Glacial Acetic Acid in Advanced Functional Nanomaterials
AUG 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Glacial Acetic Acid in Nanomaterials: Background and Objectives
Glacial acetic acid has emerged as a crucial component in the development of advanced functional nanomaterials, marking a significant milestone in the field of nanotechnology. The evolution of this technology can be traced back to the early 2000s when researchers began exploring the potential of acetic acid in nanostructure synthesis. Over the past two decades, the application of glacial acetic acid in nanomaterial production has expanded exponentially, driven by its unique properties and versatility.
The primary objective of research in this area is to harness the capabilities of glacial acetic acid to enhance the synthesis, modification, and functionalization of nanomaterials. This includes improving the control over nanoparticle size, shape, and surface properties, which are critical factors in determining the performance of nanomaterials in various applications. Additionally, researchers aim to develop more efficient and environmentally friendly synthesis methods using glacial acetic acid as a key reagent.
One of the most significant trends in this field is the increasing focus on green synthesis approaches. Glacial acetic acid, being a relatively benign and biodegradable compound, aligns well with the principles of sustainable chemistry. This has led to a surge in research aimed at replacing more hazardous chemicals with acetic acid in nanomaterial production processes, contributing to the development of eco-friendly nanomaterials.
The technological evolution in this domain has been characterized by a shift from simple nanoparticle synthesis to the creation of complex, multifunctional nanomaterials. Researchers have made substantial progress in utilizing glacial acetic acid for the fabrication of nanocomposites, core-shell structures, and hierarchical nanoarchitectures. These advanced structures offer enhanced properties and functionalities, opening up new possibilities in fields such as energy storage, catalysis, and biomedicine.
Another key aspect of the technological progression is the integration of glacial acetic acid-based synthesis methods with other advanced technologies. This includes the combination with microfluidic systems for precise control over reaction conditions, the use of ultrasonic or microwave irradiation to enhance reaction kinetics, and the incorporation of in situ characterization techniques for real-time monitoring of nanostructure formation.
Looking ahead, the research goals in this field are focused on further expanding the application scope of glacial acetic acid in nanomaterial synthesis. This includes exploring its potential in the production of novel 2D materials, developing acetic acid-based strategies for surface functionalization of nanomaterials, and investigating its role in the assembly of hierarchical nanostructures. Additionally, there is a growing interest in understanding the fundamental mechanisms by which glacial acetic acid influences nanoparticle formation and growth, which could lead to more precise control over nanomaterial properties.
The primary objective of research in this area is to harness the capabilities of glacial acetic acid to enhance the synthesis, modification, and functionalization of nanomaterials. This includes improving the control over nanoparticle size, shape, and surface properties, which are critical factors in determining the performance of nanomaterials in various applications. Additionally, researchers aim to develop more efficient and environmentally friendly synthesis methods using glacial acetic acid as a key reagent.
One of the most significant trends in this field is the increasing focus on green synthesis approaches. Glacial acetic acid, being a relatively benign and biodegradable compound, aligns well with the principles of sustainable chemistry. This has led to a surge in research aimed at replacing more hazardous chemicals with acetic acid in nanomaterial production processes, contributing to the development of eco-friendly nanomaterials.
The technological evolution in this domain has been characterized by a shift from simple nanoparticle synthesis to the creation of complex, multifunctional nanomaterials. Researchers have made substantial progress in utilizing glacial acetic acid for the fabrication of nanocomposites, core-shell structures, and hierarchical nanoarchitectures. These advanced structures offer enhanced properties and functionalities, opening up new possibilities in fields such as energy storage, catalysis, and biomedicine.
Another key aspect of the technological progression is the integration of glacial acetic acid-based synthesis methods with other advanced technologies. This includes the combination with microfluidic systems for precise control over reaction conditions, the use of ultrasonic or microwave irradiation to enhance reaction kinetics, and the incorporation of in situ characterization techniques for real-time monitoring of nanostructure formation.
