Thermochemical Analysis of Glacial Acetic Acid in Synthetic Applications
AUG 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Glacial Acetic Acid Thermochemistry Overview
Glacial acetic acid, a highly pure form of acetic acid with less than 1% water content, plays a crucial role in various synthetic applications due to its unique thermochemical properties. This overview delves into the fundamental aspects of glacial acetic acid's thermochemistry and its implications for synthetic processes.
The thermochemical behavior of glacial acetic acid is characterized by its high melting point (16.6°C) and boiling point (118.1°C), which contribute to its stability and versatility in organic synthesis. Its enthalpy of vaporization (23.7 kJ/mol) and enthalpy of fusion (11.7 kJ/mol) provide insights into the energy requirements for phase transitions, critical for designing efficient reaction conditions and separation processes.
One of the most significant thermochemical properties of glacial acetic acid is its ability to form strong hydrogen bonds. This characteristic influences its reactivity, solvent properties, and interaction with other molecules in synthetic applications. The hydrogen bonding network in liquid glacial acetic acid contributes to its relatively high boiling point compared to other carboxylic acids of similar molecular weight.
The heat capacity of glacial acetic acid (123.1 J/mol·K at 25°C) is an essential parameter for understanding its thermal behavior in reactions. This property affects temperature control and energy transfer during synthetic processes, particularly in exothermic or endothermic reactions where acetic acid serves as a reactant or solvent.
In terms of thermodynamic stability, glacial acetic acid exhibits a standard enthalpy of formation (ΔHf°) of -484.5 kJ/mol and a standard Gibbs free energy of formation (ΔGf°) of -389.9 kJ/mol. These values are crucial for predicting the spontaneity and equilibrium of reactions involving acetic acid, as well as for calculating reaction enthalpies and free energy changes.
The thermochemical properties of glacial acetic acid also influence its behavior as a proton donor in acid-base reactions. With a pKa of 4.76, it serves as a moderately strong acid in synthetic applications, capable of protonating various substrates and catalyzing reactions. The enthalpy of dissociation and related thermodynamic parameters play a role in understanding the energetics of these proton transfer processes.
Understanding the thermochemistry of glacial acetic acid is essential for optimizing reaction conditions, predicting product yields, and designing efficient synthetic routes. Its thermal stability, phase behavior, and energetic properties make it a versatile reagent and solvent in organic synthesis, with applications ranging from esterification reactions to the production of cellulose acetate and various pharmaceuticals.
The thermochemical behavior of glacial acetic acid is characterized by its high melting point (16.6°C) and boiling point (118.1°C), which contribute to its stability and versatility in organic synthesis. Its enthalpy of vaporization (23.7 kJ/mol) and enthalpy of fusion (11.7 kJ/mol) provide insights into the energy requirements for phase transitions, critical for designing efficient reaction conditions and separation processes.
One of the most significant thermochemical properties of glacial acetic acid is its ability to form strong hydrogen bonds. This characteristic influences its reactivity, solvent properties, and interaction with other molecules in synthetic applications. The hydrogen bonding network in liquid glacial acetic acid contributes to its relatively high boiling point compared to other carboxylic acids of similar molecular weight.
The heat capacity of glacial acetic acid (123.1 J/mol·K at 25°C) is an essential parameter for understanding its thermal behavior in reactions. This property affects temperature control and energy transfer during synthetic processes, particularly in exothermic or endothermic reactions where acetic acid serves as a reactant or solvent.
In terms of thermodynamic stability, glacial acetic acid exhibits a standard enthalpy of formation (ΔHf°) of -484.5 kJ/mol and a standard Gibbs free energy of formation (ΔGf°) of -389.9 kJ/mol. These values are crucial for predicting the spontaneity and equilibrium of reactions involving acetic acid, as well as for calculating reaction enthalpies and free energy changes.
The thermochemical properties of glacial acetic acid also influence its behavior as a proton donor in acid-base reactions. With a pKa of 4.76, it serves as a moderately strong acid in synthetic applications, capable of protonating various substrates and catalyzing reactions. The enthalpy of dissociation and related thermodynamic parameters play a role in understanding the energetics of these proton transfer processes.
