Use of Glacial Acetic Acid in High-throughput Chemical Analysis
AUG 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Glacial Acetic Acid in Chemical Analysis: Background and Objectives
Glacial acetic acid has been a cornerstone in chemical analysis for decades, playing a crucial role in various analytical techniques. Its unique properties, including high purity and low water content, make it an invaluable solvent and reagent in high-throughput chemical analysis. The evolution of this technology has been driven by the increasing demand for rapid, accurate, and cost-effective analytical methods across industries such as pharmaceuticals, environmental monitoring, and food safety.
The use of glacial acetic acid in high-throughput chemical analysis has its roots in traditional wet chemistry techniques. However, the advent of automated systems and advanced instrumentation has revolutionized its application, enabling the processing of large sample volumes with unprecedented speed and precision. This technological progression has been marked by significant milestones, including the development of flow injection analysis, sequential injection analysis, and lab-on-a-chip devices, all of which have incorporated glacial acetic acid in various capacities.
The primary objective of utilizing glacial acetic acid in high-throughput chemical analysis is to enhance the efficiency and reliability of analytical processes. By leveraging its excellent solvating properties and ability to form stable complexes with many analytes, researchers aim to improve detection limits, increase sample throughput, and reduce interference from matrix effects. Additionally, the use of glacial acetic acid often allows for simpler sample preparation procedures, contributing to overall process optimization.
Current research in this field focuses on several key areas. These include the development of novel analytical methods that exploit the unique characteristics of glacial acetic acid, the integration of green chemistry principles to minimize environmental impact, and the exploration of synergistic effects when combining glacial acetic acid with other reagents or analytical techniques. Furthermore, there is a growing interest in understanding the fundamental interactions between glacial acetic acid and various analytes at the molecular level, which could lead to more targeted and efficient analytical protocols.
As the field of high-throughput chemical analysis continues to evolve, the role of glacial acetic acid is expected to expand and adapt. Emerging trends suggest a move towards miniaturization, increased automation, and the incorporation of artificial intelligence in analytical processes. These advancements are likely to further enhance the utility of glacial acetic acid, potentially leading to breakthroughs in areas such as real-time monitoring, personalized medicine, and advanced materials characterization.
The use of glacial acetic acid in high-throughput chemical analysis has its roots in traditional wet chemistry techniques. However, the advent of automated systems and advanced instrumentation has revolutionized its application, enabling the processing of large sample volumes with unprecedented speed and precision. This technological progression has been marked by significant milestones, including the development of flow injection analysis, sequential injection analysis, and lab-on-a-chip devices, all of which have incorporated glacial acetic acid in various capacities.
The primary objective of utilizing glacial acetic acid in high-throughput chemical analysis is to enhance the efficiency and reliability of analytical processes. By leveraging its excellent solvating properties and ability to form stable complexes with many analytes, researchers aim to improve detection limits, increase sample throughput, and reduce interference from matrix effects. Additionally, the use of glacial acetic acid often allows for simpler sample preparation procedures, contributing to overall process optimization.
Current research in this field focuses on several key areas. These include the development of novel analytical methods that exploit the unique characteristics of glacial acetic acid, the integration of green chemistry principles to minimize environmental impact, and the exploration of synergistic effects when combining glacial acetic acid with other reagents or analytical techniques. Furthermore, there is a growing interest in understanding the fundamental interactions between glacial acetic acid and various analytes at the molecular level, which could lead to more targeted and efficient analytical protocols.
As the field of high-throughput chemical analysis continues to evolve, the role of glacial acetic acid is expected to expand and adapt. Emerging trends suggest a move towards miniaturization, increased automation, and the incorporation of artificial intelligence in analytical processes. These advancements are likely to further enhance the utility of glacial acetic acid, potentially leading to breakthroughs in areas such as real-time monitoring, personalized medicine, and advanced materials characterization.
Market Demand for High-throughput Chemical Analysis
The market demand for high-throughput chemical analysis has been steadily increasing across various industries, driven by the need for faster, more efficient, and cost-effective analytical processes. This demand is particularly pronounced in pharmaceutical research and development, environmental monitoring, food safety testing, and industrial quality control.
