Role of Glacial Acetic Acid in Biodegradable Plastics Synthesis
AUG 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Glacial Acetic Acid in Bioplastics: Background and Objectives
Glacial acetic acid has emerged as a crucial component in the synthesis of biodegradable plastics, marking a significant milestone in the evolution of sustainable materials. The development of biodegradable plastics represents a response to the growing environmental concerns associated with conventional petroleum-based plastics. As global plastic pollution continues to escalate, the need for environmentally friendly alternatives has become increasingly urgent.
The journey of biodegradable plastics can be traced back to the early 20th century, with the discovery of polyhydroxyalkanoates (PHAs) in 1926. However, it wasn't until the 1970s that serious research into biodegradable plastics gained momentum. The role of glacial acetic acid in this field has become more prominent in recent years, as researchers have recognized its potential to enhance the properties and production processes of various bioplastic formulations.
Glacial acetic acid, a pure form of acetic acid with less than 1% water content, serves multiple functions in biodegradable plastic synthesis. It acts as a solvent, catalyst, and reactant in various polymerization processes. Its unique properties, including its ability to dissolve many organic compounds and its relatively low boiling point, make it an ideal candidate for bioplastic production.
The primary objective of incorporating glacial acetic acid in biodegradable plastics synthesis is to improve the material properties of the resulting polymers. These improvements include enhanced biodegradability, increased tensile strength, and improved thermal stability. Additionally, glacial acetic acid can facilitate the blending of different biopolymers, leading to composite materials with superior characteristics.
Another key goal is to optimize the production processes of biodegradable plastics. Glacial acetic acid can potentially reduce reaction times, lower energy requirements, and increase overall efficiency in polymer synthesis. This aligns with the broader objective of making biodegradable plastics more economically viable and competitive with traditional plastics.
As research in this field progresses, the focus is shifting towards developing more sustainable sources of glacial acetic acid. Current production methods primarily rely on petrochemical feedstocks, which somewhat contradicts the eco-friendly nature of biodegradable plastics. Therefore, exploring bio-based routes for acetic acid production has become a critical area of investigation.
The integration of glacial acetic acid in biodegradable plastics synthesis represents a convergence of chemical engineering, materials science, and environmental sustainability. As we move forward, the challenge lies in refining these technologies to create biodegradable plastics that not only match but surpass the performance of conventional plastics, while maintaining a minimal environmental footprint throughout their lifecycle.
The journey of biodegradable plastics can be traced back to the early 20th century, with the discovery of polyhydroxyalkanoates (PHAs) in 1926. However, it wasn't until the 1970s that serious research into biodegradable plastics gained momentum. The role of glacial acetic acid in this field has become more prominent in recent years, as researchers have recognized its potential to enhance the properties and production processes of various bioplastic formulations.
Glacial acetic acid, a pure form of acetic acid with less than 1% water content, serves multiple functions in biodegradable plastic synthesis. It acts as a solvent, catalyst, and reactant in various polymerization processes. Its unique properties, including its ability to dissolve many organic compounds and its relatively low boiling point, make it an ideal candidate for bioplastic production.
The primary objective of incorporating glacial acetic acid in biodegradable plastics synthesis is to improve the material properties of the resulting polymers. These improvements include enhanced biodegradability, increased tensile strength, and improved thermal stability. Additionally, glacial acetic acid can facilitate the blending of different biopolymers, leading to composite materials with superior characteristics.
Another key goal is to optimize the production processes of biodegradable plastics. Glacial acetic acid can potentially reduce reaction times, lower energy requirements, and increase overall efficiency in polymer synthesis. This aligns with the broader objective of making biodegradable plastics more economically viable and competitive with traditional plastics.
As research in this field progresses, the focus is shifting towards developing more sustainable sources of glacial acetic acid. Current production methods primarily rely on petrochemical feedstocks, which somewhat contradicts the eco-friendly nature of biodegradable plastics. Therefore, exploring bio-based routes for acetic acid production has become a critical area of investigation.