Understanding the thermochemistry of glacial acetic acid is essential for optimizing reaction conditions, predicting product yields, and designing efficient synthetic routes. Its thermal stability, phase behavior, and energetic properties make it a versatile reagent and solvent in organic synthesis, with applications ranging from esterification reactions to the production of cellulose acetate and various pharmaceuticals.
Industrial Demand Analysis
The industrial demand for glacial acetic acid in synthetic applications has been steadily growing, driven by its versatile use across multiple sectors. The chemical industry remains the largest consumer, utilizing glacial acetic acid as a key raw material in the production of various chemicals and polymers. Vinyl acetate monomer (VAM) production, a crucial component in adhesives, paints, and coatings, accounts for a significant portion of the demand.
The pharmaceutical sector also contributes substantially to the demand for glacial acetic acid. Its use in the synthesis of active pharmaceutical ingredients (APIs) and as a solvent in drug formulations has increased with the expansion of the global pharmaceutical market. The growing emphasis on generic drugs and the rise of contract manufacturing organizations (CMOs) in emerging economies have further boosted this demand.
In the textile industry, glacial acetic acid plays a vital role in the production of cellulose acetate fibers and in dyeing processes. The increasing demand for synthetic fabrics and the growth of the fashion industry in developing countries have positively impacted the consumption of glacial acetic acid in this sector.
The food industry represents another significant market for glacial acetic acid, particularly in the production of food additives and preservatives. The rising consumer preference for packaged and convenience foods has led to an increased demand for acetic acid-based preservatives, driving market growth in this segment.
Emerging applications in the electronics industry, such as the production of electronic-grade chemicals and cleaning agents for semiconductor manufacturing, have opened new avenues for glacial acetic acid consumption. The rapid expansion of the electronics sector, especially in Asia-Pacific regions, has contributed to this growing demand.
Geographically, Asia-Pacific dominates the global demand for glacial acetic acid, with China being the largest consumer and producer. The region's robust industrial growth, particularly in chemicals, textiles, and electronics, has been the primary driver. North America and Europe follow, with steady demand from established chemical and pharmaceutical industries.
Market analysts project a compound annual growth rate (CAGR) for glacial acetic acid demand in the range of 4-5% over the next five years. This growth is expected to be fueled by increasing industrialization in developing economies, technological advancements in synthetic applications, and the expansion of end-use industries. However, fluctuations in raw material prices and environmental regulations regarding acetic acid production may pose challenges to market growth.
The pharmaceutical sector also contributes substantially to the demand for glacial acetic acid. Its use in the synthesis of active pharmaceutical ingredients (APIs) and as a solvent in drug formulations has increased with the expansion of the global pharmaceutical market. The growing emphasis on generic drugs and the rise of contract manufacturing organizations (CMOs) in emerging economies have further boosted this demand.
In the textile industry, glacial acetic acid plays a vital role in the production of cellulose acetate fibers and in dyeing processes. The increasing demand for synthetic fabrics and the growth of the fashion industry in developing countries have positively impacted the consumption of glacial acetic acid in this sector.
The food industry represents another significant market for glacial acetic acid, particularly in the production of food additives and preservatives. The rising consumer preference for packaged and convenience foods has led to an increased demand for acetic acid-based preservatives, driving market growth in this segment.
Emerging applications in the electronics industry, such as the production of electronic-grade chemicals and cleaning agents for semiconductor manufacturing, have opened new avenues for glacial acetic acid consumption. The rapid expansion of the electronics sector, especially in Asia-Pacific regions, has contributed to this growing demand.
Geographically, Asia-Pacific dominates the global demand for glacial acetic acid, with China being the largest consumer and producer. The region's robust industrial growth, particularly in chemicals, textiles, and electronics, has been the primary driver. North America and Europe follow, with steady demand from established chemical and pharmaceutical industries.
Market analysts project a compound annual growth rate (CAGR) for glacial acetic acid demand in the range of 4-5% over the next five years. This growth is expected to be fueled by increasing industrialization in developing economies, technological advancements in synthetic applications, and the expansion of end-use industries. However, fluctuations in raw material prices and environmental regulations regarding acetic acid production may pose challenges to market growth.
