Glacial Acetic Acid's Role in Fine Chemical Synthesis Optimization
AUG 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
GAA in Fine Chemicals
Glacial Acetic Acid (GAA) plays a crucial role in the optimization of fine chemical synthesis, serving as a versatile reagent, solvent, and catalyst in numerous industrial processes. Its high purity and low water content make it an ideal choice for reactions sensitive to moisture or requiring precise control over reaction conditions.
In the pharmaceutical industry, GAA is extensively used in the synthesis of various active pharmaceutical ingredients (APIs). It acts as an acetylating agent in the production of aspirin and other acetylated compounds, enabling efficient and cost-effective manufacturing processes. Additionally, GAA serves as a solvent for many organic reactions, facilitating the dissolution of reactants and promoting homogeneous reaction conditions.
The petrochemical sector also benefits significantly from GAA's properties. It is employed in the production of vinyl acetate monomer, a key precursor for adhesives, coatings, and packaging materials. GAA's ability to form esters with alcohols is exploited in the synthesis of various fragrances and flavoring agents, contributing to the development of high-value products in the cosmetics and food industries.
In the field of agrochemicals, GAA plays a vital role in the synthesis of herbicides, pesticides, and plant growth regulators. Its acidic nature and acetylating properties enable the modification of complex organic molecules, leading to the creation of more effective and environmentally friendly crop protection agents.
The electronics industry also relies on GAA for the production of high-purity chemicals used in semiconductor manufacturing. Its ability to dissolve metal oxides and act as an etching agent makes it indispensable in the fabrication of printed circuit boards and microchips.
Furthermore, GAA's role extends to the textile industry, where it is used in the production of cellulose acetate fibers and as a dyeing auxiliary. Its acidic properties aid in pH adjustment during various textile processing steps, ensuring optimal conditions for dye fixation and fabric treatment.
The optimization of fine chemical synthesis through the use of GAA is evident in its ability to improve reaction yields, enhance product purity, and reduce processing steps. Its low boiling point facilitates easy removal from reaction mixtures, simplifying purification processes. Moreover, GAA's compatibility with a wide range of organic compounds makes it an excellent choice for multi-step syntheses, reducing the need for solvent exchanges and minimizing waste generation.
In the pharmaceutical industry, GAA is extensively used in the synthesis of various active pharmaceutical ingredients (APIs). It acts as an acetylating agent in the production of aspirin and other acetylated compounds, enabling efficient and cost-effective manufacturing processes. Additionally, GAA serves as a solvent for many organic reactions, facilitating the dissolution of reactants and promoting homogeneous reaction conditions.
The petrochemical sector also benefits significantly from GAA's properties. It is employed in the production of vinyl acetate monomer, a key precursor for adhesives, coatings, and packaging materials. GAA's ability to form esters with alcohols is exploited in the synthesis of various fragrances and flavoring agents, contributing to the development of high-value products in the cosmetics and food industries.
In the field of agrochemicals, GAA plays a vital role in the synthesis of herbicides, pesticides, and plant growth regulators. Its acidic nature and acetylating properties enable the modification of complex organic molecules, leading to the creation of more effective and environmentally friendly crop protection agents.
The electronics industry also relies on GAA for the production of high-purity chemicals used in semiconductor manufacturing. Its ability to dissolve metal oxides and act as an etching agent makes it indispensable in the fabrication of printed circuit boards and microchips.
Furthermore, GAA's role extends to the textile industry, where it is used in the production of cellulose acetate fibers and as a dyeing auxiliary. Its acidic properties aid in pH adjustment during various textile processing steps, ensuring optimal conditions for dye fixation and fabric treatment.
The optimization of fine chemical synthesis through the use of GAA is evident in its ability to improve reaction yields, enhance product purity, and reduce processing steps. Its low boiling point facilitates easy removal from reaction mixtures, simplifying purification processes. Moreover, GAA's compatibility with a wide range of organic compounds makes it an excellent choice for multi-step syntheses, reducing the need for solvent exchanges and minimizing waste generation.