Looking ahead, the research goals in this field are focused on further expanding the application scope of glacial acetic acid in nanomaterial synthesis. This includes exploring its potential in the production of novel 2D materials, developing acetic acid-based strategies for surface functionalization of nanomaterials, and investigating its role in the assembly of hierarchical nanostructures. Additionally, there is a growing interest in understanding the fundamental mechanisms by which glacial acetic acid influences nanoparticle formation and growth, which could lead to more precise control over nanomaterial properties.
Market Analysis for Advanced Functional Nanomaterials
The market for advanced functional nanomaterials has been experiencing significant growth in recent years, driven by increasing demand across various industries such as electronics, energy, healthcare, and environmental applications. The integration of glacial acetic acid in the synthesis and modification of these nanomaterials has opened up new possibilities for enhancing their properties and expanding their applications.
In the electronics sector, the use of glacial acetic acid-modified nanomaterials has shown promise in improving the performance of electronic devices, particularly in the development of flexible and wearable electronics. The market for these applications is expected to grow rapidly as consumer demand for smart wearables and Internet of Things (IoT) devices continues to rise.
The energy industry has also been a key driver in the adoption of advanced functional nanomaterials. Glacial acetic acid-based nanomaterials have demonstrated potential in improving energy storage and conversion technologies, such as batteries and solar cells. As the global focus on renewable energy sources intensifies, the demand for these materials is projected to increase substantially.
In the healthcare sector, the application of glacial acetic acid in the synthesis of nanomaterials has led to advancements in drug delivery systems, biosensors, and imaging technologies. The growing emphasis on personalized medicine and early disease detection is expected to fuel the demand for these innovative nanomaterials in the coming years.
Environmental applications represent another significant market opportunity for advanced functional nanomaterials. Glacial acetic acid-modified nanomaterials have shown promise in water purification, air filtration, and environmental remediation technologies. As global concerns about environmental pollution and sustainability grow, the demand for these materials is likely to expand.
The Asia-Pacific region has emerged as a key market for advanced functional nanomaterials, with countries like China, Japan, and South Korea leading in research and development efforts. North America and Europe also remain important markets, driven by strong investments in nanotechnology research and a robust industrial base.
Despite the promising market outlook, challenges such as high production costs, scalability issues, and regulatory concerns need to be addressed to fully realize the potential of these materials. As research and development efforts continue to advance, it is expected that these barriers will be gradually overcome, leading to wider adoption across industries.
In conclusion, the market for advanced functional nanomaterials incorporating glacial acetic acid shows significant growth potential across multiple sectors. As technological advancements continue and new applications emerge, the demand for these innovative materials is expected to rise, creating opportunities for both established players and new entrants in the market.
In the electronics sector, the use of glacial acetic acid-modified nanomaterials has shown promise in improving the performance of electronic devices, particularly in the development of flexible and wearable electronics. The market for these applications is expected to grow rapidly as consumer demand for smart wearables and Internet of Things (IoT) devices continues to rise.
The energy industry has also been a key driver in the adoption of advanced functional nanomaterials. Glacial acetic acid-based nanomaterials have demonstrated potential in improving energy storage and conversion technologies, such as batteries and solar cells. As the global focus on renewable energy sources intensifies, the demand for these materials is projected to increase substantially.
In the healthcare sector, the application of glacial acetic acid in the synthesis of nanomaterials has led to advancements in drug delivery systems, biosensors, and imaging technologies. The growing emphasis on personalized medicine and early disease detection is expected to fuel the demand for these innovative nanomaterials in the coming years.
Environmental applications represent another significant market opportunity for advanced functional nanomaterials. Glacial acetic acid-modified nanomaterials have shown promise in water purification, air filtration, and environmental remediation technologies. As global concerns about environmental pollution and sustainability grow, the demand for these materials is likely to expand.
