The Role of Glacial Acetic Acid in Advanced Electroplating Techniques
AUG 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electroplating Evolution
Electroplating has undergone significant evolution since its inception in the early 19th century. The process, which involves the deposition of a metal coating onto a conductive surface through electrochemical reactions, has seen numerous advancements in techniques, materials, and applications over time.
In the early stages, electroplating was primarily used for decorative purposes, with gold and silver being the most commonly plated metals. The process was largely manual and inconsistent, resulting in varying quality of finishes. As industrial applications grew, the need for more precise and efficient plating methods became apparent.
The mid-20th century marked a turning point in electroplating technology. The introduction of automated plating lines significantly improved consistency and productivity. This period also saw the development of new electrolyte formulations, including the use of complexing agents and additives to enhance deposit properties and control the plating process more effectively.
The late 20th century brought about a revolution in electroplating with the advent of pulse plating techniques. This method, which involves the application of current in short pulses rather than continuously, allowed for better control over deposit properties and improved throwing power. Pulse plating opened up new possibilities for creating specialized coatings with enhanced characteristics.
In recent decades, the focus has shifted towards environmentally friendly and sustainable electroplating processes. This has led to the development of alternatives to traditional cyanide-based baths and the exploration of less toxic electrolyte formulations. The use of glacial acetic acid in advanced electroplating techniques is part of this trend, offering a more environmentally benign option for certain applications.
The integration of computer control and real-time monitoring systems has further refined electroplating processes. These advancements allow for precise control of parameters such as current density, temperature, and electrolyte composition, resulting in higher quality and more consistent plating outcomes.
Nanotechnology has also made its mark on electroplating evolution. The ability to create nanostructured coatings through electrodeposition has opened up new avenues for enhancing material properties, such as improved wear resistance, corrosion protection, and even self-healing capabilities.
As we move forward, the evolution of electroplating continues to be driven by the demands for more sophisticated, efficient, and sustainable processes. The role of glacial acetic acid in advanced techniques represents just one aspect of this ongoing development, reflecting the industry's commitment to innovation and environmental responsibility.
In the early stages, electroplating was primarily used for decorative purposes, with gold and silver being the most commonly plated metals. The process was largely manual and inconsistent, resulting in varying quality of finishes. As industrial applications grew, the need for more precise and efficient plating methods became apparent.
The mid-20th century marked a turning point in electroplating technology. The introduction of automated plating lines significantly improved consistency and productivity. This period also saw the development of new electrolyte formulations, including the use of complexing agents and additives to enhance deposit properties and control the plating process more effectively.
The late 20th century brought about a revolution in electroplating with the advent of pulse plating techniques. This method, which involves the application of current in short pulses rather than continuously, allowed for better control over deposit properties and improved throwing power. Pulse plating opened up new possibilities for creating specialized coatings with enhanced characteristics.
In recent decades, the focus has shifted towards environmentally friendly and sustainable electroplating processes. This has led to the development of alternatives to traditional cyanide-based baths and the exploration of less toxic electrolyte formulations. The use of glacial acetic acid in advanced electroplating techniques is part of this trend, offering a more environmentally benign option for certain applications.
The integration of computer control and real-time monitoring systems has further refined electroplating processes. These advancements allow for precise control of parameters such as current density, temperature, and electrolyte composition, resulting in higher quality and more consistent plating outcomes.
Nanotechnology has also made its mark on electroplating evolution. The ability to create nanostructured coatings through electrodeposition has opened up new avenues for enhancing material properties, such as improved wear resistance, corrosion protection, and even self-healing capabilities.
As we move forward, the evolution of electroplating continues to be driven by the demands for more sophisticated, efficient, and sustainable processes. The role of glacial acetic acid in advanced techniques represents just one aspect of this ongoing development, reflecting the industry's commitment to innovation and environmental responsibility.
