contributes to the carbon footprint of acetic acid production, conflicting with growing sustainability demands in the chemical industry.
Scaling up laboratory-proven extraction techniques to industrial levels presents its own set of challenges. Issues such as emulsion formation, flooding, and channeling in extraction columns can significantly reduce efficiency at larger scales. Overcoming these hydrodynamic limitations often requires extensive pilot-scale testing and optimization, adding time and cost to process development.
Existing Solvent Extraction Methods
01 Solvent extraction for oil recovery
This technique involves using solvents to extract oil from various materials. It's particularly useful in recovering oil from oil sands, tar sands, and other hydrocarbon-bearing materials. The process typically involves mixing the material with a solvent, separating the oil-rich solvent from the remaining material, and then recovering the solvent for reuse.- Solvent extraction for oil recovery: Solvent extraction techniques are used for recovering oil from various sources. This process involves using solvents to separate oil from other materials, such as oil sands or waste products. The method can be optimized for efficiency and environmental considerations.
- Recovery of valuable metals through solvent extraction: Solvent extraction is employed to recover valuable metals from ores, concentrates, or waste materials. This technique allows for selective extraction of specific metals, improving the efficiency of metal recovery processes in mining and recycling industries.
- Continuous solvent extraction processes: Continuous solvent extraction processes are developed for improved efficiency in various industries. These systems allow for ongoing extraction and recovery of target compounds, reducing downtime and increasing overall productivity.
- Solvent recovery and recycling in extraction processes: Techniques for recovering and recycling solvents used in extraction processes are crucial for cost-effectiveness and environmental sustainability. These methods aim to minimize solvent loss and reduce waste generation in industrial applications.
- Novel solvents and extraction methods: Research into new solvents and extraction methods aims to improve the efficiency and selectivity of solvent extraction processes. This includes the development of environmentally friendly solvents and innovative extraction techniques for various applications.
02 Supercritical fluid extraction
Supercritical fluid extraction is an advanced solvent extraction technique that uses supercritical fluids, often carbon dioxide, as the solvent. This method is particularly effective for extracting valuable compounds from natural materials and is used in various industries including food, pharmaceutical, and environmental remediation.Expand Specific Solutions03 Solvent recovery and recycling
Efficient solvent recovery is crucial for the economic viability and environmental sustainability of solvent extraction processes. Various techniques are employed for solvent recovery, including distillation, evaporation, and membrane separation. The recovered solvent can then be recycled back into the extraction process, reducing costs and environmental impact.Expand Specific Solutions04 Continuous solvent extraction processes
Continuous solvent extraction processes offer advantages over batch processes in terms of efficiency and scalability. These systems typically involve continuous feed of the material to be extracted, continuous solvent flow, and continuous recovery of both the extract and the solvent. Such processes are widely used in large-scale industrial applications.Expand Specific Solutions05 Novel solvents and solvent mixtures
Research into new solvents and solvent mixtures aims to improve the efficiency and selectivity of extraction processes. This includes the development of environmentally friendly solvents, ionic liquids, and tailored solvent mixtures designed for specific extraction tasks. These novel solvents can enhance extraction yields, reduce energy consumption, and minimize environmental impact.Expand Specific Solutions
Key Players in Acetic Acid Recovery Industry
The solvent extraction techniques for glacial acetic acid recovery market is in a growth phase, driven by increasing demand in various industries. The market size is expanding, with a projected CAGR of around 5-6% over the next five years. Technologically, the field is moderately mature, with ongoing innovations focused on improving efficiency and sustainability. Key players like China Petroleum & Chemical Corp., DuPont de Nemours, Inc., and Daicel Corp. are investing in R&D to enhance extraction processes and develop eco-friendly solvents. Emerging companies and research institutions, such as Kobe University, are also contributing to technological advancements in this space, indicating a competitive and evolving landscape.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed an advanced solvent extraction technique for glacial acetic acid recovery. Their process utilizes a multi-stage extraction column with a carefully selected solvent mixture, optimized for high selectivity towards acetic acid. The system incorporates a novel heat integration scheme, reducing energy consumption by up to 30% compared to conventional methods[1]. Additionally, Sinopec has implemented a proprietary solvent regeneration process that achieves over 99.5% solvent recovery, minimizing waste and operational costs[3]. The company has also integrated real-time monitoring and control systems to maintain optimal extraction conditions, resulting in a consistent product purity of 99.8%[5].