The integration of glacial acetic acid in biodegradable plastics synthesis represents a convergence of chemical engineering, materials science, and environmental sustainability. As we move forward, the challenge lies in refining these technologies to create biodegradable plastics that not only match but surpass the performance of conventional plastics, while maintaining a minimal environmental footprint throughout their lifecycle.
Market Analysis for Biodegradable Plastics
The market for biodegradable plastics has experienced significant growth in recent years, driven by increasing environmental concerns and regulatory pressures to reduce plastic waste. The global biodegradable plastics market was valued at approximately $4.5 billion in 2020 and is projected to reach $7.8 billion by 2025, growing at a CAGR of 11.6% during the forecast period.
The packaging industry remains the largest consumer of biodegradable plastics, accounting for over 60% of the total market share. This sector's demand is primarily fueled by the food and beverage industry, which is increasingly adopting eco-friendly packaging solutions. The agriculture and horticulture sectors are also emerging as significant consumers, utilizing biodegradable plastics for mulch films and plant pots.
Geographically, Europe leads the biodegradable plastics market, followed by North America and Asia-Pacific. Europe's dominance is attributed to stringent regulations on single-use plastics and a high level of environmental awareness among consumers. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by rapid industrialization and increasing adoption of sustainable practices in countries like China and India.
The market is characterized by the presence of both established players and new entrants, leading to intense competition and innovation. Key market players include NatureWorks LLC, BASF SE, Novamont S.p.A., and Total Corbion PLA. These companies are investing heavily in research and development to improve the properties and reduce the production costs of biodegradable plastics.
Consumer awareness and willingness to pay for eco-friendly products are growing, particularly among millennials and Gen Z consumers. This trend is encouraging retailers and brand owners to switch to biodegradable packaging options, further driving market growth. However, the higher cost of biodegradable plastics compared to conventional plastics remains a significant barrier to widespread adoption.
Government regulations and initiatives play a crucial role in shaping the market landscape. Many countries have implemented bans or taxes on single-use plastics, indirectly boosting the demand for biodegradable alternatives. For instance, the European Union's Single-Use Plastics Directive, which aims to reduce plastic waste, has created new opportunities for biodegradable plastic manufacturers.
The COVID-19 pandemic has had a mixed impact on the biodegradable plastics market. While it initially disrupted supply chains and manufacturing processes, the increased focus on hygiene and safety has led to a surge in demand for disposable packaging, potentially accelerating the shift towards biodegradable options in the long term.
The packaging industry remains the largest consumer of biodegradable plastics, accounting for over 60% of the total market share. This sector's demand is primarily fueled by the food and beverage industry, which is increasingly adopting eco-friendly packaging solutions. The agriculture and horticulture sectors are also emerging as significant consumers, utilizing biodegradable plastics for mulch films and plant pots.
Geographically, Europe leads the biodegradable plastics market, followed by North America and Asia-Pacific. Europe's dominance is attributed to stringent regulations on single-use plastics and a high level of environmental awareness among consumers. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by rapid industrialization and increasing adoption of sustainable practices in countries like China and India.
The market is characterized by the presence of both established players and new entrants, leading to intense competition and innovation. Key market players include NatureWorks LLC, BASF SE, Novamont S.p.A., and Total Corbion PLA. These companies are investing heavily in research and development to improve the properties and reduce the production costs of biodegradable plastics.
Consumer awareness and willingness to pay for eco-friendly products are growing, particularly among millennials and Gen Z consumers. This trend is encouraging retailers and brand owners to switch to biodegradable packaging options, further driving market growth. However, the higher cost of biodegradable plastics compared to conventional plastics remains a significant barrier to widespread adoption.
Government regulations and initiatives play a crucial role in shaping the market landscape. Many countries have implemented bans or taxes on single-use plastics, indirectly boosting the demand for biodegradable alternatives. For instance, the European Union's Single-Use Plastics Directive, which aims to reduce plastic waste, has created new opportunities for biodegradable plastic manufacturers.