Market Demand Analysis
The market demand for glacial acetic acid in fine chemical synthesis optimization has been steadily growing, driven by the increasing need for high-purity chemicals in various industries. The pharmaceutical sector, in particular, has emerged as a significant consumer of glacial acetic acid, utilizing it as a key reagent in the synthesis of active pharmaceutical ingredients (APIs) and intermediate compounds. This demand is further amplified by the global trend towards personalized medicine and the development of complex drug molecules, which often require precise and efficient synthetic processes.
In the agrochemical industry, glacial acetic acid plays a crucial role in the production of herbicides, pesticides, and plant growth regulators. As the agricultural sector faces challenges such as climate change and the need for increased crop yields, the demand for innovative and environmentally friendly agrochemicals continues to rise, subsequently driving the market for glacial acetic acid.
The electronics industry also contributes significantly to the market demand for glacial acetic acid in fine chemical synthesis. Its use in the production of high-purity solvents for cleaning electronic components and in the manufacture of advanced materials for semiconductors and display technologies has seen a notable increase. This trend is expected to continue as the electronics industry expands and evolves, particularly with the advent of 5G technology and the Internet of Things (IoT).
Another growing market segment for glacial acetic acid is the production of specialty polymers and advanced materials. These materials find applications in diverse fields such as aerospace, automotive, and renewable energy technologies. The optimization of synthesis processes using glacial acetic acid has enabled the development of materials with enhanced properties, meeting the demanding requirements of these high-tech industries.
The cosmetics and personal care industry has also shown an increasing demand for fine chemicals synthesized using glacial acetic acid. This sector's focus on natural and sustainable ingredients has led to the development of novel synthesis routes for cosmetic actives and fragrances, where glacial acetic acid serves as an essential reagent or solvent.
Geographically, the Asia-Pacific region, particularly China and India, has emerged as a major consumer and producer of glacial acetic acid for fine chemical synthesis. The rapid industrialization and growth of the chemical manufacturing sector in these countries have significantly contributed to the global market demand. North America and Europe continue to be important markets, driven by their established pharmaceutical and specialty chemical industries.
The market demand for glacial acetic acid in fine chemical synthesis optimization is also influenced by sustainability concerns and regulatory pressures. There is a growing emphasis on developing greener synthesis processes and reducing the environmental impact of chemical manufacturing. This has led to research into more efficient use of glacial acetic acid, recycling methods, and the exploration of bio-based alternatives, which may shape the future market dynamics of this essential chemical.
In the agrochemical industry, glacial acetic acid plays a crucial role in the production of herbicides, pesticides, and plant growth regulators. As the agricultural sector faces challenges such as climate change and the need for increased crop yields, the demand for innovative and environmentally friendly agrochemicals continues to rise, subsequently driving the market for glacial acetic acid.
The electronics industry also contributes significantly to the market demand for glacial acetic acid in fine chemical synthesis. Its use in the production of high-purity solvents for cleaning electronic components and in the manufacture of advanced materials for semiconductors and display technologies has seen a notable increase. This trend is expected to continue as the electronics industry expands and evolves, particularly with the advent of 5G technology and the Internet of Things (IoT).
Another growing market segment for glacial acetic acid is the production of specialty polymers and advanced materials. These materials find applications in diverse fields such as aerospace, automotive, and renewable energy technologies. The optimization of synthesis processes using glacial acetic acid has enabled the development of materials with enhanced properties, meeting the demanding requirements of these high-tech industries.
The cosmetics and personal care industry has also shown an increasing demand for fine chemicals synthesized using glacial acetic acid. This sector's focus on natural and sustainable ingredients has led to the development of novel synthesis routes for cosmetic actives and fragrances, where glacial acetic acid serves as an essential reagent or solvent.
Geographically, the Asia-Pacific region, particularly China and India, has emerged as a major consumer and producer of glacial acetic acid for fine chemical synthesis. The rapid industrialization and growth of the chemical manufacturing sector in these countries have significantly contributed to the global market demand. North America and Europe continue to be important markets, driven by their established pharmaceutical and specialty chemical industries.
The market demand for glacial acetic acid in fine chemical synthesis optimization is also influenced by sustainability concerns and regulatory pressures. There is a growing emphasis on developing greener synthesis processes and reducing the environmental impact of chemical manufacturing. This has led to research into more efficient use of glacial acetic acid, recycling methods, and the exploration of bio-based alternatives, which may shape the future market dynamics of this essential chemical.