Current Challenges in Thermochemical Analysis
The thermochemical analysis of glacial acetic acid in synthetic applications faces several significant challenges that hinder its widespread adoption and reliability. One of the primary obstacles is the high volatility of acetic acid, which complicates accurate measurements and analysis. The rapid evaporation of the compound at room temperature can lead to inconsistent results and difficulties in maintaining sample integrity throughout the analytical process.
Another challenge lies in the corrosive nature of glacial acetic acid. This property not only poses safety concerns for researchers but also affects the longevity and accuracy of analytical equipment. Specialized materials and protective measures are required to prevent degradation of instruments and ensure the safety of personnel, adding complexity and cost to the analysis process.
The hygroscopic nature of glacial acetic acid presents an additional hurdle. Its strong affinity for water means that even trace amounts of moisture can significantly alter the composition and properties of the sample. This sensitivity to environmental conditions necessitates stringent control measures and can introduce variability in results across different laboratory settings.
Furthermore, the thermochemical analysis of glacial acetic acid is complicated by its tendency to form azeotropes with water and other solvents. These azeotropic mixtures exhibit constant boiling points, making it challenging to separate and analyze the pure compound. This phenomenon can lead to inaccuracies in determining thermodynamic properties and reaction kinetics in synthetic applications.
The temperature-dependent behavior of glacial acetic acid adds another layer of complexity to its analysis. As the temperature changes, the physical and chemical properties of the acid can vary significantly, affecting reaction rates, equilibrium constants, and overall synthetic outcomes. Developing robust analytical methods that account for these temperature-induced variations remains a persistent challenge.
Lastly, the integration of thermochemical data into predictive models for synthetic applications is hindered by the complex interactions between glacial acetic acid and other reactants or catalysts. The acid's ability to act as both a solvent and a reagent in many reactions complicates the interpretation of thermochemical data and its application in reaction optimization and scale-up processes.
Addressing these challenges requires a multidisciplinary approach, combining advances in analytical instrumentation, materials science, and computational modeling. Overcoming these obstacles will be crucial for enhancing the reliability and applicability of thermochemical analysis of glacial acetic acid in diverse synthetic applications.
Another challenge lies in the corrosive nature of glacial acetic acid. This property not only poses safety concerns for researchers but also affects the longevity and accuracy of analytical equipment. Specialized materials and protective measures are required to prevent degradation of instruments and ensure the safety of personnel, adding complexity and cost to the analysis process.
The hygroscopic nature of glacial acetic acid presents an additional hurdle. Its strong affinity for water means that even trace amounts of moisture can significantly alter the composition and properties of the sample. This sensitivity to environmental conditions necessitates stringent control measures and can introduce variability in results across different laboratory settings.
Furthermore, the thermochemical analysis of glacial acetic acid is complicated by its tendency to form azeotropes with water and other solvents. These azeotropic mixtures exhibit constant boiling points, making it challenging to separate and analyze the pure compound. This phenomenon can lead to inaccuracies in determining thermodynamic properties and reaction kinetics in synthetic applications.
The temperature-dependent behavior of glacial acetic acid adds another layer of complexity to its analysis. As the temperature changes, the physical and chemical properties of the acid can vary significantly, affecting reaction rates, equilibrium constants, and overall synthetic outcomes. Developing robust analytical methods that account for these temperature-induced variations remains a persistent challenge.
Lastly, the integration of thermochemical data into predictive models for synthetic applications is hindered by the complex interactions between glacial acetic acid and other reactants or catalysts. The acid's ability to act as both a solvent and a reagent in many reactions complicates the interpretation of thermochemical data and its application in reaction optimization and scale-up processes.
Addressing these challenges requires a multidisciplinary approach, combining advances in analytical instrumentation, materials science, and computational modeling. Overcoming these obstacles will be crucial for enhancing the reliability and applicability of thermochemical analysis of glacial acetic acid in diverse synthetic applications.
