Glacial Acetic Acid in the Synthesis of Novel Nanostructures
AUG 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Glacial Acetic Acid in Nanostructure Synthesis: Background and Objectives
Glacial acetic acid has emerged as a versatile and powerful tool in the synthesis of novel nanostructures, marking a significant milestone in the field of nanotechnology. This organic compound, known for its high purity and low water content, has been instrumental in advancing the development of nanomaterials with unique properties and applications.
The journey of glacial acetic acid in nanostructure synthesis began in the early 2000s when researchers discovered its potential as a solvent and reactant in various nanofabrication processes. Since then, its use has expanded rapidly, encompassing a wide range of nanostructures including metal nanoparticles, carbon nanotubes, and complex oxide nanoarchitectures.
One of the key advantages of glacial acetic acid lies in its ability to control the morphology and size of nanostructures during synthesis. Its mild acidity and excellent solvating properties allow for precise manipulation of reaction kinetics, leading to the formation of nanostructures with well-defined shapes and sizes. This level of control is crucial for tailoring the properties of nanomaterials for specific applications.
The evolution of glacial acetic acid's role in nanostructure synthesis has been closely tied to advancements in characterization techniques. As analytical methods such as high-resolution transmission electron microscopy (HRTEM) and X-ray diffraction (XRD) have improved, researchers have gained deeper insights into the mechanisms by which glacial acetic acid influences nanostructure formation. This understanding has, in turn, driven further innovations in synthesis protocols.
Recent years have seen a surge in research focused on using glacial acetic acid for the green synthesis of nanostructures. Its relatively low toxicity and biodegradability make it an attractive alternative to more hazardous solvents traditionally used in nanomaterial production. This aligns well with the growing emphasis on sustainable and environmentally friendly manufacturing processes in the nanotechnology sector.
The primary objective of current research on glacial acetic acid in nanostructure synthesis is to expand its applicability across a broader range of materials and to fine-tune its role in controlling nanostructure properties. Researchers are exploring novel reaction conditions, including temperature variations, pressure modulations, and the introduction of additives, to unlock new possibilities in nanostructure design.
Another key goal is to develop scalable synthesis methods that can translate the laboratory success of glacial acetic acid-based processes to industrial-scale production. This involves optimizing reaction parameters for larger batch sizes and addressing challenges related to uniformity and reproducibility in scaled-up syntheses.
The journey of glacial acetic acid in nanostructure synthesis began in the early 2000s when researchers discovered its potential as a solvent and reactant in various nanofabrication processes. Since then, its use has expanded rapidly, encompassing a wide range of nanostructures including metal nanoparticles, carbon nanotubes, and complex oxide nanoarchitectures.
One of the key advantages of glacial acetic acid lies in its ability to control the morphology and size of nanostructures during synthesis. Its mild acidity and excellent solvating properties allow for precise manipulation of reaction kinetics, leading to the formation of nanostructures with well-defined shapes and sizes. This level of control is crucial for tailoring the properties of nanomaterials for specific applications.
The evolution of glacial acetic acid's role in nanostructure synthesis has been closely tied to advancements in characterization techniques. As analytical methods such as high-resolution transmission electron microscopy (HRTEM) and X-ray diffraction (XRD) have improved, researchers have gained deeper insights into the mechanisms by which glacial acetic acid influences nanostructure formation. This understanding has, in turn, driven further innovations in synthesis protocols.
Recent years have seen a surge in research focused on using glacial acetic acid for the green synthesis of nanostructures. Its relatively low toxicity and biodegradability make it an attractive alternative to more hazardous solvents traditionally used in nanomaterial production. This aligns well with the growing emphasis on sustainable and environmentally friendly manufacturing processes in the nanotechnology sector.
The primary objective of current research on glacial acetic acid in nanostructure synthesis is to expand its applicability across a broader range of materials and to fine-tune its role in controlling nanostructure properties. Researchers are exploring novel reaction conditions, including temperature variations, pressure modulations, and the introduction of additives, to unlock new possibilities in nanostructure design.
Another key goal is to develop scalable synthesis methods that can translate the laboratory success of glacial acetic acid-based processes to industrial-scale production. This involves optimizing reaction parameters for larger batch sizes and addressing challenges related to uniformity and reproducibility in scaled-up syntheses.