In the pharmaceutical sector, high-throughput chemical analysis plays a crucial role in drug discovery and development. The ability to rapidly screen large compound libraries for potential drug candidates has become essential in accelerating the drug development pipeline. This has led to a growing demand for advanced analytical technologies that can handle high sample volumes while maintaining accuracy and precision.
Environmental monitoring agencies and regulatory bodies are also driving the demand for high-throughput chemical analysis. With increasing concerns about environmental pollutants and their impact on human health, there is a pressing need for rapid and reliable analytical methods to detect and quantify a wide range of contaminants in air, water, and soil samples. This has resulted in a surge in demand for automated, high-throughput analytical systems capable of processing large numbers of environmental samples efficiently.
The food and beverage industry is another significant contributor to the market demand for high-throughput chemical analysis. With stringent food safety regulations and growing consumer awareness, food manufacturers and regulatory agencies require rapid and accurate methods for detecting contaminants, additives, and adulterants in food products. High-throughput analytical techniques enable the screening of large batches of food samples, ensuring compliance with safety standards and maintaining consumer trust.
In the industrial sector, quality control processes have become increasingly reliant on high-throughput chemical analysis. Manufacturing companies across various industries, including automotive, electronics, and materials science, require rapid analytical methods to ensure product quality and consistency. This has led to a growing demand for automated analytical systems that can handle high sample volumes and provide real-time results.
The market demand for high-throughput chemical analysis is also being fueled by advancements in analytical instrumentation and data processing capabilities. The integration of artificial intelligence and machine learning algorithms with analytical platforms has enabled faster data analysis and interpretation, further driving the adoption of high-throughput techniques.
As industries continue to prioritize efficiency and productivity, the demand for high-throughput chemical analysis is expected to grow further. This trend is likely to drive innovation in analytical technologies, leading to the development of more advanced, automated, and integrated analytical systems capable of handling increasingly complex analytical challenges across various sectors.
In the pharmaceutical sector, high-throughput chemical analysis plays a crucial role in drug discovery and development. The ability to rapidly screen large compound libraries for potential drug candidates has become essential in accelerating the drug development pipeline. This has led to a growing demand for advanced analytical technologies that can handle high sample volumes while maintaining accuracy and precision.
Environmental monitoring agencies and regulatory bodies are also driving the demand for high-throughput chemical analysis. With increasing concerns about environmental pollutants and their impact on human health, there is a pressing need for rapid and reliable analytical methods to detect and quantify a wide range of contaminants in air, water, and soil samples. This has resulted in a surge in demand for automated, high-throughput analytical systems capable of processing large numbers of environmental samples efficiently.
The food and beverage industry is another significant contributor to the market demand for high-throughput chemical analysis. With stringent food safety regulations and growing consumer awareness, food manufacturers and regulatory agencies require rapid and accurate methods for detecting contaminants, additives, and adulterants in food products. High-throughput analytical techniques enable the screening of large batches of food samples, ensuring compliance with safety standards and maintaining consumer trust.
In the industrial sector, quality control processes have become increasingly reliant on high-throughput chemical analysis. Manufacturing companies across various industries, including automotive, electronics, and materials science, require rapid analytical methods to ensure product quality and consistency. This has led to a growing demand for automated analytical systems that can handle high sample volumes and provide real-time results.
The market demand for high-throughput chemical analysis is also being fueled by advancements in analytical instrumentation and data processing capabilities. The integration of artificial intelligence and machine learning algorithms with analytical platforms has enabled faster data analysis and interpretation, further driving the adoption of high-throughput techniques.
As industries continue to prioritize efficiency and productivity, the demand for high-throughput chemical analysis is expected to grow further. This trend is likely to drive innovation in analytical technologies, leading to the development of more advanced, automated, and integrated analytical systems capable of handling increasingly complex analytical challenges across various sectors.
Current State and Challenges in Glacial Acetic Acid Usage
Glacial acetic acid has become an indispensable component in high-throughput chemical analysis, offering unique properties that facilitate efficient and accurate analytical processes. The current state of its usage reflects a balance between its advantages and the challenges associated with its implementation.