Market Demand Analysis
The market demand for advanced electroplating techniques utilizing glacial acetic acid has shown significant growth in recent years, driven by the increasing need for high-performance surface coatings across various industries. The electronics sector, in particular, has emerged as a key driver of this demand, with the rising production of smartphones, tablets, and other consumer electronics requiring sophisticated plating solutions for improved durability and functionality.
Automotive manufacturing represents another substantial market for advanced electroplating techniques. The industry's shift towards electric vehicles and the growing emphasis on lightweight materials have created new opportunities for innovative plating processes that can enhance corrosion resistance and reduce overall vehicle weight. This trend is expected to continue as automakers strive to meet stringent environmental regulations and improve fuel efficiency.
The aerospace industry has also contributed to the expanding market for advanced electroplating techniques. With the constant push for lighter and more durable components, aerospace manufacturers are increasingly turning to specialized plating solutions to protect critical parts from extreme conditions while maintaining optimal performance. This demand is particularly pronounced in the commercial aviation sector, where the need for long-lasting, high-performance coatings is paramount.
In the medical device industry, the use of glacial acetic acid in electroplating has gained traction due to its ability to create biocompatible surfaces with enhanced properties. The growing prevalence of implantable devices and the need for antimicrobial coatings in healthcare settings have further bolstered the demand for advanced plating techniques in this sector.
The renewable energy sector, especially solar panel manufacturing, has emerged as a promising market for advanced electroplating. The use of specialized plating processes can improve the efficiency and longevity of solar cells, contributing to the overall growth of the solar energy industry. As governments worldwide continue to invest in clean energy solutions, this market segment is poised for substantial expansion.
Geographically, Asia-Pacific has emerged as the largest market for advanced electroplating techniques, driven by the region's robust electronics manufacturing sector and rapid industrialization. North America and Europe follow closely, with their strong presence in automotive, aerospace, and medical device industries contributing significantly to market growth.
Looking ahead, the market for advanced electroplating techniques utilizing glacial acetic acid is projected to maintain its upward trajectory. Factors such as the ongoing miniaturization of electronic components, the increasing adoption of electric vehicles, and the growing demand for sustainable manufacturing processes are expected to fuel this growth. Additionally, the development of new materials and the need for more efficient plating solutions in emerging technologies like 5G infrastructure and Internet of Things (IoT) devices are likely to create fresh opportunities for innovation and market expansion in the coming years.
Automotive manufacturing represents another substantial market for advanced electroplating techniques. The industry's shift towards electric vehicles and the growing emphasis on lightweight materials have created new opportunities for innovative plating processes that can enhance corrosion resistance and reduce overall vehicle weight. This trend is expected to continue as automakers strive to meet stringent environmental regulations and improve fuel efficiency.
The aerospace industry has also contributed to the expanding market for advanced electroplating techniques. With the constant push for lighter and more durable components, aerospace manufacturers are increasingly turning to specialized plating solutions to protect critical parts from extreme conditions while maintaining optimal performance. This demand is particularly pronounced in the commercial aviation sector, where the need for long-lasting, high-performance coatings is paramount.
In the medical device industry, the use of glacial acetic acid in electroplating has gained traction due to its ability to create biocompatible surfaces with enhanced properties. The growing prevalence of implantable devices and the need for antimicrobial coatings in healthcare settings have further bolstered the demand for advanced plating techniques in this sector.
The renewable energy sector, especially solar panel manufacturing, has emerged as a promising market for advanced electroplating. The use of specialized plating processes can improve the efficiency and longevity of solar cells, contributing to the overall growth of the solar energy industry. As governments worldwide continue to invest in clean energy solutions, this market segment is poised for substantial expansion.
Geographically, Asia-Pacific has emerged as the largest market for advanced electroplating techniques, driven by the region's robust electronics manufacturing sector and rapid industrialization. North America and Europe follow closely, with their strong presence in automotive, aerospace, and medical device industries contributing significantly to market growth.