The COVID-19 pandemic has had a mixed impact on the biodegradable plastics market. While it initially disrupted supply chains and manufacturing processes, the increased focus on hygiene and safety has led to a surge in demand for disposable packaging, potentially accelerating the shift towards biodegradable options in the long term.
Current Challenges in Bioplastic Synthesis
The synthesis of biodegradable plastics faces several significant challenges that hinder widespread adoption and commercialization. One of the primary obstacles is the high production cost compared to conventional petroleum-based plastics. The raw materials and processing techniques required for bioplastic synthesis are often more expensive, making it difficult for these materials to compete in the market.
Another major challenge is the variability in material properties. Biodegradable plastics can exhibit inconsistent mechanical and thermal characteristics, which can limit their applicability in certain industries. This variability stems from the natural origin of the raw materials and the complexity of the synthesis processes.
The role of glacial acetic acid in biodegradable plastics synthesis presents its own set of challenges. While it serves as an important reagent in various bioplastic production methods, its use can lead to issues with product purity and consistency. The high acidity of glacial acetic acid can cause unwanted side reactions or degradation of the biopolymer chains, affecting the final product's quality and performance.
Environmental concerns also arise from the use of glacial acetic acid in large-scale production. Proper handling and disposal of this corrosive substance are crucial to prevent ecological damage and ensure worker safety. Additionally, the production of glacial acetic acid itself can have a significant carbon footprint, potentially offsetting some of the environmental benefits of biodegradable plastics.
Scalability remains a persistent challenge in bioplastic synthesis. Many promising laboratory-scale processes struggle to maintain efficiency and product quality when scaled up to industrial levels. This is particularly true for processes involving glacial acetic acid, as its reactivity and corrosive nature can complicate large-scale reactor design and material handling systems.
The biodegradability of the final product is another area of concern. While marketed as biodegradable, some bioplastics may not degrade as quickly or completely as desired under real-world conditions. The presence of residual acetic acid or its derivatives in the final product can affect the degradation process, potentially leading to incomplete breakdown or the release of harmful byproducts into the environment.
Regulatory hurdles also pose challenges to the bioplastics industry. The lack of standardized testing methods and certification processes for biodegradability claims can create confusion in the market and hinder consumer acceptance. Furthermore, the use of glacial acetic acid in food-contact applications may face additional scrutiny from regulatory bodies, requiring extensive testing and documentation to ensure product safety.
Another major challenge is the variability in material properties. Biodegradable plastics can exhibit inconsistent mechanical and thermal characteristics, which can limit their applicability in certain industries. This variability stems from the natural origin of the raw materials and the complexity of the synthesis processes.
The role of glacial acetic acid in biodegradable plastics synthesis presents its own set of challenges. While it serves as an important reagent in various bioplastic production methods, its use can lead to issues with product purity and consistency. The high acidity of glacial acetic acid can cause unwanted side reactions or degradation of the biopolymer chains, affecting the final product's quality and performance.
Environmental concerns also arise from the use of glacial acetic acid in large-scale production. Proper handling and disposal of this corrosive substance are crucial to prevent ecological damage and ensure worker safety. Additionally, the production of glacial acetic acid itself can have a significant carbon footprint, potentially offsetting some of the environmental benefits of biodegradable plastics.
Scalability remains a persistent challenge in bioplastic synthesis. Many promising laboratory-scale processes struggle to maintain efficiency and product quality when scaled up to industrial levels. This is particularly true for processes involving glacial acetic acid, as its reactivity and corrosive nature can complicate large-scale reactor design and material handling systems.
The biodegradability of the final product is another area of concern. While marketed as biodegradable, some bioplastics may not degrade as quickly or completely as desired under real-world conditions. The presence of residual acetic acid or its derivatives in the final product can affect the degradation process, potentially leading to incomplete breakdown or the release of harmful byproducts into the environment.