Market Demand for Novel Nanostructures
The market demand for novel nanostructures synthesized using glacial acetic acid has been steadily increasing across various industries. This growth is primarily driven by the unique properties and potential applications of these nanostructures in fields such as electronics, energy storage, healthcare, and environmental remediation.
In the electronics sector, there is a growing need for advanced materials that can enhance the performance of devices while reducing their size and power consumption. Novel nanostructures offer promising solutions for developing next-generation semiconductors, flexible electronics, and high-capacity data storage devices. The market for these applications is expected to expand significantly in the coming years, fueled by the ongoing digital transformation and the rise of Internet of Things (IoT) technologies.
The energy storage industry is another key driver of demand for novel nanostructures. With the global push towards renewable energy and electric vehicles, there is an urgent need for more efficient and high-capacity energy storage solutions. Nanostructures synthesized using glacial acetic acid have shown potential in improving the performance of batteries and supercapacitors, leading to increased interest from both established energy companies and innovative startups.
In the healthcare sector, novel nanostructures are gaining attention for their potential applications in drug delivery, biosensing, and tissue engineering. The ability to create precisely controlled nanostructures using glacial acetic acid opens up new possibilities for targeted drug delivery systems and advanced diagnostic tools. This has led to increased investment in research and development from pharmaceutical companies and medical device manufacturers.
Environmental remediation is another area where the demand for novel nanostructures is growing. These materials show promise in water purification, air filtration, and pollution control applications. As environmental regulations become stricter and public awareness of ecological issues increases, the market for advanced nanomaterials in this sector is expected to expand rapidly.
The global market for nanotechnology-based products, including those utilizing novel nanostructures, is projected to experience substantial growth in the coming years. This growth is driven by increasing research and development activities, government funding for nanotechnology initiatives, and the expanding range of applications across various industries. As the synthesis methods using glacial acetic acid continue to improve and new applications are discovered, the market demand for these novel nanostructures is likely to accelerate further.
In the electronics sector, there is a growing need for advanced materials that can enhance the performance of devices while reducing their size and power consumption. Novel nanostructures offer promising solutions for developing next-generation semiconductors, flexible electronics, and high-capacity data storage devices. The market for these applications is expected to expand significantly in the coming years, fueled by the ongoing digital transformation and the rise of Internet of Things (IoT) technologies.
The energy storage industry is another key driver of demand for novel nanostructures. With the global push towards renewable energy and electric vehicles, there is an urgent need for more efficient and high-capacity energy storage solutions. Nanostructures synthesized using glacial acetic acid have shown potential in improving the performance of batteries and supercapacitors, leading to increased interest from both established energy companies and innovative startups.
In the healthcare sector, novel nanostructures are gaining attention for their potential applications in drug delivery, biosensing, and tissue engineering. The ability to create precisely controlled nanostructures using glacial acetic acid opens up new possibilities for targeted drug delivery systems and advanced diagnostic tools. This has led to increased investment in research and development from pharmaceutical companies and medical device manufacturers.
Environmental remediation is another area where the demand for novel nanostructures is growing. These materials show promise in water purification, air filtration, and pollution control applications. As environmental regulations become stricter and public awareness of ecological issues increases, the market for advanced nanomaterials in this sector is expected to expand rapidly.
The global market for nanotechnology-based products, including those utilizing novel nanostructures, is projected to experience substantial growth in the coming years. This growth is driven by increasing research and development activities, government funding for nanotechnology initiatives, and the expanding range of applications across various industries. As the synthesis methods using glacial acetic acid continue to improve and new applications are discovered, the market demand for these novel nanostructures is likely to accelerate further.
Current State and Challenges in Nanostructure Synthesis
The synthesis of novel nanostructures using glacial acetic acid has gained significant attention in recent years. Currently, researchers are exploring various approaches to harness the unique properties of this organic compound in nanostructure fabrication. The field has witnessed remarkable progress, with several breakthrough techniques emerging in the past decade.
One of the primary advantages of using glacial acetic acid in nanostructure synthesis is its ability to act as both a solvent and a reactant. This dual functionality allows for more controlled and efficient synthesis processes. Recent studies have demonstrated successful applications in the production of metal oxide nanoparticles, carbon-based nanostructures, and hybrid organic-inorganic nanomaterials.
Despite these advancements, several challenges persist in the field. The precise control of nanostructure morphology and size distribution remains a significant hurdle. Researchers are grappling with issues related to the reproducibility of synthesis methods, especially when scaling up production for industrial applications.