Looking ahead, the market for advanced electroplating techniques utilizing glacial acetic acid is projected to maintain its upward trajectory. Factors such as the ongoing miniaturization of electronic components, the increasing adoption of electric vehicles, and the growing demand for sustainable manufacturing processes are expected to fuel this growth. Additionally, the development of new materials and the need for more efficient plating solutions in emerging technologies like 5G infrastructure and Internet of Things (IoT) devices are likely to create fresh opportunities for innovation and market expansion in the coming years.
Glacial Acetic Acid
Glacial acetic acid, also known as anhydrous acetic acid, is a crucial component in advanced electroplating techniques. This organic compound, with its chemical formula CH3COOH, plays a multifaceted role in enhancing the efficiency and quality of electroplating processes. Its high purity and low water content make it an ideal choice for various electroplating applications.
In electroplating, glacial acetic acid primarily functions as an electrolyte additive. It helps to improve the conductivity of the plating solution, facilitating the efficient transfer of metal ions from the anode to the cathode. This enhanced conductivity leads to more uniform metal deposition, resulting in smoother and more consistent plating surfaces. Additionally, the acid's ability to form complexes with metal ions contributes to better control over the plating process, allowing for finer adjustments in deposition rates and layer thicknesses.
The use of glacial acetic acid in electroplating solutions also aids in maintaining the pH balance of the bath. This pH control is crucial for optimizing the plating conditions and ensuring the stability of the metal ions in solution. By regulating the acidity of the plating bath, glacial acetic acid helps prevent the formation of hydroxides and other undesirable precipitates that could compromise the quality of the plated surface.
Furthermore, glacial acetic acid serves as an effective cleaning agent in the pre-plating process. Its ability to dissolve oxides and other contaminants on the surface of the substrate ensures proper adhesion of the plated metal. This cleaning action is particularly beneficial for metals that are prone to oxidation, such as copper and iron, as it helps create a clean, reactive surface for optimal plating results.
In advanced electroplating techniques, glacial acetic acid is often used in combination with other additives to achieve specific surface properties. For instance, it can be employed in the production of bright and ductile nickel deposits, where it works synergistically with other organic compounds to influence the crystal structure of the deposited metal. This results in improved corrosion resistance and enhanced aesthetic appeal of the plated surface.
The role of glacial acetic acid extends to specialized electroplating processes such as alloy plating and composite electrodeposition. In these applications, it aids in the co-deposition of different metal species or the incorporation of non-metallic particles into the plated layer. By modifying the electrochemical behavior of the plating bath, glacial acetic acid enables the creation of advanced functional coatings with tailored properties for specific industrial applications.
In electroplating, glacial acetic acid primarily functions as an electrolyte additive. It helps to improve the conductivity of the plating solution, facilitating the efficient transfer of metal ions from the anode to the cathode. This enhanced conductivity leads to more uniform metal deposition, resulting in smoother and more consistent plating surfaces. Additionally, the acid's ability to form complexes with metal ions contributes to better control over the plating process, allowing for finer adjustments in deposition rates and layer thicknesses.
The use of glacial acetic acid in electroplating solutions also aids in maintaining the pH balance of the bath. This pH control is crucial for optimizing the plating conditions and ensuring the stability of the metal ions in solution. By regulating the acidity of the plating bath, glacial acetic acid helps prevent the formation of hydroxides and other undesirable precipitates that could compromise the quality of the plated surface.
Furthermore, glacial acetic acid serves as an effective cleaning agent in the pre-plating process. Its ability to dissolve oxides and other contaminants on the surface of the substrate ensures proper adhesion of the plated metal. This cleaning action is particularly beneficial for metals that are prone to oxidation, such as copper and iron, as it helps create a clean, reactive surface for optimal plating results.
In advanced electroplating techniques, glacial acetic acid is often used in combination with other additives to achieve specific surface properties. For instance, it can be employed in the production of bright and ductile nickel deposits, where it works synergistically with other organic compounds to influence the crystal structure of the deposited metal. This results in improved corrosion resistance and enhanced aesthetic appeal of the plated surface.