Regulatory hurdles also pose challenges to the bioplastics industry. The lack of standardized testing methods and certification processes for biodegradability claims can create confusion in the market and hinder consumer acceptance. Furthermore, the use of glacial acetic acid in food-contact applications may face additional scrutiny from regulatory bodies, requiring extensive testing and documentation to ensure product safety.
Existing Glacial Acetic Acid-based Synthesis Methods
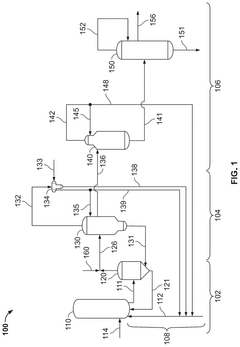
01 Production methods of glacial acetic acid
Various methods are employed to produce glacial acetic acid, including oxidation of acetaldehyde, fermentation processes, and catalytic reactions. These methods often involve specific reaction conditions, catalysts, and purification steps to achieve high-purity glacial acetic acid.- Production methods of glacial acetic acid: Various methods are employed to produce glacial acetic acid, including oxidation of acetaldehyde, fermentation processes, and catalytic reactions. These methods often involve specific reaction conditions, catalysts, and purification steps to achieve high purity acetic acid.
- Applications in chemical synthesis: Glacial acetic acid serves as a crucial reagent and solvent in numerous chemical synthesis processes. It is used in the production of various organic compounds, pharmaceuticals, and industrial chemicals, often acting as an acidic catalyst or reaction medium.
- Purification and concentration techniques: Specialized techniques are employed to purify and concentrate acetic acid to achieve glacial purity (>99.5%). These may include distillation, crystallization, and membrane separation processes, often utilizing specific equipment and operating conditions.
- Industrial equipment and processes: The production and handling of glacial acetic acid require specialized industrial equipment and processes. This includes corrosion-resistant materials, safety systems, and specific reactor designs to manage the highly corrosive nature of the compound.
- Safety and environmental considerations: Handling glacial acetic acid involves significant safety and environmental considerations due to its corrosive nature and potential environmental impact. Innovations in this area focus on improved containment, spill prevention, and waste treatment methods to ensure safe and environmentally responsible use of the compound.
02 Applications in chemical synthesis
Glacial acetic acid serves as a crucial reagent and solvent in numerous chemical synthesis processes. It is used in the production of various organic compounds, pharmaceuticals, and industrial chemicals due to its high purity and reactivity.Expand Specific Solutions03 Purification and concentration techniques
Specialized techniques are employed to purify and concentrate acetic acid to achieve glacial purity. These may include distillation, crystallization, and membrane separation processes, often utilizing specific equipment and operating conditions.Expand Specific Solutions04 Industrial equipment and processes
Specialized industrial equipment and processes are designed for the handling, storage, and processing of glacial acetic acid. This includes corrosion-resistant materials, safety systems, and process control mechanisms to ensure efficient and safe operations.Expand Specific Solutions05 Safety and environmental considerations
Handling glacial acetic acid requires strict safety measures and environmental considerations due to its corrosive nature and potential health hazards. This includes proper storage, transportation, and disposal methods, as well as the implementation of safety protocols in industrial settings.Expand Specific Solutions
Key Players in Biodegradable Plastics Industry
The role of glacial acetic acid in biodegradable plastics synthesis is an emerging field with significant potential for growth. The market is in its early stages, characterized by increasing research and development activities. While the exact market size is not specified, it is expected to expand rapidly due to growing environmental concerns and demand for sustainable materials. The technology is still evolving, with varying levels of maturity among key players. Companies like CJ CheilJedang Corp., SK Innovation Co., Ltd., and Kureha Corp. are at the forefront, leveraging their expertise in chemical and materials science to advance biodegradable plastics synthesis using glacial acetic acid. Academic institutions such as the University of Florida and Universidad Nacional Autónoma de México are also contributing to the field through research collaborations and innovations.