State-of-the-Art Analytical Methods
01 Thermochemical properties of glacial acetic acid
Glacial acetic acid exhibits specific thermochemical properties that are important for various industrial processes. These properties include its melting point, boiling point, heat capacity, and enthalpy of vaporization. Understanding these properties is crucial for designing and optimizing chemical processes involving glacial acetic acid.- Thermochemical properties of glacial acetic acid: Glacial acetic acid exhibits specific thermochemical properties, including its melting point, boiling point, and heat capacity. These properties are crucial for understanding its behavior in various chemical processes and applications. The thermochemical characteristics of glacial acetic acid influence its reactivity and stability under different temperature conditions.
- Production methods affecting thermochemical properties: Different production methods for glacial acetic acid can impact its thermochemical properties. Factors such as purity levels, synthesis routes, and purification techniques can influence the final product's thermodynamic characteristics. Understanding these production-related effects is essential for controlling and optimizing the thermochemical properties of glacial acetic acid.
- Thermochemical analysis techniques for glacial acetic acid: Various analytical techniques are employed to study the thermochemical properties of glacial acetic acid. These may include differential scanning calorimetry, thermogravimetric analysis, and spectroscopic methods. Such techniques provide valuable data on heat capacity, phase transitions, and thermal stability, contributing to a comprehensive understanding of the compound's thermochemical behavior.
- Applications utilizing thermochemical properties: The unique thermochemical properties of glacial acetic acid are exploited in various industrial applications. These properties influence its use as a solvent, reagent, and intermediate in chemical processes. Understanding and leveraging these thermochemical characteristics enable the development of efficient and effective applications across multiple industries.
- Safety considerations related to thermochemical properties: The thermochemical properties of glacial acetic acid necessitate specific safety considerations in handling, storage, and transportation. Its flammability, reactivity, and potential for thermal decomposition require appropriate safety measures. Understanding these properties is crucial for developing proper risk assessment and management strategies in industrial and laboratory settings.
02 Production methods affecting thermochemical properties
Different production methods for glacial acetic acid can influence its thermochemical properties. Factors such as purity levels, synthesis routes, and purification techniques can impact the final product's thermodynamic characteristics. Optimizing these production methods can lead to improved thermochemical properties for specific applications.Expand Specific Solutions03 Measurement and analysis of thermochemical properties
Accurate measurement and analysis of glacial acetic acid's thermochemical properties are essential for quality control and process optimization. Various analytical techniques and instruments are used to determine these properties, including calorimetry, thermogravimetric analysis, and differential scanning calorimetry.Expand Specific Solutions04 Applications utilizing thermochemical properties
The unique thermochemical properties of glacial acetic acid make it suitable for various industrial applications. These properties are exploited in processes such as esterification, acetylation, and as a solvent in chemical reactions. Understanding and leveraging these properties can lead to more efficient and cost-effective industrial processes.Expand Specific Solutions05 Environmental and safety considerations
The thermochemical properties of glacial acetic acid have implications for environmental and safety considerations. These properties influence its handling, storage, and disposal requirements. Understanding these aspects is crucial for developing safe and environmentally friendly processes involving glacial acetic acid.Expand Specific Solutions
Key Players in Analytical Chemistry
The thermochemical analysis of glacial acetic acid in synthetic applications is a mature field with established players and ongoing research. The market is in a growth phase, driven by increasing demand in pharmaceutical and industrial sectors. Key players include Celanese International Corp., Lion Corp., and Kureha Corp., who have significant market presence and technological expertise. Academic institutions like MIT and research organizations such as CSIR contribute to advancing the field. The market size is substantial, with applications spanning from pharmaceuticals to industrial chemicals. Technological advancements focus on improving efficiency and sustainability in synthetic processes, with companies like Arkema France SA and Akzo Nobel Chemicals International BV leading innovation efforts.
Celanese International Corp.
Technical Solution: Celanese has developed advanced thermochemical analysis techniques for glacial acetic acid in synthetic applications. Their approach involves high-precision calorimetry and spectroscopic methods to study the thermodynamic properties and reactivity of glacial acetic acid under various conditions. They have implemented in-situ monitoring systems to track heat flow and molecular interactions during synthetic processes, allowing for real-time optimization of reaction parameters[1]. Celanese has also pioneered the use of computational modeling to predict the behavior of glacial acetic acid in complex synthetic environments, enabling more efficient process design and scale-up[3].