GAA Technical Challenges
Glacial Acetic Acid (GAA) plays a crucial role in fine chemical synthesis optimization, but its application faces several technical challenges. One of the primary issues is the corrosive nature of GAA, which can lead to equipment degradation and potential safety hazards. This necessitates the use of specialized materials and protective measures, increasing production costs and complexity.
Another significant challenge is the control of reaction selectivity when using GAA as a solvent or reagent. The high acidity of GAA can lead to unwanted side reactions, affecting product purity and yield. Researchers must develop sophisticated reaction control strategies to mitigate these effects and ensure optimal product formation.
The recovery and purification of GAA from reaction mixtures present additional technical hurdles. Efficient separation techniques are required to recycle GAA, reducing waste and improving process economics. However, the high boiling point and tendency to form azeotropes with water complicate traditional distillation methods.
Environmental concerns also pose challenges in GAA utilization. Stringent regulations on volatile organic compound (VOC) emissions necessitate the development of containment and treatment systems for GAA vapors. This adds another layer of complexity to process design and operation in fine chemical synthesis.
The energy-intensive nature of GAA production and handling is another technical challenge. Maintaining the glacial state of acetic acid requires careful temperature control throughout the process, leading to increased energy consumption. Improving energy efficiency in GAA-related processes is crucial for sustainable fine chemical synthesis.
Scaling up GAA-based processes from laboratory to industrial scale presents its own set of challenges. Maintaining reaction efficiency and product quality while increasing production volumes often requires significant process modifications and optimization efforts. Engineers must address heat transfer, mixing, and mass transfer issues that become more pronounced at larger scales.
Lastly, the development of alternative, more environmentally friendly solvents and reagents poses a challenge to the continued use of GAA in fine chemical synthesis. Researchers are exploring green chemistry approaches, such as using ionic liquids or supercritical fluids, which may eventually replace GAA in certain applications. This ongoing shift necessitates continuous innovation in GAA-based processes to maintain their relevance and competitiveness in the evolving landscape of fine chemical synthesis.
Another significant challenge is the control of reaction selectivity when using GAA as a solvent or reagent. The high acidity of GAA can lead to unwanted side reactions, affecting product purity and yield. Researchers must develop sophisticated reaction control strategies to mitigate these effects and ensure optimal product formation.
The recovery and purification of GAA from reaction mixtures present additional technical hurdles. Efficient separation techniques are required to recycle GAA, reducing waste and improving process economics. However, the high boiling point and tendency to form azeotropes with water complicate traditional distillation methods.
Environmental concerns also pose challenges in GAA utilization. Stringent regulations on volatile organic compound (VOC) emissions necessitate the development of containment and treatment systems for GAA vapors. This adds another layer of complexity to process design and operation in fine chemical synthesis.
The energy-intensive nature of GAA production and handling is another technical challenge. Maintaining the glacial state of acetic acid requires careful temperature control throughout the process, leading to increased energy consumption. Improving energy efficiency in GAA-related processes is crucial for sustainable fine chemical synthesis.
Scaling up GAA-based processes from laboratory to industrial scale presents its own set of challenges. Maintaining reaction efficiency and product quality while increasing production volumes often requires significant process modifications and optimization efforts. Engineers must address heat transfer, mixing, and mass transfer issues that become more pronounced at larger scales.
Lastly, the development of alternative, more environmentally friendly solvents and reagents poses a challenge to the continued use of GAA in fine chemical synthesis. Researchers are exploring green chemistry approaches, such as using ionic liquids or supercritical fluids, which may eventually replace GAA in certain applications. This ongoing shift necessitates continuous innovation in GAA-based processes to maintain their relevance and competitiveness in the evolving landscape of fine chemical synthesis.