The role of glacial acetic acid extends to specialized electroplating processes such as alloy plating and composite electrodeposition. In these applications, it aids in the co-deposition of different metal species or the incorporation of non-metallic particles into the plated layer. By modifying the electrochemical behavior of the plating bath, glacial acetic acid enables the creation of advanced functional coatings with tailored properties for specific industrial applications.
Current Techniques
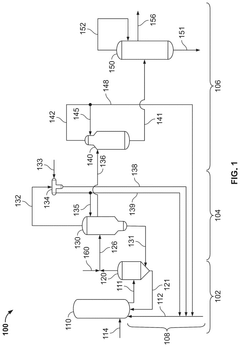
01 Production methods of glacial acetic acid
Various methods are employed to produce glacial acetic acid, including oxidation of acetaldehyde, fermentation processes, and catalytic reactions. These methods often involve specific reaction conditions, catalysts, and purification steps to achieve high purity acetic acid.- Production methods of glacial acetic acid: Various methods are employed to produce glacial acetic acid, including oxidation of acetaldehyde, fermentation processes, and catalytic reactions. These methods often involve specific reaction conditions, catalysts, and purification steps to achieve high purity acetic acid.
- Purification and concentration techniques: Purification and concentration of acetic acid to achieve glacial grade often involves distillation, crystallization, and membrane separation processes. These techniques aim to remove impurities and increase the acid concentration to near 100%.
- Applications in chemical synthesis: Glacial acetic acid serves as a crucial reagent and solvent in various chemical synthesis processes. It is used in the production of vinyl acetate monomer, acetic anhydride, and other organic compounds, playing a vital role in industrial chemistry.
- Storage and handling systems: Specialized storage and handling systems are designed for glacial acetic acid due to its corrosive nature. These systems often include corrosion-resistant materials, safety features, and specific designs to maintain the acid's purity during storage and transportation.
- Environmental and safety considerations: Handling glacial acetic acid requires strict safety measures and environmental considerations. This includes proper disposal methods, emission control systems, and safety equipment to protect workers and the environment from potential hazards associated with the acid.
02 Applications in chemical synthesis
Glacial acetic acid serves as a crucial reagent and solvent in numerous chemical synthesis processes. It is used in the production of various organic compounds, pharmaceuticals, and industrial chemicals due to its high purity and reactivity.Expand Specific Solutions03 Purification and concentration techniques
Specialized techniques are employed to purify and concentrate acetic acid to achieve glacial purity. These may include distillation, crystallization, and membrane separation processes, often utilizing specific equipment and operating conditions.Expand Specific Solutions04 Industrial equipment and processes
Specialized industrial equipment and processes are designed for the handling, storage, and processing of glacial acetic acid. This includes corrosion-resistant materials, safety systems, and process control mechanisms to ensure efficient and safe operations.Expand Specific Solutions05 Safety and environmental considerations
Handling glacial acetic acid requires strict safety measures and environmental considerations. This includes proper storage, transportation, and disposal methods, as well as techniques for neutralization and treatment of acetic acid waste to minimize environmental impact.Expand Specific Solutions
Industry Leaders
The glacial acetic acid market in advanced electroplating techniques is in a growth phase, driven by increasing demand for high-performance electronic components and automotive applications. The market size is expanding, with a projected CAGR of 5-7% over the next five years. Technologically, the field is advancing rapidly, with companies like Atotech Deutschland GmbH & Co. KG and PPG Industries Ohio, Inc. leading innovation in surface-finishing solutions. Johnson Matthey Plc and Merck Patent GmbH are also making significant strides in developing new catalysts and chemical formulations for electroplating processes. Academic institutions such as Zhejiang University and National Tsing-Hua University are contributing to fundamental research, fostering industry-academia collaborations to push the boundaries of electroplating technology.