Strengths: Comprehensive analytical capabilities, real-time process optimization, and predictive modeling. Weaknesses: Potentially high implementation costs and complexity for smaller-scale operations.
Arkema France SA
Technical Solution: Arkema has developed a novel thermochemical analysis platform specifically for glacial acetic acid applications in polymer synthesis. Their system combines differential scanning calorimetry (DSC) with Fourier-transform infrared spectroscopy (FTIR) to simultaneously measure heat flow and chemical changes during reactions[2]. This integrated approach allows for precise characterization of reaction kinetics and thermodynamics. Arkema has also implemented machine learning algorithms to analyze the complex data sets generated, enabling rapid identification of optimal reaction conditions and prediction of product properties[4]. Their technology has been successfully applied to improve the efficiency and quality of various acetic acid-based polymer productions.
Strengths: Integrated analytical approach, advanced data analysis capabilities. Weaknesses: May be less versatile for non-polymer applications.
Innovative Approaches in Thermochemistry
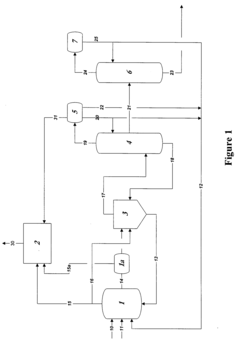
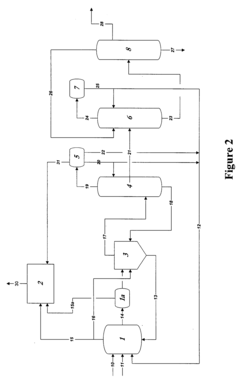
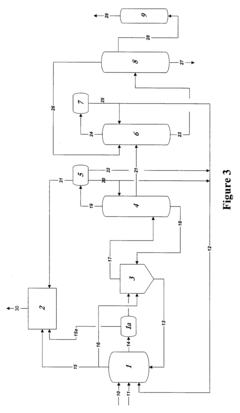
Reaction product of rhodium-catalyzed methanol carbonylation
PatentWO2008153708A2
Innovation
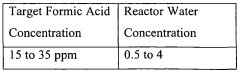
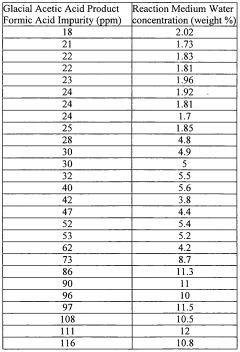
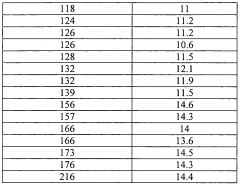
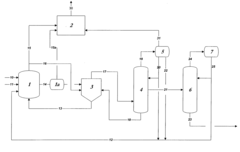
- Maintaining a reactor water concentration of 0.5 to 14 weight % and using an iodide salt with 2 to 20 wt% ionic iodide, 1 to 20 wt% methyl iodide, and 0.5 to 30 wt% methyl acetate in the reaction medium, while employing a Group VIII metal catalyst, to produce glacial acetic acid with controlled aldehyde and iodide concentrations.
Control of impurities in product glacial acetic acid of rhodium-catalyzed methanol carbonylation
PatentInactiveUS20090187043A1
Innovation
- A method involving a rhodium-catalyzed carbonylation process with controlled water and iodide concentrations, combined with the use of a macroreticular strong-acid cation exchange resin and silver or mercury exchanged cation exchange substrates to manage impurities, ensuring high purity glacial acetic acid production.
Environmental Impact Assessment
The environmental impact assessment of thermochemical analysis of glacial acetic acid in synthetic applications reveals several significant considerations. The production and use of glacial acetic acid can have substantial environmental implications, primarily due to its corrosive nature and potential for atmospheric emissions.