The Asia-Pacific region has emerged as a key market for advanced functional nanomaterials, with countries like China, Japan, and South Korea leading in research and development efforts. North America and Europe also remain important markets, driven by strong investments in nanotechnology research and a robust industrial base.
Despite the promising market outlook, challenges such as high production costs, scalability issues, and regulatory concerns need to be addressed to fully realize the potential of these materials. As research and development efforts continue to advance, it is expected that these barriers will be gradually overcome, leading to wider adoption across industries.
In conclusion, the market for advanced functional nanomaterials incorporating glacial acetic acid shows significant growth potential across multiple sectors. As technological advancements continue and new applications emerge, the demand for these innovative materials is expected to rise, creating opportunities for both established players and new entrants in the market.
Current Challenges in Glacial Acetic Acid Utilization
Despite the widespread use of glacial acetic acid in the synthesis of advanced functional nanomaterials, several challenges persist in its utilization. One of the primary concerns is the corrosive nature of glacial acetic acid, which can lead to equipment degradation and potential safety hazards in laboratory and industrial settings. This necessitates the use of specialized handling equipment and storage facilities, increasing operational costs and complexity.
Another significant challenge lies in the purification process of glacial acetic acid. Achieving and maintaining high purity levels is crucial for its application in nanomaterial synthesis, as impurities can significantly affect the properties and performance of the resulting materials. The purification process often involves energy-intensive distillation techniques, which contribute to increased production costs and environmental concerns.
The volatility of glacial acetic acid poses additional challenges in terms of storage and transportation. Its low boiling point and high vapor pressure require careful handling to prevent loss through evaporation and maintain product quality. This volatility also raises environmental and health concerns, as acetic acid vapors can be harmful if inhaled or exposed to skin and eyes.
In the context of nanomaterial synthesis, controlling the reaction kinetics when using glacial acetic acid can be challenging. The high reactivity of the acid can lead to rapid and sometimes uncontrolled reactions, making it difficult to achieve precise control over nanoparticle size, shape, and composition. This challenge is particularly pronounced in the synthesis of complex nanostructures or when attempting to achieve uniform particle distributions.
The environmental impact of glacial acetic acid production and use is another area of concern. Traditional production methods often rely on petrochemical feedstocks, contributing to carbon emissions and resource depletion. There is a growing need for more sustainable production routes and greener alternatives that can match the performance of glacial acetic acid in nanomaterial synthesis.
Scalability remains a significant challenge in the use of glacial acetic acid for large-scale production of functional nanomaterials. While laboratory-scale syntheses have demonstrated promising results, translating these processes to industrial scales while maintaining product quality and cost-effectiveness is often problematic. This scaling issue is compounded by the aforementioned challenges related to handling, purification, and reaction control.
Lastly, the regulatory landscape surrounding the use of glacial acetic acid in nanomaterial production is becoming increasingly complex. Stricter environmental and safety regulations are being implemented in many regions, necessitating adaptations in production processes and potentially limiting the use of glacial acetic acid in certain applications or industries.
Another significant challenge lies in the purification process of glacial acetic acid. Achieving and maintaining high purity levels is crucial for its application in nanomaterial synthesis, as impurities can significantly affect the properties and performance of the resulting materials. The purification process often involves energy-intensive distillation techniques, which contribute to increased production costs and environmental concerns.
The volatility of glacial acetic acid poses additional challenges in terms of storage and transportation. Its low boiling point and high vapor pressure require careful handling to prevent loss through evaporation and maintain product quality. This volatility also raises environmental and health concerns, as acetic acid vapors can be harmful if inhaled or exposed to skin and eyes.
In the context of nanomaterial synthesis, controlling the reaction kinetics when using glacial acetic acid can be challenging. The high reactivity of the acid can lead to rapid and sometimes uncontrolled reactions, making it difficult to achieve precise control over nanoparticle size, shape, and composition. This challenge is particularly pronounced in the synthesis of complex nanostructures or when attempting to achieve uniform particle distributions.