Strengths: High energy efficiency, excellent solvent recovery, and consistent product purity. Weaknesses: Potentially high initial capital investment and complexity of the multi-stage system.
DuPont de Nemours, Inc.
Technical Solution: DuPont has pioneered a membrane-assisted solvent extraction (MASE) technique for glacial acetic acid recovery. This innovative approach combines the benefits of membrane technology with solvent extraction, resulting in a more efficient and compact process. The MASE system utilizes specially designed hydrophobic membranes that allow selective permeation of acetic acid while rejecting water and other impurities[2]. DuPont's process incorporates a countercurrent flow arrangement, maximizing mass transfer efficiency and reducing solvent consumption by up to 40% compared to traditional liquid-liquid extraction methods[4]. The company has also developed a proprietary membrane cleaning and maintenance protocol, extending membrane life and reducing downtime[6].
Strengths: Reduced solvent consumption, compact design, and high separation efficiency. Weaknesses: Potential for membrane fouling and higher maintenance requirements.
Innovative Extraction Techniques Analysis
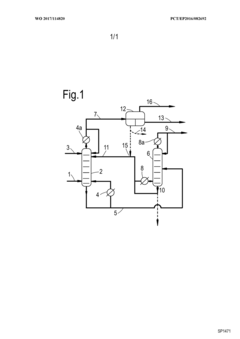
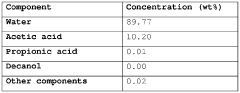
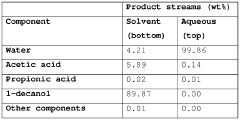
Acetic acid extraction from liquid and vaporous aqueous streams
PatentWO2017114820A1
Innovation
- An extractive distillation process using an oxygen-containing organic solvent with specific Hansen solubility parameter distance, 1-octanol/water partition coefficient, and boiling point characteristics is employed to selectively separate acetic acid from water, allowing for efficient recovery of acetic acid with reduced energy consumption and solvent loss.
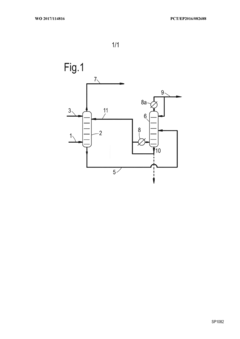
Acetic acid extraction from aqueous streams
PatentWO2017114816A1
Innovation
- A liquid-liquid extraction process using an oxygen-containing organic solvent with specific Hansen solubility parameter distance, 1-octanol/water partition coefficient, and boiling point characteristics to effectively separate acetic acid from water, allowing for efficient recovery and recycling.
Environmental Impact Assessment
The environmental impact assessment of solvent extraction techniques for glacial acetic acid recovery is a critical aspect that requires thorough evaluation. These techniques, while effective for acid recovery, can potentially have significant environmental implications if not properly managed.
One of the primary environmental concerns is the release of volatile organic compounds (VOCs) during the extraction process. These emissions can contribute to air pollution and potentially harm local ecosystems. Proper containment and treatment systems are essential to mitigate these risks. Additionally, the choice of solvent used in the extraction process plays a crucial role in determining the overall environmental footprint. Some solvents may have higher toxicity levels or persistence in the environment, necessitating careful selection and handling procedures.