During the manufacturing process of glacial acetic acid, there is a risk of air pollution through the release of volatile organic compounds (VOCs) and acetic acid vapors. These emissions can contribute to the formation of ground-level ozone and photochemical smog, potentially impacting local air quality and human health. Furthermore, the production often involves energy-intensive processes, leading to increased greenhouse gas emissions and contributing to climate change concerns.
Water pollution is another critical environmental aspect to consider. Improper handling or disposal of glacial acetic acid can result in contamination of water bodies, affecting aquatic ecosystems and potentially entering the food chain. The high acidity of the compound can disrupt the pH balance of water systems, causing harm to flora and fauna.
Soil contamination is also a potential risk associated with the use and disposal of glacial acetic acid in synthetic applications. Accidental spills or improper waste management practices can lead to soil acidification, negatively impacting soil fertility and microbial communities essential for ecosystem health.
The transportation and storage of glacial acetic acid pose additional environmental risks. Accidents during transport can result in spills, leading to localized environmental damage and potential human health hazards. Proper containment and handling protocols are crucial to mitigate these risks.
In terms of resource consumption, the production of glacial acetic acid requires significant energy inputs and raw materials. This can contribute to resource depletion and increased carbon footprint, particularly if non-renewable energy sources are utilized in the manufacturing process.
However, it is important to note that advancements in green chemistry and sustainable manufacturing practices are addressing some of these environmental concerns. The development of more efficient production methods, improved catalysts, and the use of renewable feedstocks are helping to reduce the environmental impact of glacial acetic acid production and use in synthetic applications.
Recycling and proper waste management strategies are also crucial in minimizing the environmental footprint of glacial acetic acid. Implementing closed-loop systems and recovery processes can significantly reduce waste generation and the need for raw materials, thereby lessening the overall environmental impact.
In conclusion, while the thermochemical analysis and use of glacial acetic acid in synthetic applications present several environmental challenges, ongoing research and technological advancements are continually improving its sustainability profile. Balancing the benefits of its applications with responsible environmental stewardship remains a key focus for industries utilizing this important chemical compound.
During the manufacturing process of glacial acetic acid, there is a risk of air pollution through the release of volatile organic compounds (VOCs) and acetic acid vapors. These emissions can contribute to the formation of ground-level ozone and photochemical smog, potentially impacting local air quality and human health. Furthermore, the production often involves energy-intensive processes, leading to increased greenhouse gas emissions and contributing to climate change concerns.
Water pollution is another critical environmental aspect to consider. Improper handling or disposal of glacial acetic acid can result in contamination of water bodies, affecting aquatic ecosystems and potentially entering the food chain. The high acidity of the compound can disrupt the pH balance of water systems, causing harm to flora and fauna.
Soil contamination is also a potential risk associated with the use and disposal of glacial acetic acid in synthetic applications. Accidental spills or improper waste management practices can lead to soil acidification, negatively impacting soil fertility and microbial communities essential for ecosystem health.
The transportation and storage of glacial acetic acid pose additional environmental risks. Accidents during transport can result in spills, leading to localized environmental damage and potential human health hazards. Proper containment and handling protocols are crucial to mitigate these risks.
In terms of resource consumption, the production of glacial acetic acid requires significant energy inputs and raw materials. This can contribute to resource depletion and increased carbon footprint, particularly if non-renewable energy sources are utilized in the manufacturing process.
However, it is important to note that advancements in green chemistry and sustainable manufacturing practices are addressing some of these environmental concerns. The development of more efficient production methods, improved catalysts, and the use of renewable feedstocks are helping to reduce the environmental impact of glacial acetic acid production and use in synthetic applications.
Recycling and proper waste management strategies are also crucial in minimizing the environmental footprint of glacial acetic acid. Implementing closed-loop systems and recovery processes can significantly reduce waste generation and the need for raw materials, thereby lessening the overall environmental impact.
In conclusion, while the thermochemical analysis and use of glacial acetic acid in synthetic applications present several environmental challenges, ongoing research and technological advancements are continually improving its sustainability profile. Balancing the benefits of its applications with responsible environmental stewardship remains a key focus for industries utilizing this important chemical compound.