Another critical challenge is the environmental impact of using glacial acetic acid in large-scale nanostructure synthesis. While it is less toxic compared to many other solvents, concerns about its proper disposal and potential ecological effects have been raised. This has led to increased efforts in developing green synthesis methods that minimize the use of harmful chemicals.
The stability of nanostructures synthesized using glacial acetic acid is another area of ongoing research. Some studies have reported issues with long-term stability, particularly in aqueous environments. This poses limitations on potential applications, especially in biomedical and environmental fields where nanostructure integrity over time is crucial.
Researchers are also facing difficulties in achieving uniform surface functionalization of nanostructures synthesized with glacial acetic acid. This challenge impacts the development of nanostructures for specific applications, such as targeted drug delivery or selective catalysis.
The integration of glacial acetic acid-based synthesis methods with other advanced manufacturing techniques, such as 3D printing or microfluidics, presents both opportunities and challenges. While these combinations show promise for creating complex nanostructured materials, they also introduce new variables that need to be carefully controlled and understood.
Lastly, the characterization of nanostructures produced using glacial acetic acid remains a challenge. Existing analytical techniques sometimes struggle to provide accurate information about the internal structure and composition of these nanomaterials, particularly for complex or hybrid nanostructures. This limitation hinders the optimization of synthesis processes and the full understanding of structure-property relationships.
One of the primary advantages of using glacial acetic acid in nanostructure synthesis is its ability to act as both a solvent and a reactant. This dual functionality allows for more controlled and efficient synthesis processes. Recent studies have demonstrated successful applications in the production of metal oxide nanoparticles, carbon-based nanostructures, and hybrid organic-inorganic nanomaterials.
Despite these advancements, several challenges persist in the field. The precise control of nanostructure morphology and size distribution remains a significant hurdle. Researchers are grappling with issues related to the reproducibility of synthesis methods, especially when scaling up production for industrial applications.
Another critical challenge is the environmental impact of using glacial acetic acid in large-scale nanostructure synthesis. While it is less toxic compared to many other solvents, concerns about its proper disposal and potential ecological effects have been raised. This has led to increased efforts in developing green synthesis methods that minimize the use of harmful chemicals.
The stability of nanostructures synthesized using glacial acetic acid is another area of ongoing research. Some studies have reported issues with long-term stability, particularly in aqueous environments. This poses limitations on potential applications, especially in biomedical and environmental fields where nanostructure integrity over time is crucial.
Researchers are also facing difficulties in achieving uniform surface functionalization of nanostructures synthesized with glacial acetic acid. This challenge impacts the development of nanostructures for specific applications, such as targeted drug delivery or selective catalysis.
The integration of glacial acetic acid-based synthesis methods with other advanced manufacturing techniques, such as 3D printing or microfluidics, presents both opportunities and challenges. While these combinations show promise for creating complex nanostructured materials, they also introduce new variables that need to be carefully controlled and understood.
Lastly, the characterization of nanostructures produced using glacial acetic acid remains a challenge. Existing analytical techniques sometimes struggle to provide accurate information about the internal structure and composition of these nanomaterials, particularly for complex or hybrid nanostructures. This limitation hinders the optimization of synthesis processes and the full understanding of structure-property relationships.
Existing Methods Using Glacial Acetic Acid in Synthesis
01 Synthesis of glacial acetic acid nanostructures
Various methods are employed to synthesize glacial acetic acid nanostructures, including chemical vapor deposition, sol-gel processes, and electrospinning. These techniques allow for the creation of nanofibers, nanoparticles, and other nanostructured forms of glacial acetic acid, which can exhibit unique properties compared to bulk materials.- Synthesis of glacial acetic acid nanostructures: Various methods are employed to synthesize glacial acetic acid nanostructures, including chemical vapor deposition, sol-gel processes, and electrospinning. These techniques allow for the creation of nanofibers, nanoparticles, and other nanostructured forms of glacial acetic acid, which can exhibit unique properties compared to bulk materials.
- Applications in catalysis and chemical reactions: Glacial acetic acid nanostructures are utilized as catalysts or catalyst supports in various chemical reactions. Their high surface area and unique properties enhance reaction rates and selectivity. These nanostructures are particularly useful in organic synthesis, esterification reactions, and as components in heterogeneous catalysis systems.