Current GAA Solutions
01 Process optimization for glacial acetic acid production
Various methods are employed to optimize the production process of glacial acetic acid. These include improving reaction conditions, enhancing catalytic efficiency, and refining separation techniques. Such optimizations aim to increase yield, reduce energy consumption, and improve product purity.- Process optimization for glacial acetic acid production: Various methods are employed to optimize the production process of glacial acetic acid. These include improving reaction conditions, enhancing catalytic efficiency, and refining separation techniques. Such optimizations aim to increase yield, reduce energy consumption, and improve product purity.
- Equipment design for glacial acetic acid production: Specialized equipment designs are crucial for efficient glacial acetic acid production. This includes innovative reactor designs, advanced distillation columns, and improved heat exchange systems. These equipment modifications aim to enhance process efficiency, reduce operational costs, and ensure product quality.
- Purification and concentration techniques: Various purification and concentration techniques are employed to obtain high-purity glacial acetic acid. These may include advanced distillation methods, crystallization processes, and membrane separation technologies. The goal is to achieve the highest possible concentration and purity of acetic acid while minimizing impurities.
- Catalytic systems for acetic acid synthesis: Development of novel catalytic systems plays a crucial role in optimizing glacial acetic acid production. This includes research into heterogeneous and homogeneous catalysts, catalyst support materials, and promoters. The aim is to improve reaction rates, selectivity, and catalyst longevity.
- Waste reduction and environmental considerations: Optimization efforts also focus on reducing waste and improving the environmental profile of glacial acetic acid production. This includes developing closed-loop systems, implementing energy recovery techniques, and exploring greener production routes. The goal is to minimize environmental impact while maintaining production efficiency.
02 Purification and concentration techniques
Advanced purification and concentration methods are developed to achieve high-purity glacial acetic acid. These techniques may involve distillation, crystallization, or membrane separation processes. The focus is on removing impurities and increasing the acetic acid concentration to meet industry standards.Expand Specific Solutions03 Equipment and apparatus design
Specialized equipment and apparatus are designed to enhance the production and handling of glacial acetic acid. This includes reactors, distillation columns, heat exchangers, and storage tanks optimized for acetic acid processing. The equipment is engineered to improve efficiency, safety, and product quality.Expand Specific Solutions04 Catalytic systems for acetic acid synthesis
Development of novel catalytic systems to improve the synthesis of glacial acetic acid. This involves research into heterogeneous and homogeneous catalysts, catalyst supports, and reaction mechanisms. The goal is to enhance selectivity, increase conversion rates, and prolong catalyst life.Expand Specific Solutions05 Process control and monitoring systems
Implementation of advanced process control and monitoring systems for glacial acetic acid production. This includes the use of sensors, real-time analytics, and automation technologies to optimize process parameters, ensure consistent quality, and improve overall production efficiency.Expand Specific Solutions
Key Industry Players
The market for glacial acetic acid in fine chemical synthesis optimization is in a mature stage, with a stable global market size estimated at several billion dollars annually. The technology is well-established, with major players like BASF Corp., Evonik Operations GmbH, and Daicel Corp. leading the field. These companies have extensive experience in chemical manufacturing and process optimization. The competitive landscape is characterized by ongoing efforts to improve efficiency, reduce costs, and develop more sustainable production methods. Emerging trends include the integration of advanced catalysts, process intensification techniques, and the exploration of bio-based acetic acid production, driven by environmental concerns and the push for greener chemistry solutions.
BASF Corp.
Technical Solution: BASF has developed an innovative approach to optimize fine chemical synthesis using glacial acetic acid. Their method involves a catalytic oxidation process that utilizes glacial acetic acid as both a solvent and a reactant[1]. This process enhances the selectivity and yield of target compounds, particularly in the synthesis of aromatic carboxylic acids and their derivatives[2]. BASF's technology employs a proprietary metal catalyst system that operates efficiently under mild conditions, reducing energy consumption and improving overall process economics[3].
Strengths: High selectivity, improved yields, and reduced energy consumption. Weaknesses: Potential catalyst cost and recovery issues, limited to specific reaction types.
Evonik Operations GmbH
Technical Solution: Evonik has pioneered a novel approach to fine chemical synthesis optimization using glacial acetic acid as a key component. Their method focuses on the development of a continuous flow reactor system that maximizes the efficiency of acetic acid-mediated reactions[4]. This technology enables precise control over reaction parameters, resulting in improved product quality and consistency. Evonik's process also incorporates an innovative solvent recovery system, which significantly reduces waste and improves the overall sustainability of the synthesis[5].