Purac Biochem BV
Technical Solution: Purac Biochem has developed an innovative approach to biodegradable plastics synthesis using glacial acetic acid as a key component in their process. Their method focuses on the production of poly(lactic acid) (PLA) and its copolymers. Glacial acetic acid is utilized in the purification and modification of lactic acid monomers, as well as in the ring-opening polymerization of lactide to form high molecular weight PLA. Purac has optimized their process to achieve a high optical purity of L-lactide (>99.5%), which is crucial for producing PLA with excellent mechanical and thermal properties[9]. The company has also developed a proprietary catalyst system that allows for controlled polymerization and enables the production of PLA with tailored molecular weights ranging from 50,000 to 300,000 g/mol[10].
Strengths: High-purity, high-performance PLA suitable for a wide range of applications, from packaging to medical devices. Controlled polymerization process allows for tailored material properties. Weaknesses: Relatively high production costs compared to conventional plastics, limited heat resistance of PLA in some applications.
Arkema France SA
Technical Solution: Arkema has developed an innovative approach to biodegradable plastics synthesis incorporating glacial acetic acid. Their process focuses on the production of polyvinyl alcohol (PVOH) derivatives, using glacial acetic acid in a controlled hydrolysis reaction of polyvinyl acetate. This method allows for precise tuning of the degree of hydrolysis, typically ranging from 80% to 99%, which directly influences the biodegradability and water solubility of the final product[5]. Arkema has also developed a proprietary blending technique that combines their PVOH derivatives with other biodegradable polymers, enhancing overall material performance. The company has successfully scaled this technology for industrial production, with capacities reaching several thousand tons per year[6].
Strengths: Highly tunable material properties, established large-scale production capabilities, and versatility in end-product applications. Weaknesses: Potential sensitivity to humidity during storage and use, and higher raw material costs compared to conventional plastics.
Innovations in Acetic Acid Utilization for Bioplastics
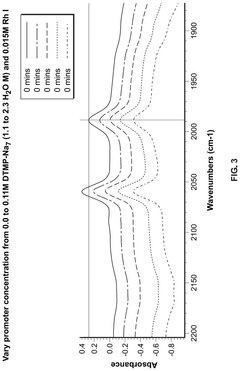
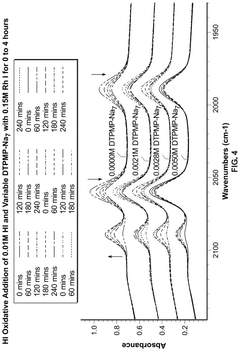
Polyphoshates and polyphosphonates as rate promoters for the glacial acetic acid process
PatentPendingUS20250074856A1
Innovation
- The process involves using a reaction mixture comprising a carbonylation catalyst, water, and specific rate-promoting compounds such as Group I and Group II polyphosphate and polyphosphonate salts, which are added at an iodide to promoter molar ratio greater than 2, to enhance the rate of acetic acid formation while reducing the amount of water required.
Manufacture of glacial acetic acid from dilute acetic acid
PatentInactiveGB315397A
Innovation
- A two-stage process where dilute acetic acid is first concentrated to 60% strength through freezing, followed by additional concentration methods such as cold extractions, azeotropic mixtures, or absorption with high-boiling extracting agents, allowing for efficient separation of glacial acetic acid with reduced heat application.
Environmental Impact Assessment
The environmental impact assessment of glacial acetic acid in biodegradable plastics synthesis reveals both positive and negative implications. On the positive side, the use of glacial acetic acid contributes to the production of biodegradable plastics, which offer a more sustainable alternative to conventional petroleum-based plastics. These biodegradable materials can significantly reduce plastic pollution and its long-term environmental consequences.
Glacial acetic acid, when used in biodegradable plastics synthesis, helps create polymers that can decompose naturally in the environment. This decomposition process is typically faster and more complete compared to traditional plastics, resulting in less accumulation of plastic waste in ecosystems. The breakdown products of these biodegradable plastics are generally less harmful to wildlife and marine life, reducing the risk of entanglement and ingestion of plastic debris.