- Incorporation in composite materials: Glacial acetic acid nanostructures are incorporated into composite materials to enhance their properties. These nanocomposites find applications in areas such as polymer reinforcement, coatings, and functional materials. The addition of acetic acid nanostructures can improve mechanical strength, thermal stability, and chemical resistance of the composite materials.
- Use in drug delivery systems: Nanostructures based on glacial acetic acid are explored for potential applications in drug delivery systems. Their ability to encapsulate or carry drug molecules, combined with their biocompatibility and controlled release properties, makes them promising candidates for targeted drug delivery and sustained release formulations.
- Environmental and energy applications: Glacial acetic acid nanostructures are investigated for environmental remediation and energy-related applications. These include their use in water treatment processes, as adsorbents for pollutant removal, and as components in energy storage devices such as supercapacitors or batteries. The high surface area and unique properties of these nanostructures contribute to their effectiveness in these applications.
02 Applications in catalysis and chemical reactions
Glacial acetic acid nanostructures are utilized as catalysts or catalyst supports in various chemical reactions. Their high surface area and unique properties enhance catalytic activity, selectivity, and efficiency in processes such as esterification, acetylation, and oxidation reactions.Expand Specific Solutions03 Use in advanced materials and composites
Glacial acetic acid nanostructures are incorporated into advanced materials and composites to improve their mechanical, thermal, and chemical properties. These nanostructures can enhance the strength, durability, and functionality of polymers, coatings, and other materials used in various industries.Expand Specific Solutions04 Environmental and energy applications
Nanostructures derived from glacial acetic acid find applications in environmental remediation and energy-related technologies. They are used in the development of advanced filtration systems, adsorbents for pollutant removal, and components in energy storage and conversion devices.Expand Specific Solutions05 Characterization and analysis techniques
Various analytical and characterization techniques are employed to study the properties and structure of glacial acetic acid nanostructures. These include electron microscopy, spectroscopy, and surface analysis methods, which provide insights into the morphology, composition, and behavior of these nanomaterials.Expand Specific Solutions
Key Players in Nanostructure Research and Development
The research on Glacial Acetic Acid in nanostructure synthesis is in an early developmental stage, with significant potential for growth. The market size is relatively small but expanding as applications in materials science and nanotechnology increase. The technology's maturity is still evolving, with academic institutions leading research efforts. Key players include Technische Universität München, Northwestern University, and Zhejiang University, focusing on fundamental research. Companies like GlaxoSmithKline and Infineon Technologies are exploring potential industrial applications. The competitive landscape is characterized by collaboration between academia and industry, with a focus on developing novel synthesis methods and exploring diverse applications for these nanostructures.
Donghua University
Technical Solution: Donghua University has developed an innovative approach using glacial acetic acid for the synthesis of polymer-based nanostructures. Their method involves the use of glacial acetic acid as a solvent and catalyst in the polymerization process, enabling the formation of unique nanostructures with controlled architectures[7]. The university's research has focused on the synthesis of nanofibers and nanospheres from various polymers, including polyacrylonitrile (PAN) and polyvinylidene fluoride (PVDF). By manipulating the concentration of glacial acetic acid and reaction conditions, they have achieved precise control over the diameter, morphology, and surface properties of the nanostructures[8]. This technique has been successfully applied in the development of advanced materials for applications such as filtration membranes, energy storage devices, and tissue engineering scaffolds[9].
Strengths: Versatility in synthesizing polymer-based nanostructures, control over nanostructure properties, and potential for diverse applications. Weaknesses: Limited to polymer-based materials and potential challenges in scaling up the production process.
Northwestern University
Technical Solution: Northwestern University has made significant advancements in the use of glacial acetic acid for the synthesis of metal nanoparticles and alloy nanostructures. Their approach utilizes glacial acetic acid as a reducing agent and stabilizer in the synthesis process, allowing for the formation of well-defined nanostructures with controlled size and composition[10]. The university's research has focused on the synthesis of noble metal nanoparticles, such as gold and silver, as well as bimetallic alloy nanostructures. By adjusting the concentration of glacial acetic acid and reaction parameters, they have achieved precise control over the shape and size distribution of the nanoparticles[11]. Additionally, Northwestern University has explored the use of glacial acetic acid in the synthesis of core-shell nanostructures, demonstrating enhanced catalytic properties and stability compared to conventional synthesis methods[12].