Strengths: Continuous process, improved product quality, and enhanced sustainability. Weaknesses: High initial investment costs and potential scalability challenges for certain reactions.
GAA Innovation Patents
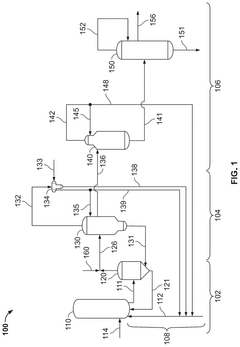
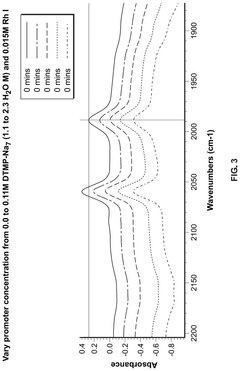
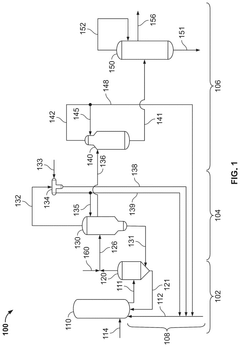
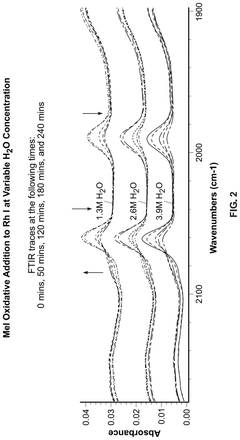
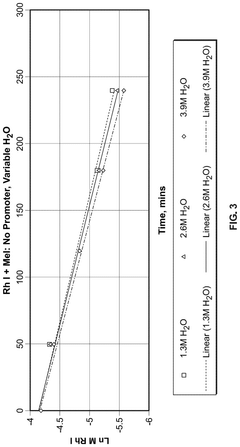
Polyphoshates and polyphosphonates as rate promoters for the glacial acetic acid process
PatentPendingUS20250074856A1
Innovation
- The process involves using a reaction mixture comprising a carbonylation catalyst, water, and specific rate-promoting compounds such as Group I and Group II polyphosphate and polyphosphonate salts, which are added at an iodide to promoter molar ratio greater than 2, to enhance the rate of acetic acid formation while reducing the amount of water required.
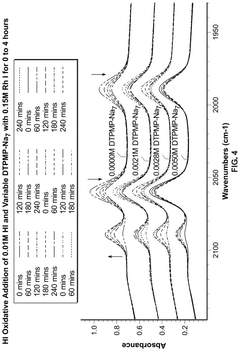
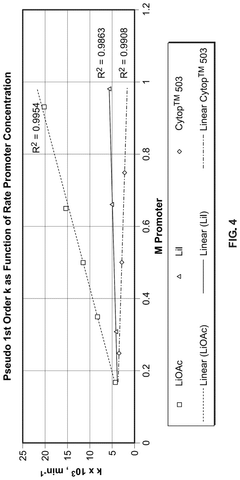
Aminopolycarboxylates as rate promoters for the glacial acetic acid process
PatentPendingUS20250074857A1
Innovation
- The process involves using a reaction mixture comprising a carbonylation catalyst, water, and specific rate-promoting compounds, such as Group I and Group II aminopolycarboxylate salts, to enhance the rate of acetic acid formation while reducing the amount of water required and suppressing undesirable oxidative addition reactions.
Environmental Impact
The use of glacial acetic acid in fine chemical synthesis optimization has significant environmental implications that warrant careful consideration. As a widely used solvent and reagent in various chemical processes, its environmental impact extends across multiple dimensions.
Firstly, the production of glacial acetic acid itself contributes to environmental concerns. The traditional method of manufacturing acetic acid through methanol carbonylation requires substantial energy input and generates greenhouse gas emissions. However, recent advancements in biotechnological processes, such as fermentation using genetically engineered microorganisms, offer more sustainable alternatives with reduced carbon footprints.