However, the production and use of glacial acetic acid also present environmental challenges. The manufacturing process of glacial acetic acid often involves fossil fuel-based feedstocks, contributing to greenhouse gas emissions and climate change. Additionally, the production may require significant energy inputs, further increasing its carbon footprint.
The use of glacial acetic acid in industrial processes can lead to potential air and water pollution if not properly managed. Accidental releases or improper disposal of acetic acid can cause localized environmental damage, affecting soil and water quality. Proper handling, storage, and disposal protocols are crucial to mitigate these risks.
From a life cycle perspective, the environmental benefits of biodegradable plastics synthesized using glacial acetic acid must be weighed against the impacts of their production. While the end-of-life phase shows clear advantages over conventional plastics, the production phase may have comparable or potentially higher environmental impacts in terms of energy use and emissions.
It is important to consider the sourcing of glacial acetic acid as well. Efforts to produce acetic acid from renewable resources, such as biomass fermentation, could significantly improve the overall environmental profile of the biodegradable plastics synthesis process. This shift towards bio-based acetic acid production aligns with circular economy principles and could enhance the sustainability credentials of the resulting biodegradable plastics.
In conclusion, while glacial acetic acid plays a crucial role in advancing biodegradable plastics technology, its environmental impact is complex. The benefits of reduced plastic pollution and improved end-of-life outcomes must be balanced against the production-related environmental costs. Ongoing research and innovation in greener production methods for both acetic acid and biodegradable plastics are essential to maximize the environmental benefits of this technology.
Glacial acetic acid, when used in biodegradable plastics synthesis, helps create polymers that can decompose naturally in the environment. This decomposition process is typically faster and more complete compared to traditional plastics, resulting in less accumulation of plastic waste in ecosystems. The breakdown products of these biodegradable plastics are generally less harmful to wildlife and marine life, reducing the risk of entanglement and ingestion of plastic debris.
However, the production and use of glacial acetic acid also present environmental challenges. The manufacturing process of glacial acetic acid often involves fossil fuel-based feedstocks, contributing to greenhouse gas emissions and climate change. Additionally, the production may require significant energy inputs, further increasing its carbon footprint.
The use of glacial acetic acid in industrial processes can lead to potential air and water pollution if not properly managed. Accidental releases or improper disposal of acetic acid can cause localized environmental damage, affecting soil and water quality. Proper handling, storage, and disposal protocols are crucial to mitigate these risks.
From a life cycle perspective, the environmental benefits of biodegradable plastics synthesized using glacial acetic acid must be weighed against the impacts of their production. While the end-of-life phase shows clear advantages over conventional plastics, the production phase may have comparable or potentially higher environmental impacts in terms of energy use and emissions.
It is important to consider the sourcing of glacial acetic acid as well. Efforts to produce acetic acid from renewable resources, such as biomass fermentation, could significantly improve the overall environmental profile of the biodegradable plastics synthesis process. This shift towards bio-based acetic acid production aligns with circular economy principles and could enhance the sustainability credentials of the resulting biodegradable plastics.
In conclusion, while glacial acetic acid plays a crucial role in advancing biodegradable plastics technology, its environmental impact is complex. The benefits of reduced plastic pollution and improved end-of-life outcomes must be balanced against the production-related environmental costs. Ongoing research and innovation in greener production methods for both acetic acid and biodegradable plastics are essential to maximize the environmental benefits of this technology.
Regulatory Framework for Bioplastic Production
The regulatory framework for bioplastic production plays a crucial role in shaping the industry's growth and ensuring environmental sustainability. As the demand for biodegradable plastics increases, governments and international organizations have been developing and implementing regulations to guide the production, use, and disposal of these materials.
At the international level, organizations such as the United Nations Environment Programme (UNEP) and the European Union (EU) have established guidelines and directives for bioplastic production. The EU, in particular, has been at the forefront of regulatory efforts with its Circular Economy Action Plan, which includes specific measures for biodegradable and compostable plastics.