Atotech Deutschland GmbH & Co. KG
Technical Solution: Atotech has developed advanced electroplating techniques utilizing glacial acetic acid as a key component in their electrolyte formulations. Their process involves a precise control of acetic acid concentration, typically ranging from 5-15% by volume[1], to enhance the conductivity and stability of the plating bath. This approach allows for improved metal deposition rates and uniformity, particularly in challenging applications such as high-aspect-ratio through-hole plating in printed circuit boards[2]. Atotech's proprietary additives, when combined with glacial acetic acid, create a synergistic effect that promotes better throwing power and reduces the risk of void formation in fine features[3].
Strengths: Enhanced plating uniformity, improved throwing power, and reduced void formation. Weaknesses: Potential for increased process complexity and higher raw material costs due to the use of high-purity glacial acetic acid.
PPG Industries Ohio, Inc.
Technical Solution: PPG Industries has innovated in the field of advanced electroplating by incorporating glacial acetic acid into their pretreatment processes. Their technique involves using acetic acid as a surface activator and chelating agent, typically in concentrations of 1-5% by weight[4]. This pretreatment step enhances the adhesion of subsequent metal layers and improves the overall corrosion resistance of the plated parts. PPG's method also includes a proprietary rinse system that effectively removes excess acetic acid while maintaining the activated surface state, resulting in more consistent plating results across various substrate materials[5].
Strengths: Improved adhesion and corrosion resistance, versatility across different substrates. Weaknesses: Additional process step may increase production time and costs.
Key Innovations
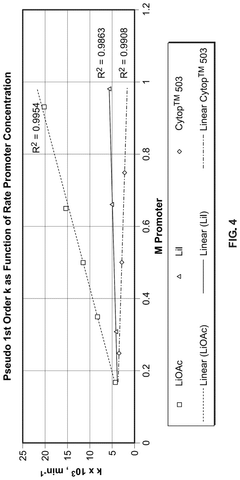
Aminopolycarboxylates as rate promoters for the glacial acetic acid process
PatentPendingUS20250074857A1
Innovation
- The process involves using a reaction mixture comprising a carbonylation catalyst, water, and specific rate-promoting compounds, such as Group I and Group II aminopolycarboxylate salts, to enhance the rate of acetic acid formation while reducing the amount of water required and suppressing undesirable oxidative addition reactions.
Low bromine content glacial acetic acid
PatentInactiveUS4278503A
Innovation
- A process involving thermal conversion of 3-bromo-2-butanone to 1-butene-3-one and inorganic bromides, followed by cryogenic fractional crystallization, reduces 3-bromo-2-butanone contamination by concentrating the aqueous acid mixture and rejecting the impurity, using decompression and heat treatment steps before distillative removal of organic impurities.
Environmental Impact
The use of glacial acetic acid in advanced electroplating techniques raises significant environmental concerns that require careful consideration and mitigation strategies. The primary environmental impact stems from the potential release of acetic acid into aquatic ecosystems, which can lead to acidification and harm aquatic life. Even small quantities of acetic acid can alter the pH balance of water bodies, affecting the survival and reproduction of fish, plants, and microorganisms.
Furthermore, the production and disposal of glacial acetic acid contribute to air pollution and greenhouse gas emissions. The manufacturing process often involves fossil fuel consumption, leading to carbon dioxide emissions and other air pollutants. Improper disposal or accidental spills of acetic acid can contaminate soil and groundwater, posing risks to terrestrial ecosystems and human health.
In electroplating facilities, the use of glacial acetic acid may result in the generation of hazardous waste streams. These waste streams can contain heavy metals and other toxic substances, which require specialized treatment and disposal methods to prevent environmental contamination. The potential for workplace exposure to acetic acid vapors also raises occupational health and safety concerns, necessitating robust safety protocols and ventilation systems.