In terms of its application in fine chemical synthesis, glacial acetic acid's high volatility poses potential air pollution risks. Proper handling and containment measures are crucial to minimize atmospheric releases. Additionally, its corrosive nature necessitates the use of specialized equipment and storage facilities, which may have indirect environmental impacts through increased resource consumption for infrastructure.
Water pollution is another critical concern associated with glacial acetic acid usage. Improper disposal or accidental spills can lead to the acidification of water bodies, adversely affecting aquatic ecosystems. Implementing robust wastewater treatment systems and adhering to stringent disposal protocols are essential for mitigating these risks.
On a positive note, the optimization of fine chemical synthesis processes using glacial acetic acid can potentially lead to improved resource efficiency and reduced waste generation. By enhancing reaction selectivity and yield, the overall environmental footprint of chemical production can be minimized. Furthermore, the development of novel catalytic systems that enable milder reaction conditions and lower acetic acid consumption contributes to greener chemistry practices.
The recyclability of glacial acetic acid presents an opportunity for reducing its environmental impact. Implementing effective recovery and purification techniques can significantly decrease the need for fresh acetic acid, thereby conserving resources and minimizing waste. However, the energy requirements for such recycling processes must be carefully balanced against the environmental benefits gained.
As environmental regulations become increasingly stringent, the chemical industry is exploring alternatives to glacial acetic acid in certain applications. Bio-based solvents and ionic liquids are emerging as potential substitutes with lower environmental impacts. However, comprehensive life cycle assessments are necessary to ensure that these alternatives truly offer superior environmental performance across their entire production and use cycle.
In conclusion, while glacial acetic acid plays a crucial role in optimizing fine chemical synthesis, its environmental impact necessitates a holistic approach to its use and management. Balancing the benefits of improved synthesis efficiency with the need for environmental protection requires ongoing research, innovation, and responsible industrial practices.
Firstly, the production of glacial acetic acid itself contributes to environmental concerns. The traditional method of manufacturing acetic acid through methanol carbonylation requires substantial energy input and generates greenhouse gas emissions. However, recent advancements in biotechnological processes, such as fermentation using genetically engineered microorganisms, offer more sustainable alternatives with reduced carbon footprints.
In terms of its application in fine chemical synthesis, glacial acetic acid's high volatility poses potential air pollution risks. Proper handling and containment measures are crucial to minimize atmospheric releases. Additionally, its corrosive nature necessitates the use of specialized equipment and storage facilities, which may have indirect environmental impacts through increased resource consumption for infrastructure.
Water pollution is another critical concern associated with glacial acetic acid usage. Improper disposal or accidental spills can lead to the acidification of water bodies, adversely affecting aquatic ecosystems. Implementing robust wastewater treatment systems and adhering to stringent disposal protocols are essential for mitigating these risks.
On a positive note, the optimization of fine chemical synthesis processes using glacial acetic acid can potentially lead to improved resource efficiency and reduced waste generation. By enhancing reaction selectivity and yield, the overall environmental footprint of chemical production can be minimized. Furthermore, the development of novel catalytic systems that enable milder reaction conditions and lower acetic acid consumption contributes to greener chemistry practices.
The recyclability of glacial acetic acid presents an opportunity for reducing its environmental impact. Implementing effective recovery and purification techniques can significantly decrease the need for fresh acetic acid, thereby conserving resources and minimizing waste. However, the energy requirements for such recycling processes must be carefully balanced against the environmental benefits gained.
As environmental regulations become increasingly stringent, the chemical industry is exploring alternatives to glacial acetic acid in certain applications. Bio-based solvents and ionic liquids are emerging as potential substitutes with lower environmental impacts. However, comprehensive life cycle assessments are necessary to ensure that these alternatives truly offer superior environmental performance across their entire production and use cycle.
In conclusion, while glacial acetic acid plays a crucial role in optimizing fine chemical synthesis, its environmental impact necessitates a holistic approach to its use and management. Balancing the benefits of improved synthesis efficiency with the need for environmental protection requires ongoing research, innovation, and responsible industrial practices.