Safety Protocols and Regulations
The handling and use of glacial acetic acid in synthetic applications require strict adherence to safety protocols and regulations due to its corrosive and flammable nature. Proper personal protective equipment (PPE) is essential, including chemical-resistant gloves, safety goggles, and lab coats. Respiratory protection may be necessary when working with large quantities or in poorly ventilated areas. All operations involving glacial acetic acid should be conducted in a fume hood to prevent exposure to vapors.
Storage regulations mandate that glacial acetic acid be kept in tightly sealed containers in a cool, dry, and well-ventilated area, away from sources of heat and ignition. Incompatible materials, such as oxidizing agents and strong bases, must be stored separately to prevent hazardous reactions. Spill response protocols should be in place, including the availability of appropriate absorbent materials and neutralizing agents.
Waste disposal of glacial acetic acid and its solutions must comply with local, state, and federal regulations. Typically, it cannot be disposed of down the drain and requires specialized chemical waste handling procedures. Proper labeling and documentation are crucial for all containers and waste streams containing glacial acetic acid.
In thermochemical analysis applications, additional safety measures are necessary. Temperature control systems must be regularly maintained and calibrated to prevent overheating. Emergency shutdown procedures should be established and practiced. When conducting reactions or analyses at elevated temperatures, pressure relief systems may be required to mitigate the risk of container rupture.
Training programs for personnel working with glacial acetic acid should cover safe handling practices, emergency procedures, and the proper use of safety equipment. Regular safety audits and inspections are essential to ensure compliance with established protocols and to identify potential hazards before incidents occur.
Transportation of glacial acetic acid is subject to strict regulations, including proper packaging, labeling, and documentation requirements. Vehicles used for transport must meet specific safety standards and drivers must be trained in hazardous materials handling.
Environmental considerations are also critical. Facilities using glacial acetic acid must have containment systems to prevent release into the environment. Air quality monitoring may be necessary to ensure that vapor concentrations remain below permissible exposure limits. Wastewater treatment systems should be designed to handle potential acetic acid contamination.
Continuous improvement of safety protocols is vital. This includes staying updated on regulatory changes, incorporating lessons learned from incidents in the industry, and regularly reviewing and updating standard operating procedures. Collaboration with safety professionals and regulatory agencies can help ensure that all safety measures are current and effective.
Storage regulations mandate that glacial acetic acid be kept in tightly sealed containers in a cool, dry, and well-ventilated area, away from sources of heat and ignition. Incompatible materials, such as oxidizing agents and strong bases, must be stored separately to prevent hazardous reactions. Spill response protocols should be in place, including the availability of appropriate absorbent materials and neutralizing agents.
Waste disposal of glacial acetic acid and its solutions must comply with local, state, and federal regulations. Typically, it cannot be disposed of down the drain and requires specialized chemical waste handling procedures. Proper labeling and documentation are crucial for all containers and waste streams containing glacial acetic acid.
In thermochemical analysis applications, additional safety measures are necessary. Temperature control systems must be regularly maintained and calibrated to prevent overheating. Emergency shutdown procedures should be established and practiced. When conducting reactions or analyses at elevated temperatures, pressure relief systems may be required to mitigate the risk of container rupture.
Training programs for personnel working with glacial acetic acid should cover safe handling practices, emergency procedures, and the proper use of safety equipment. Regular safety audits and inspections are essential to ensure compliance with established protocols and to identify potential hazards before incidents occur.
Transportation of glacial acetic acid is subject to strict regulations, including proper packaging, labeling, and documentation requirements. Vehicles used for transport must meet specific safety standards and drivers must be trained in hazardous materials handling.
Environmental considerations are also critical. Facilities using glacial acetic acid must have containment systems to prevent release into the environment. Air quality monitoring may be necessary to ensure that vapor concentrations remain below permissible exposure limits. Wastewater treatment systems should be designed to handle potential acetic acid contamination.
Continuous improvement of safety protocols is vital. This includes staying updated on regulatory changes, incorporating lessons learned from incidents in the industry, and regularly reviewing and updating standard operating procedures. Collaboration with safety professionals and regulatory agencies can help ensure that all safety measures are current and effective.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!