To address these environmental challenges, the electroplating industry is increasingly adopting cleaner production techniques and waste minimization strategies. These include implementing closed-loop systems to recycle and reuse acetic acid, investing in more efficient plating processes that reduce chemical consumption, and exploring alternative, less hazardous substances as substitutes for glacial acetic acid where possible.
Regulatory frameworks play a crucial role in mitigating the environmental impact of glacial acetic acid use in electroplating. Many countries have implemented strict regulations governing the handling, storage, and disposal of acetic acid and other hazardous chemicals used in industrial processes. Compliance with these regulations often requires companies to invest in advanced treatment technologies and monitoring systems to ensure that emissions and effluents meet environmental standards.
Research and development efforts are ongoing to develop more environmentally friendly electroplating techniques that reduce or eliminate the need for glacial acetic acid. These include the exploration of bio-based alternatives, the use of ionic liquids, and the development of novel electroplating bath formulations that achieve similar or superior results with less environmental impact. Such innovations are crucial for the long-term sustainability of the electroplating industry and its ability to meet increasingly stringent environmental regulations.
Furthermore, the production and disposal of glacial acetic acid contribute to air pollution and greenhouse gas emissions. The manufacturing process often involves fossil fuel consumption, leading to carbon dioxide emissions and other air pollutants. Improper disposal or accidental spills of acetic acid can contaminate soil and groundwater, posing risks to terrestrial ecosystems and human health.
In electroplating facilities, the use of glacial acetic acid may result in the generation of hazardous waste streams. These waste streams can contain heavy metals and other toxic substances, which require specialized treatment and disposal methods to prevent environmental contamination. The potential for workplace exposure to acetic acid vapors also raises occupational health and safety concerns, necessitating robust safety protocols and ventilation systems.
To address these environmental challenges, the electroplating industry is increasingly adopting cleaner production techniques and waste minimization strategies. These include implementing closed-loop systems to recycle and reuse acetic acid, investing in more efficient plating processes that reduce chemical consumption, and exploring alternative, less hazardous substances as substitutes for glacial acetic acid where possible.
Regulatory frameworks play a crucial role in mitigating the environmental impact of glacial acetic acid use in electroplating. Many countries have implemented strict regulations governing the handling, storage, and disposal of acetic acid and other hazardous chemicals used in industrial processes. Compliance with these regulations often requires companies to invest in advanced treatment technologies and monitoring systems to ensure that emissions and effluents meet environmental standards.
Research and development efforts are ongoing to develop more environmentally friendly electroplating techniques that reduce or eliminate the need for glacial acetic acid. These include the exploration of bio-based alternatives, the use of ionic liquids, and the development of novel electroplating bath formulations that achieve similar or superior results with less environmental impact. Such innovations are crucial for the long-term sustainability of the electroplating industry and its ability to meet increasingly stringent environmental regulations.
Quality Control
Quality control plays a crucial role in advanced electroplating techniques utilizing glacial acetic acid. The implementation of robust quality control measures ensures the consistency, reliability, and performance of electroplated products. In the context of glacial acetic acid-based electroplating, quality control encompasses several key aspects that require careful monitoring and management.
Firstly, the purity and concentration of glacial acetic acid must be rigorously controlled. Any variations in these parameters can significantly impact the electroplating process and the resulting surface finish. Regular testing of the acid's composition, including pH levels and contaminant concentrations, is essential to maintain optimal plating conditions. Advanced analytical techniques such as high-performance liquid chromatography (HPLC) and atomic absorption spectroscopy (AAS) are commonly employed for this purpose.
The electroplating bath composition is another critical factor that demands close attention. The precise balance of glacial acetic acid, metal salts, and other additives must be maintained to achieve the desired plating characteristics. Continuous monitoring of bath parameters, including temperature, pH, and metal ion concentrations, is necessary to ensure consistent plating quality. Automated systems equipped with real-time sensors and feedback mechanisms can help maintain these parameters within specified tolerances.