The environmental impact of glacial acetic acid production and use is another area of concern. Traditional production methods often rely on petrochemical feedstocks, contributing to carbon emissions and resource depletion. There is a growing need for more sustainable production routes and greener alternatives that can match the performance of glacial acetic acid in nanomaterial synthesis.
Scalability remains a significant challenge in the use of glacial acetic acid for large-scale production of functional nanomaterials. While laboratory-scale syntheses have demonstrated promising results, translating these processes to industrial scales while maintaining product quality and cost-effectiveness is often problematic. This scaling issue is compounded by the aforementioned challenges related to handling, purification, and reaction control.
Lastly, the regulatory landscape surrounding the use of glacial acetic acid in nanomaterial production is becoming increasingly complex. Stricter environmental and safety regulations are being implemented in many regions, necessitating adaptations in production processes and potentially limiting the use of glacial acetic acid in certain applications or industries.
Existing Applications of Glacial Acetic Acid in Nanomaterials
01 Production methods of glacial acetic acid
Various methods are employed to produce glacial acetic acid, including oxidation of acetaldehyde, fermentation processes, and catalytic reactions. These methods often involve specific reaction conditions, catalysts, and purification steps to achieve high purity acetic acid.- Production methods of glacial acetic acid: Various methods are employed to produce glacial acetic acid, including oxidation of acetaldehyde, fermentation processes, and catalytic reactions. These methods often involve specific reaction conditions, catalysts, and purification steps to achieve high purity acetic acid.
- Applications in chemical synthesis: Glacial acetic acid serves as a crucial reagent and solvent in numerous chemical synthesis processes. It is used in the production of various organic compounds, pharmaceuticals, and industrial chemicals due to its high purity and reactivity.
- Purification and concentration techniques: Specialized techniques are employed to purify and concentrate acetic acid to achieve the glacial form. These may include distillation, crystallization, and membrane separation processes, ensuring high purity levels suitable for industrial and laboratory use.
- Storage and handling considerations: Due to its corrosive nature and hygroscopic properties, glacial acetic acid requires specific storage and handling procedures. Specialized containers, safety equipment, and environmental controls are necessary to maintain its purity and ensure safe usage in various applications.
- Industrial applications and equipment: Glacial acetic acid finds extensive use in various industries, including textiles, food processing, and petrochemicals. Specialized equipment and processes have been developed to utilize glacial acetic acid efficiently in these industrial applications, considering its unique properties and handling requirements.
02 Applications in chemical synthesis
Glacial acetic acid serves as a crucial reagent and solvent in numerous chemical synthesis processes. It is used in the production of various organic compounds, pharmaceuticals, and industrial chemicals, often as an acetylating agent or acidic catalyst.Expand Specific Solutions03 Purification and quality control
Achieving and maintaining high purity of glacial acetic acid is essential for many applications. Various purification techniques, including distillation, crystallization, and membrane separation, are employed. Quality control measures ensure the acid meets stringent purity standards.Expand Specific Solutions04 Industrial equipment and processes
Specialized equipment and processes are designed for the handling, storage, and use of glacial acetic acid in industrial settings. This includes corrosion-resistant materials, safety systems, and efficient production lines tailored to acetic acid's properties.Expand Specific Solutions05 Environmental and safety considerations
The use and production of glacial acetic acid require careful attention to environmental impact and safety measures. This includes waste treatment, emission control, personal protective equipment, and safe handling procedures to mitigate risks associated with its corrosive and flammable nature.Expand Specific Solutions
Key Players in Functional Nanomaterial Industry
The research on Glacial Acetic Acid in Advanced Functional Nanomaterials is in a developing stage, with growing market potential and increasing technological maturity. The field is characterized by a mix of academic institutions and industry players, indicating a collaborative ecosystem. Key academic contributors include Zhejiang University, Northwestern University, and the Chinese Academy of Science Institute of Chemistry, suggesting a strong research foundation. Industry involvement from companies like Akzo Nobel Chemicals International BV and FUJIFILM Corp. points to practical applications and commercialization efforts. The presence of diverse global participants, including institutions from China, the US, and Europe, highlights the international nature of this research area and its potential for widespread impact in nanomaterial development.