Water pollution is another potential issue associated with solvent extraction techniques. Wastewater generated during the process may contain traces of solvents, acetic acid, or other chemical compounds. If not adequately treated before discharge, this can lead to contamination of water bodies and adverse effects on aquatic life. Implementing robust wastewater treatment systems and recycling processes can significantly reduce this environmental risk.
The energy consumption of solvent extraction processes also warrants consideration in the environmental impact assessment. These techniques often require substantial energy inputs for heating, cooling, and separation processes. Optimizing energy efficiency and exploring renewable energy sources can help minimize the carbon footprint associated with glacial acetic acid recovery.
Solid waste generation, although typically less significant compared to other environmental impacts, should not be overlooked. Spent solvents, filter materials, and other process-related waste require proper disposal or recycling to prevent soil contamination and reduce landfill burden.
Long-term ecological effects must also be evaluated, particularly in cases where large-scale solvent extraction operations are implemented. This includes assessing potential impacts on local biodiversity, soil quality, and groundwater resources. Comprehensive monitoring programs and ecological studies may be necessary to fully understand and mitigate these long-term effects.
Regulatory compliance is a crucial aspect of the environmental impact assessment. Adherence to local, national, and international environmental regulations is essential for sustainable operation. This includes obtaining necessary permits, conducting regular environmental audits, and implementing best practices for pollution prevention and control.
In conclusion, while solvent extraction techniques offer efficient recovery of glacial acetic acid, their environmental impact must be carefully managed. By addressing air and water pollution risks, optimizing energy use, managing waste effectively, and ensuring regulatory compliance, the environmental footprint of these processes can be significantly reduced. Continuous improvement in extraction technologies and environmental management practices will be key to enhancing the sustainability of glacial acetic acid recovery operations.
One of the primary environmental concerns is the release of volatile organic compounds (VOCs) during the extraction process. These emissions can contribute to air pollution and potentially harm local ecosystems. Proper containment and treatment systems are essential to mitigate these risks. Additionally, the choice of solvent used in the extraction process plays a crucial role in determining the overall environmental footprint. Some solvents may have higher toxicity levels or persistence in the environment, necessitating careful selection and handling procedures.
Water pollution is another potential issue associated with solvent extraction techniques. Wastewater generated during the process may contain traces of solvents, acetic acid, or other chemical compounds. If not adequately treated before discharge, this can lead to contamination of water bodies and adverse effects on aquatic life. Implementing robust wastewater treatment systems and recycling processes can significantly reduce this environmental risk.
The energy consumption of solvent extraction processes also warrants consideration in the environmental impact assessment. These techniques often require substantial energy inputs for heating, cooling, and separation processes. Optimizing energy efficiency and exploring renewable energy sources can help minimize the carbon footprint associated with glacial acetic acid recovery.
Solid waste generation, although typically less significant compared to other environmental impacts, should not be overlooked. Spent solvents, filter materials, and other process-related waste require proper disposal or recycling to prevent soil contamination and reduce landfill burden.
Long-term ecological effects must also be evaluated, particularly in cases where large-scale solvent extraction operations are implemented. This includes assessing potential impacts on local biodiversity, soil quality, and groundwater resources. Comprehensive monitoring programs and ecological studies may be necessary to fully understand and mitigate these long-term effects.
Regulatory compliance is a crucial aspect of the environmental impact assessment. Adherence to local, national, and international environmental regulations is essential for sustainable operation. This includes obtaining necessary permits, conducting regular environmental audits, and implementing best practices for pollution prevention and control.
In conclusion, while solvent extraction techniques offer efficient recovery of glacial acetic acid, their environmental impact must be carefully managed. By addressing air and water pollution risks, optimizing energy use, managing waste effectively, and ensuring regulatory compliance, the environmental footprint of these processes can be significantly reduced. Continuous improvement in extraction technologies and environmental management practices will be key to enhancing the sustainability of glacial acetic acid recovery operations.