Strengths: Precise control over nanoparticle size and composition, versatility in synthesizing various metal and alloy nanostructures, and potential for catalytic applications. Weaknesses: Limited to metal-based nanostructures and potential challenges in large-scale production.
Core Innovations in Glacial Acetic Acid-Based Synthesis
Novel DNA nanostructures that promote cell-cell interaction and use thereof
PatentWO2010040091A1
Innovation
- Development of nucleic acid nanostructures with aptamers bound to specific receptors on immune cells and tumor cells, facilitating targeted interactions between immune cells and tumor cells, including the use of multivalent aptamers and DNA nanostructures to enhance binding affinity and specificity.
nanostructure
PatentWO2025093877A1
Innovation
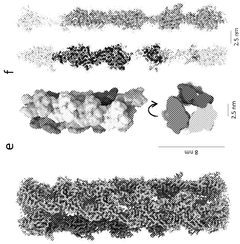
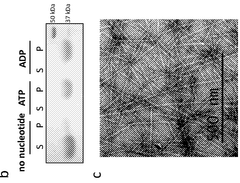
- Development of novel bacterial actin homologues and modified versions that can form nanostructures controlled by ATP presence and hydrolysis, allowing for functionalization and targeted delivery of therapeutic agents.
Environmental Impact and Safety Considerations
The use of Glacial Acetic Acid (GAA) in the synthesis of novel nanostructures presents both environmental and safety challenges that require careful consideration. GAA is a corrosive and flammable substance, necessitating stringent safety protocols during handling and storage. Proper ventilation systems and personal protective equipment are essential to mitigate the risks associated with its vapors, which can cause respiratory irritation and other health issues upon prolonged exposure.
From an environmental perspective, the production and disposal of GAA can have significant impacts. Its manufacture often involves petrochemical processes, contributing to carbon emissions and resource depletion. Improper disposal of GAA or its byproducts can lead to soil and water contamination, potentially harming ecosystems and biodiversity. Therefore, implementing closed-loop systems and efficient recycling methods is crucial to minimize environmental footprint.
In the context of nanostructure synthesis, the environmental impact extends beyond GAA itself. The process may generate nanomaterials with unknown long-term effects on the environment. There is growing concern about the potential for nanoparticles to accumulate in food chains and disrupt ecological balances. Consequently, rigorous risk assessments and lifecycle analyses are necessary to understand and mitigate these potential environmental hazards.
Safety considerations in nanostructure synthesis using GAA also encompass the unique properties of nanomaterials. The small size of nanoparticles increases their reactivity and potential for absorption through skin or inhalation, posing occupational health risks. Implementing robust containment measures, such as glove boxes and specialized filtration systems, is essential to protect researchers and laboratory personnel.
To address these challenges, the development of greener synthesis methods is gaining traction. This includes exploring alternatives to GAA or optimizing processes to reduce its usage. Additionally, the principles of green chemistry are being applied to nanostructure synthesis, focusing on atom economy, energy efficiency, and the use of renewable feedstocks.
Regulatory frameworks are evolving to keep pace with the rapid advancements in nanotechnology. Compliance with these regulations, which often include specific guidelines for nanomaterial handling and disposal, is crucial for research institutions and industries working with GAA in nanostructure synthesis. Furthermore, ongoing research into the environmental fate and toxicology of nanomaterials is essential to inform policy-making and ensure the sustainable development of this technology.
From an environmental perspective, the production and disposal of GAA can have significant impacts. Its manufacture often involves petrochemical processes, contributing to carbon emissions and resource depletion. Improper disposal of GAA or its byproducts can lead to soil and water contamination, potentially harming ecosystems and biodiversity. Therefore, implementing closed-loop systems and efficient recycling methods is crucial to minimize environmental footprint.
In the context of nanostructure synthesis, the environmental impact extends beyond GAA itself. The process may generate nanomaterials with unknown long-term effects on the environment. There is growing concern about the potential for nanoparticles to accumulate in food chains and disrupt ecological balances. Consequently, rigorous risk assessments and lifecycle analyses are necessary to understand and mitigate these potential environmental hazards.
Safety considerations in nanostructure synthesis using GAA also encompass the unique properties of nanomaterials. The small size of nanoparticles increases their reactivity and potential for absorption through skin or inhalation, posing occupational health risks. Implementing robust containment measures, such as glove boxes and specialized filtration systems, is essential to protect researchers and laboratory personnel.