Zhejiang University
Technical Solution: Zhejiang University has made notable contributions to the research on glacial acetic acid in advanced functional nanomaterials. Their team has developed a facile and environmentally friendly approach using glacial acetic acid for the synthesis of metal oxide nanostructures with controlled morphologies [2]. This method has been successfully applied to create various metal oxide nanomaterials, including TiO2, ZnO, and Fe3O4, with enhanced photocatalytic and magnetic properties. The university has also explored the use of glacial acetic acid as a capping agent in the preparation of quantum dots, resulting in improved optical properties and stability [4]. Furthermore, they have investigated the role of glacial acetic acid in the surface modification of carbon nanotubes, leading to enhanced dispersibility and compatibility with polymer matrices for advanced composite materials [6].
Strengths: Versatile synthesis methods for metal oxide nanostructures and quantum dots. Weaknesses: Limited research on the long-term stability of modified nanomaterials in various applications.
Tianjin University
Technical Solution: Tianjin University has made significant progress in the research of glacial acetic acid for advanced functional nanomaterials. They have developed a novel approach using glacial acetic acid as a structure-directing agent in the synthesis of mesoporous silica nanoparticles with controlled pore sizes and morphologies [7]. This method has been applied to create drug delivery systems with improved loading capacity and release kinetics. The university has also investigated the use of glacial acetic acid in the preparation of metal-organic framework (MOF) thin films with enhanced gas separation properties [9]. Additionally, they have explored the role of glacial acetic acid in the surface functionalization of carbon nanotubes, leading to improved dispersion and compatibility with various polymer matrices for advanced composite materials [11].
Strengths: Expertise in mesoporous silica synthesis and MOF thin film preparation. Weaknesses: Limited research on the scalability of their synthesis methods for industrial applications.
Innovative Approaches in Glacial Acetic Acid-based Synthesis
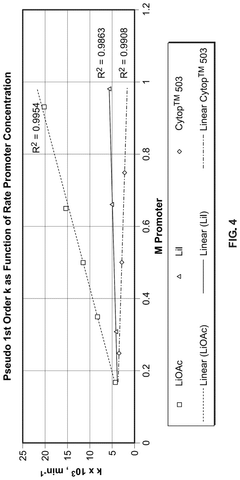
Aminopolycarboxylates as rate promoters for the glacial acetic acid process
PatentPendingUS20250074857A1
Innovation
- The process involves using a reaction mixture comprising a carbonylation catalyst, water, and specific rate-promoting compounds, such as Group I and Group II aminopolycarboxylate salts, to enhance the rate of acetic acid formation while reducing the amount of water required and suppressing undesirable oxidative addition reactions.
Low bromine content glacial acetic acid
PatentInactiveUS4278503A
Innovation
- A process involving thermal conversion of 3-bromo-2-butanone to 1-butene-3-one and inorganic bromides, followed by cryogenic fractional crystallization, reduces 3-bromo-2-butanone contamination by concentrating the aqueous acid mixture and rejecting the impurity, using decompression and heat treatment steps before distillative removal of organic impurities.
Environmental Impact of Glacial Acetic Acid in Nanomaterial Production
The use of glacial acetic acid in the production of advanced functional nanomaterials has raised concerns about its environmental impact. As a widely used solvent and reagent in nanomaterial synthesis, glacial acetic acid plays a crucial role in various processes, including the preparation of metal oxide nanoparticles and carbon-based nanomaterials. However, its potential environmental consequences must be carefully considered and addressed.
One of the primary environmental concerns associated with glacial acetic acid is its potential for water pollution. When released into aquatic ecosystems, acetic acid can lower the pH of water bodies, leading to acidification. This can have detrimental effects on aquatic flora and fauna, disrupting the delicate balance of ecosystems. Moreover, the increased acidity can mobilize heavy metals from sediments, further exacerbating water pollution issues.