Regulatory Compliance
The use of glacial acetic acid in fine chemical synthesis optimization is subject to various regulatory requirements that must be carefully considered and adhered to. These regulations are designed to ensure the safety of workers, protect the environment, and maintain product quality standards.
Occupational safety and health regulations play a crucial role in the handling and use of glacial acetic acid. Organizations such as the Occupational Safety and Health Administration (OSHA) in the United States and the European Agency for Safety and Health at Work (EU-OSHA) in Europe have established strict guidelines for the safe handling, storage, and disposal of this corrosive substance. These regulations typically mandate the use of appropriate personal protective equipment (PPE), proper ventilation systems, and emergency response protocols.
Environmental regulations also significantly impact the use of glacial acetic acid in chemical synthesis. Agencies like the Environmental Protection Agency (EPA) in the US and the European Environment Agency (EEA) in Europe have set limits on emissions and waste disposal related to acetic acid production and use. Companies must comply with these regulations by implementing proper waste management systems, monitoring air and water quality, and obtaining necessary permits for their operations.
Product quality and safety regulations are another critical aspect of regulatory compliance in fine chemical synthesis using glacial acetic acid. Regulatory bodies such as the Food and Drug Administration (FDA) in the US and the European Medicines Agency (EMA) in Europe have established Good Manufacturing Practice (GMP) guidelines that must be followed when producing chemicals for pharmaceutical or food applications. These guidelines ensure that products meet specific quality and purity standards.
Transportation of glacial acetic acid is also heavily regulated due to its corrosive nature. Organizations like the Department of Transportation (DOT) in the US and the European Agreement concerning the International Carriage of Dangerous Goods by Road (ADR) in Europe have specific requirements for packaging, labeling, and shipping of acetic acid to prevent accidents and ensure safe transport.
Compliance with these regulations requires companies to implement comprehensive management systems, conduct regular audits, and maintain detailed documentation of their processes and procedures. Failure to comply with these regulations can result in severe penalties, including fines, legal action, and potential shutdown of operations.
As the regulatory landscape continues to evolve, companies must stay informed about changes in regulations and adapt their practices accordingly. This may involve investing in new technologies, updating standard operating procedures, and providing ongoing training to employees to ensure continued compliance with all relevant regulations.
Occupational safety and health regulations play a crucial role in the handling and use of glacial acetic acid. Organizations such as the Occupational Safety and Health Administration (OSHA) in the United States and the European Agency for Safety and Health at Work (EU-OSHA) in Europe have established strict guidelines for the safe handling, storage, and disposal of this corrosive substance. These regulations typically mandate the use of appropriate personal protective equipment (PPE), proper ventilation systems, and emergency response protocols.
Environmental regulations also significantly impact the use of glacial acetic acid in chemical synthesis. Agencies like the Environmental Protection Agency (EPA) in the US and the European Environment Agency (EEA) in Europe have set limits on emissions and waste disposal related to acetic acid production and use. Companies must comply with these regulations by implementing proper waste management systems, monitoring air and water quality, and obtaining necessary permits for their operations.
Product quality and safety regulations are another critical aspect of regulatory compliance in fine chemical synthesis using glacial acetic acid. Regulatory bodies such as the Food and Drug Administration (FDA) in the US and the European Medicines Agency (EMA) in Europe have established Good Manufacturing Practice (GMP) guidelines that must be followed when producing chemicals for pharmaceutical or food applications. These guidelines ensure that products meet specific quality and purity standards.
Transportation of glacial acetic acid is also heavily regulated due to its corrosive nature. Organizations like the Department of Transportation (DOT) in the US and the European Agreement concerning the International Carriage of Dangerous Goods by Road (ADR) in Europe have specific requirements for packaging, labeling, and shipping of acetic acid to prevent accidents and ensure safe transport.
Compliance with these regulations requires companies to implement comprehensive management systems, conduct regular audits, and maintain detailed documentation of their processes and procedures. Failure to comply with these regulations can result in severe penalties, including fines, legal action, and potential shutdown of operations.
As the regulatory landscape continues to evolve, companies must stay informed about changes in regulations and adapt their practices accordingly. This may involve investing in new technologies, updating standard operating procedures, and providing ongoing training to employees to ensure continued compliance with all relevant regulations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!