Surface preparation of the substrate is a crucial step that directly influences the quality of the electroplated coating. Thorough cleaning, degreasing, and activation of the substrate surface are essential to promote proper adhesion and uniform plating. Quality control measures in this area include visual inspections, surface roughness measurements, and wettability tests to verify the effectiveness of the preparation process.
During the electroplating process itself, several parameters require careful control. Current density, plating time, and agitation rates must be precisely regulated to achieve the desired coating thickness and properties. Advanced process control systems, incorporating computerized monitoring and adjustment capabilities, can help maintain these parameters within optimal ranges. Regular calibration and maintenance of equipment are also essential to ensure accurate and consistent performance.
Post-plating quality control involves a range of tests and inspections to verify the properties and performance of the electroplated coating. These may include adhesion tests, hardness measurements, corrosion resistance evaluations, and thickness measurements using techniques such as X-ray fluorescence (XRF) or eddy current testing. Visual inspections and microscopic examinations are also conducted to detect any surface defects or irregularities.
Statistical process control (SPC) techniques are widely employed in advanced electroplating operations to monitor and improve quality over time. By collecting and analyzing data on various process parameters and product characteristics, manufacturers can identify trends, detect anomalies, and implement corrective actions proactively. This data-driven approach helps in continuous process optimization and reduction of defects.
In conclusion, effective quality control in advanced electroplating techniques using glacial acetic acid requires a comprehensive and systematic approach. By implementing rigorous controls at every stage of the process, from raw material handling to final product inspection, manufacturers can ensure consistent, high-quality electroplated surfaces that meet or exceed industry standards and customer expectations.
Firstly, the purity and concentration of glacial acetic acid must be rigorously controlled. Any variations in these parameters can significantly impact the electroplating process and the resulting surface finish. Regular testing of the acid's composition, including pH levels and contaminant concentrations, is essential to maintain optimal plating conditions. Advanced analytical techniques such as high-performance liquid chromatography (HPLC) and atomic absorption spectroscopy (AAS) are commonly employed for this purpose.
The electroplating bath composition is another critical factor that demands close attention. The precise balance of glacial acetic acid, metal salts, and other additives must be maintained to achieve the desired plating characteristics. Continuous monitoring of bath parameters, including temperature, pH, and metal ion concentrations, is necessary to ensure consistent plating quality. Automated systems equipped with real-time sensors and feedback mechanisms can help maintain these parameters within specified tolerances.
Surface preparation of the substrate is a crucial step that directly influences the quality of the electroplated coating. Thorough cleaning, degreasing, and activation of the substrate surface are essential to promote proper adhesion and uniform plating. Quality control measures in this area include visual inspections, surface roughness measurements, and wettability tests to verify the effectiveness of the preparation process.
During the electroplating process itself, several parameters require careful control. Current density, plating time, and agitation rates must be precisely regulated to achieve the desired coating thickness and properties. Advanced process control systems, incorporating computerized monitoring and adjustment capabilities, can help maintain these parameters within optimal ranges. Regular calibration and maintenance of equipment are also essential to ensure accurate and consistent performance.
Post-plating quality control involves a range of tests and inspections to verify the properties and performance of the electroplated coating. These may include adhesion tests, hardness measurements, corrosion resistance evaluations, and thickness measurements using techniques such as X-ray fluorescence (XRF) or eddy current testing. Visual inspections and microscopic examinations are also conducted to detect any surface defects or irregularities.
Statistical process control (SPC) techniques are widely employed in advanced electroplating operations to monitor and improve quality over time. By collecting and analyzing data on various process parameters and product characteristics, manufacturers can identify trends, detect anomalies, and implement corrective actions proactively. This data-driven approach helps in continuous process optimization and reduction of defects.
In conclusion, effective quality control in advanced electroplating techniques using glacial acetic acid requires a comprehensive and systematic approach. By implementing rigorous controls at every stage of the process, from raw material handling to final product inspection, manufacturers can ensure consistent, high-quality electroplated surfaces that meet or exceed industry standards and customer expectations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!