Air pollution is another significant environmental impact of glacial acetic acid use in nanomaterial production. The volatile nature of acetic acid means that it can easily evaporate and contribute to the formation of ground-level ozone and smog. These air pollutants can have adverse effects on human health, particularly respiratory systems, and can damage vegetation and crops.
The production and disposal of glacial acetic acid also contribute to greenhouse gas emissions. The manufacturing process of acetic acid often involves the use of fossil fuels, leading to carbon dioxide emissions. Additionally, improper disposal or accidental release of acetic acid can result in the formation of methane, a potent greenhouse gas, through anaerobic decomposition.
Soil contamination is another potential environmental impact of glacial acetic acid use in nanomaterial production. Accidental spills or improper disposal can lead to soil acidification, affecting soil fertility and microbial communities. This can have long-lasting effects on terrestrial ecosystems and agricultural productivity.
To mitigate these environmental impacts, several strategies can be implemented. Firstly, the development and adoption of greener synthesis methods that reduce or eliminate the use of glacial acetic acid should be prioritized. This may involve exploring alternative solvents or reagents with lower environmental footprints. Secondly, implementing robust waste management and treatment systems can help prevent the release of acetic acid into the environment.
Furthermore, improving process efficiency and recycling techniques can minimize the overall consumption of glacial acetic acid in nanomaterial production. This not only reduces environmental impact but also offers economic benefits. Lastly, strict adherence to environmental regulations and the implementation of best practices in handling and storage can significantly reduce the risk of accidental releases and their associated environmental consequences.
One of the primary environmental concerns associated with glacial acetic acid is its potential for water pollution. When released into aquatic ecosystems, acetic acid can lower the pH of water bodies, leading to acidification. This can have detrimental effects on aquatic flora and fauna, disrupting the delicate balance of ecosystems. Moreover, the increased acidity can mobilize heavy metals from sediments, further exacerbating water pollution issues.
Air pollution is another significant environmental impact of glacial acetic acid use in nanomaterial production. The volatile nature of acetic acid means that it can easily evaporate and contribute to the formation of ground-level ozone and smog. These air pollutants can have adverse effects on human health, particularly respiratory systems, and can damage vegetation and crops.
The production and disposal of glacial acetic acid also contribute to greenhouse gas emissions. The manufacturing process of acetic acid often involves the use of fossil fuels, leading to carbon dioxide emissions. Additionally, improper disposal or accidental release of acetic acid can result in the formation of methane, a potent greenhouse gas, through anaerobic decomposition.
Soil contamination is another potential environmental impact of glacial acetic acid use in nanomaterial production. Accidental spills or improper disposal can lead to soil acidification, affecting soil fertility and microbial communities. This can have long-lasting effects on terrestrial ecosystems and agricultural productivity.
To mitigate these environmental impacts, several strategies can be implemented. Firstly, the development and adoption of greener synthesis methods that reduce or eliminate the use of glacial acetic acid should be prioritized. This may involve exploring alternative solvents or reagents with lower environmental footprints. Secondly, implementing robust waste management and treatment systems can help prevent the release of acetic acid into the environment.
Furthermore, improving process efficiency and recycling techniques can minimize the overall consumption of glacial acetic acid in nanomaterial production. This not only reduces environmental impact but also offers economic benefits. Lastly, strict adherence to environmental regulations and the implementation of best practices in handling and storage can significantly reduce the risk of accidental releases and their associated environmental consequences.
Scalability and Commercialization Prospects
The scalability and commercialization prospects for glacial acetic acid in advanced functional nanomaterials are promising, driven by the growing demand for high-performance materials across various industries. The unique properties of glacial acetic acid, such as its high purity and reactivity, make it an attractive component in the synthesis and modification of nanomaterials.