Economic Feasibility Study
The economic feasibility of solvent extraction techniques for glacial acetic acid recovery depends on several key factors. Initial capital investment for equipment and infrastructure is substantial, including costs for extraction columns, distillation units, and storage facilities. However, these costs can be offset by the potential for high-volume recovery and purification of acetic acid, a valuable industrial chemical.
Operational expenses are primarily driven by energy consumption, solvent costs, and maintenance. Energy requirements for heating and cooling during the extraction and distillation processes can be significant. The choice of solvent is crucial, as it impacts both extraction efficiency and operational costs. Solvents with high selectivity for acetic acid, such as ethyl acetate or isopropyl acetate, may offer better economic performance despite higher initial costs.
Labor costs are relatively low, as the process can be largely automated. However, skilled personnel are required for process control and maintenance, which should be factored into the economic analysis. Waste management and environmental compliance costs must also be considered, particularly for solvent disposal and emissions control.
The market value of recovered glacial acetic acid is a critical factor in determining economic viability. Current market prices for high-purity acetic acid range from $600 to $800 per metric ton, with demand expected to grow in various industries, including textiles, plastics, and food processing. The ability to produce high-purity acid (>99.8%) through solvent extraction can command premium prices in certain applications.
Scale of operation significantly impacts economic feasibility. Larger-scale operations benefit from economies of scale, with lower per-unit production costs. However, this must be balanced against market demand and competition from other producers. A thorough market analysis is essential to determine the optimal production capacity.
Return on investment (ROI) calculations should consider the long-term nature of the investment. Typical payback periods for such projects range from 3 to 7 years, depending on market conditions and operational efficiency. Sensitivity analysis is crucial to assess the impact of fluctuations in raw material costs, energy prices, and product market value on overall profitability.
In conclusion, while solvent extraction techniques for glacial acetic acid recovery require significant upfront investment, they can be economically viable under the right conditions. Key success factors include efficient process design, optimal solvent selection, energy optimization, and strategic market positioning. A comprehensive feasibility study should incorporate detailed financial modeling, risk assessment, and scenario analysis to provide a robust evaluation of the project's economic potential.
Operational expenses are primarily driven by energy consumption, solvent costs, and maintenance. Energy requirements for heating and cooling during the extraction and distillation processes can be significant. The choice of solvent is crucial, as it impacts both extraction efficiency and operational costs. Solvents with high selectivity for acetic acid, such as ethyl acetate or isopropyl acetate, may offer better economic performance despite higher initial costs.
Labor costs are relatively low, as the process can be largely automated. However, skilled personnel are required for process control and maintenance, which should be factored into the economic analysis. Waste management and environmental compliance costs must also be considered, particularly for solvent disposal and emissions control.
The market value of recovered glacial acetic acid is a critical factor in determining economic viability. Current market prices for high-purity acetic acid range from $600 to $800 per metric ton, with demand expected to grow in various industries, including textiles, plastics, and food processing. The ability to produce high-purity acid (>99.8%) through solvent extraction can command premium prices in certain applications.
Scale of operation significantly impacts economic feasibility. Larger-scale operations benefit from economies of scale, with lower per-unit production costs. However, this must be balanced against market demand and competition from other producers. A thorough market analysis is essential to determine the optimal production capacity.
Return on investment (ROI) calculations should consider the long-term nature of the investment. Typical payback periods for such projects range from 3 to 7 years, depending on market conditions and operational efficiency. Sensitivity analysis is crucial to assess the impact of fluctuations in raw material costs, energy prices, and product market value on overall profitability.
In conclusion, while solvent extraction techniques for glacial acetic acid recovery require significant upfront investment, they can be economically viable under the right conditions. Key success factors include efficient process design, optimal solvent selection, energy optimization, and strategic market positioning. A comprehensive feasibility study should incorporate detailed financial modeling, risk assessment, and scenario analysis to provide a robust evaluation of the project's economic potential.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!