To address these challenges, the development of greener synthesis methods is gaining traction. This includes exploring alternatives to GAA or optimizing processes to reduce its usage. Additionally, the principles of green chemistry are being applied to nanostructure synthesis, focusing on atom economy, energy efficiency, and the use of renewable feedstocks.
Regulatory frameworks are evolving to keep pace with the rapid advancements in nanotechnology. Compliance with these regulations, which often include specific guidelines for nanomaterial handling and disposal, is crucial for research institutions and industries working with GAA in nanostructure synthesis. Furthermore, ongoing research into the environmental fate and toxicology of nanomaterials is essential to inform policy-making and ensure the sustainable development of this technology.
Scalability and Industrial Applications
The scalability and industrial applications of using glacial acetic acid in the synthesis of novel nanostructures present both opportunities and challenges. As research progresses, the potential for large-scale production and diverse industrial uses becomes increasingly apparent.
One of the key advantages of glacial acetic acid in nanostructure synthesis is its relatively low cost and wide availability. This makes it an attractive option for industrial-scale production, potentially reducing manufacturing expenses. Additionally, the mild acidity of glacial acetic acid allows for better control over reaction conditions, which is crucial for maintaining consistency in large-scale synthesis processes.
However, scaling up the production of nanostructures using glacial acetic acid faces several hurdles. The precise control of reaction parameters, such as temperature and concentration, becomes more challenging at larger scales. This can lead to variations in nanostructure size, shape, and quality, which may impact the final product's performance and reliability.
In terms of industrial applications, the nanostructures synthesized using glacial acetic acid show promise in various sectors. In the electronics industry, these nanostructures could be used to develop more efficient and compact components. The energy sector might benefit from improved battery materials or catalysts for fuel cells. Additionally, the medical field could see advancements in drug delivery systems or diagnostic tools utilizing these novel nanostructures.
The environmental impact of large-scale production using glacial acetic acid must also be considered. While acetic acid is generally considered less harmful than many other industrial chemicals, proper waste management and recycling processes need to be implemented to minimize environmental footprint. This aspect is crucial for sustainable industrial adoption.
Research into optimizing reaction conditions and developing more efficient synthesis methods is ongoing. These efforts aim to improve yield, reduce energy consumption, and enhance the uniformity of nanostructures produced at scale. Innovations in reactor design and process automation are also key areas of focus for industrial implementation.
Collaborations between academic institutions and industry partners are driving progress in this field. These partnerships facilitate the transfer of laboratory-scale discoveries to industrial settings, addressing real-world challenges in scalability and application. As research continues, it is likely that new and unexpected applications for these nanostructures will emerge, potentially revolutionizing various industries.
One of the key advantages of glacial acetic acid in nanostructure synthesis is its relatively low cost and wide availability. This makes it an attractive option for industrial-scale production, potentially reducing manufacturing expenses. Additionally, the mild acidity of glacial acetic acid allows for better control over reaction conditions, which is crucial for maintaining consistency in large-scale synthesis processes.
However, scaling up the production of nanostructures using glacial acetic acid faces several hurdles. The precise control of reaction parameters, such as temperature and concentration, becomes more challenging at larger scales. This can lead to variations in nanostructure size, shape, and quality, which may impact the final product's performance and reliability.
In terms of industrial applications, the nanostructures synthesized using glacial acetic acid show promise in various sectors. In the electronics industry, these nanostructures could be used to develop more efficient and compact components. The energy sector might benefit from improved battery materials or catalysts for fuel cells. Additionally, the medical field could see advancements in drug delivery systems or diagnostic tools utilizing these novel nanostructures.
The environmental impact of large-scale production using glacial acetic acid must also be considered. While acetic acid is generally considered less harmful than many other industrial chemicals, proper waste management and recycling processes need to be implemented to minimize environmental footprint. This aspect is crucial for sustainable industrial adoption.
Research into optimizing reaction conditions and developing more efficient synthesis methods is ongoing. These efforts aim to improve yield, reduce energy consumption, and enhance the uniformity of nanostructures produced at scale. Innovations in reactor design and process automation are also key areas of focus for industrial implementation.
Collaborations between academic institutions and industry partners are driving progress in this field. These partnerships facilitate the transfer of laboratory-scale discoveries to industrial settings, addressing real-world challenges in scalability and application. As research continues, it is likely that new and unexpected applications for these nanostructures will emerge, potentially revolutionizing various industries.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!