One of the key factors contributing to the scalability of glacial acetic acid in nanomaterial production is its versatility. It can be used in a wide range of applications, including the synthesis of metal oxide nanoparticles, carbon nanotubes, and graphene-based materials. This versatility allows for economies of scale in production, as manufacturers can utilize the same raw material for multiple product lines.
The industrial-scale production of glacial acetic acid is well-established, with global production capacity exceeding 10 million tons annually. This existing infrastructure provides a solid foundation for scaling up its use in nanomaterial synthesis. Additionally, the relatively low cost of glacial acetic acid compared to other specialty chemicals used in nanomaterial production enhances its economic viability for large-scale applications.
From a commercialization perspective, the integration of glacial acetic acid-based nanomaterials into various products offers significant market potential. Industries such as electronics, energy storage, and healthcare are increasingly adopting advanced nanomaterials to enhance product performance. For instance, acetic acid-modified graphene oxide has shown promise in improving the efficiency of solar cells and batteries, opening up opportunities in the renewable energy sector.
The environmental considerations associated with glacial acetic acid also contribute to its commercialization prospects. As a biodegradable compound, it aligns with the growing trend towards sustainable manufacturing processes. This aspect can be leveraged in marketing strategies to appeal to environmentally conscious consumers and meet stringent regulatory requirements.
However, challenges remain in fully realizing the commercial potential of glacial acetic acid in nanomaterial production. These include optimizing reaction conditions for large-scale synthesis, ensuring consistent quality across batches, and developing cost-effective purification methods. Addressing these challenges will require continued research and development efforts, as well as collaboration between academic institutions and industry partners.
In conclusion, the scalability and commercialization prospects for glacial acetic acid in advanced functional nanomaterials are favorable, supported by its versatility, established production infrastructure, and alignment with market trends. As research progresses and manufacturing processes are refined, we can expect to see an increasing adoption of glacial acetic acid-based nanomaterials across various high-tech applications, driving innovation and economic growth in the materials science sector.
One of the key factors contributing to the scalability of glacial acetic acid in nanomaterial production is its versatility. It can be used in a wide range of applications, including the synthesis of metal oxide nanoparticles, carbon nanotubes, and graphene-based materials. This versatility allows for economies of scale in production, as manufacturers can utilize the same raw material for multiple product lines.
The industrial-scale production of glacial acetic acid is well-established, with global production capacity exceeding 10 million tons annually. This existing infrastructure provides a solid foundation for scaling up its use in nanomaterial synthesis. Additionally, the relatively low cost of glacial acetic acid compared to other specialty chemicals used in nanomaterial production enhances its economic viability for large-scale applications.
From a commercialization perspective, the integration of glacial acetic acid-based nanomaterials into various products offers significant market potential. Industries such as electronics, energy storage, and healthcare are increasingly adopting advanced nanomaterials to enhance product performance. For instance, acetic acid-modified graphene oxide has shown promise in improving the efficiency of solar cells and batteries, opening up opportunities in the renewable energy sector.
The environmental considerations associated with glacial acetic acid also contribute to its commercialization prospects. As a biodegradable compound, it aligns with the growing trend towards sustainable manufacturing processes. This aspect can be leveraged in marketing strategies to appeal to environmentally conscious consumers and meet stringent regulatory requirements.
However, challenges remain in fully realizing the commercial potential of glacial acetic acid in nanomaterial production. These include optimizing reaction conditions for large-scale synthesis, ensuring consistent quality across batches, and developing cost-effective purification methods. Addressing these challenges will require continued research and development efforts, as well as collaboration between academic institutions and industry partners.
In conclusion, the scalability and commercialization prospects for glacial acetic acid in advanced functional nanomaterials are favorable, supported by its versatility, established production infrastructure, and alignment with market trends. As research progresses and manufacturing processes are refined, we can expect to see an increasing adoption of glacial acetic acid-based nanomaterials across various high-tech applications, driving innovation and economic growth in the materials science sector.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!