National governments have also been active in creating regulatory frameworks for bioplastic production. Many countries have implemented standards for biodegradability and compostability, such as the ASTM D6400 in the United States and the EN 13432 in Europe. These standards define the criteria for materials to be classified as biodegradable or compostable, ensuring consistency and reliability in product claims.
Regulations often focus on several key areas, including raw material sourcing, production processes, and end-of-life management. For instance, some jurisdictions require a minimum percentage of bio-based content in bioplastics or mandate the use of sustainably sourced feedstocks. Production regulations may address energy efficiency, waste management, and emissions control during the manufacturing process.
Labeling and certification requirements are another critical aspect of the regulatory framework. Many countries have implemented strict guidelines for product labeling to prevent greenwashing and ensure consumers can make informed choices. Certification schemes, such as the "OK compost" label in Europe, provide third-party verification of a product's biodegradability claims.
End-of-life management regulations are particularly important for bioplastics. These may include requirements for separate collection and composting infrastructure, as well as guidelines for the proper disposal of biodegradable plastics. Some jurisdictions have also implemented extended producer responsibility (EPR) schemes, which hold manufacturers accountable for the entire lifecycle of their products.
As the bioplastics industry continues to evolve, regulatory frameworks are adapting to address new challenges and opportunities. For instance, there is growing attention to the potential environmental impacts of bioplastics, such as land use changes and competition with food crops. Regulators are working to balance the benefits of biodegradable plastics with these potential drawbacks through more comprehensive lifecycle assessments and sustainability criteria.
The role of glacial acetic acid in biodegradable plastics synthesis is also subject to regulatory considerations. As a key ingredient in some bioplastic production processes, its use may be governed by chemical safety regulations and environmental protection laws. Manufacturers must ensure compliance with these regulations throughout the production chain, from sourcing to final product.
At the international level, organizations such as the United Nations Environment Programme (UNEP) and the European Union (EU) have established guidelines and directives for bioplastic production. The EU, in particular, has been at the forefront of regulatory efforts with its Circular Economy Action Plan, which includes specific measures for biodegradable and compostable plastics.
National governments have also been active in creating regulatory frameworks for bioplastic production. Many countries have implemented standards for biodegradability and compostability, such as the ASTM D6400 in the United States and the EN 13432 in Europe. These standards define the criteria for materials to be classified as biodegradable or compostable, ensuring consistency and reliability in product claims.
Regulations often focus on several key areas, including raw material sourcing, production processes, and end-of-life management. For instance, some jurisdictions require a minimum percentage of bio-based content in bioplastics or mandate the use of sustainably sourced feedstocks. Production regulations may address energy efficiency, waste management, and emissions control during the manufacturing process.
Labeling and certification requirements are another critical aspect of the regulatory framework. Many countries have implemented strict guidelines for product labeling to prevent greenwashing and ensure consumers can make informed choices. Certification schemes, such as the "OK compost" label in Europe, provide third-party verification of a product's biodegradability claims.
End-of-life management regulations are particularly important for bioplastics. These may include requirements for separate collection and composting infrastructure, as well as guidelines for the proper disposal of biodegradable plastics. Some jurisdictions have also implemented extended producer responsibility (EPR) schemes, which hold manufacturers accountable for the entire lifecycle of their products.
As the bioplastics industry continues to evolve, regulatory frameworks are adapting to address new challenges and opportunities. For instance, there is growing attention to the potential environmental impacts of bioplastics, such as land use changes and competition with food crops. Regulators are working to balance the benefits of biodegradable plastics with these potential drawbacks through more comprehensive lifecycle assessments and sustainability criteria.
The role of glacial acetic acid in biodegradable plastics synthesis is also subject to regulatory considerations. As a key ingredient in some bioplastic production processes, its use may be governed by chemical safety regulations and environmental protection laws. Manufacturers must ensure compliance with these regulations throughout the production chain, from sourcing to final product.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!