In terms of advantages, glacial acetic acid's high purity and low water content make it an excellent solvent for many organic compounds. Its ability to dissolve a wide range of substances without interfering with analytical results has led to its widespread adoption in chromatography, spectroscopy, and other analytical techniques. The acid's relatively low boiling point also allows for easy removal from samples, minimizing contamination risks in subsequent analytical steps.
However, the use of glacial acetic acid in high-throughput chemical analysis is not without challenges. One significant concern is its corrosive nature, which can lead to equipment degradation over time. This necessitates the use of specialized materials and regular maintenance of analytical instruments, potentially increasing operational costs.
Safety considerations also pose a challenge in the widespread use of glacial acetic acid. Its strong odor and potential for skin and eye irritation require stringent safety protocols and personal protective equipment for laboratory personnel. Proper ventilation systems and handling procedures must be in place to mitigate health risks associated with prolonged exposure.
Another challenge lies in the acid's hygroscopic nature. Glacial acetic acid readily absorbs moisture from the air, which can affect its purity and, consequently, the accuracy of analytical results. This necessitates careful storage and handling practices to maintain the acid's integrity throughout the analytical process.
The environmental impact of glacial acetic acid usage is an emerging concern in the scientific community. While it is biodegradable, improper disposal can lead to localized environmental issues. Developing eco-friendly alternatives or implementing efficient recycling methods remains an ongoing challenge for researchers and industry professionals.
In the context of high-throughput analysis, optimizing the use of glacial acetic acid to achieve maximum efficiency while minimizing consumption is a persistent challenge. Researchers are continually exploring ways to reduce sample volumes and improve analytical methods to decrease the overall acid usage without compromising analytical quality.
Despite these challenges, the current state of glacial acetic acid usage in high-throughput chemical analysis remains robust. Ongoing research focuses on addressing these limitations through innovative approaches, such as developing novel analytical techniques that require smaller sample volumes or exploring alternative solvents that offer similar benefits with reduced drawbacks.
In terms of advantages, glacial acetic acid's high purity and low water content make it an excellent solvent for many organic compounds. Its ability to dissolve a wide range of substances without interfering with analytical results has led to its widespread adoption in chromatography, spectroscopy, and other analytical techniques. The acid's relatively low boiling point also allows for easy removal from samples, minimizing contamination risks in subsequent analytical steps.
However, the use of glacial acetic acid in high-throughput chemical analysis is not without challenges. One significant concern is its corrosive nature, which can lead to equipment degradation over time. This necessitates the use of specialized materials and regular maintenance of analytical instruments, potentially increasing operational costs.
Safety considerations also pose a challenge in the widespread use of glacial acetic acid. Its strong odor and potential for skin and eye irritation require stringent safety protocols and personal protective equipment for laboratory personnel. Proper ventilation systems and handling procedures must be in place to mitigate health risks associated with prolonged exposure.
Another challenge lies in the acid's hygroscopic nature. Glacial acetic acid readily absorbs moisture from the air, which can affect its purity and, consequently, the accuracy of analytical results. This necessitates careful storage and handling practices to maintain the acid's integrity throughout the analytical process.
The environmental impact of glacial acetic acid usage is an emerging concern in the scientific community. While it is biodegradable, improper disposal can lead to localized environmental issues. Developing eco-friendly alternatives or implementing efficient recycling methods remains an ongoing challenge for researchers and industry professionals.
In the context of high-throughput analysis, optimizing the use of glacial acetic acid to achieve maximum efficiency while minimizing consumption is a persistent challenge. Researchers are continually exploring ways to reduce sample volumes and improve analytical methods to decrease the overall acid usage without compromising analytical quality.
Despite these challenges, the current state of glacial acetic acid usage in high-throughput chemical analysis remains robust. Ongoing research focuses on addressing these limitations through innovative approaches, such as developing novel analytical techniques that require smaller sample volumes or exploring alternative solvents that offer similar benefits with reduced drawbacks.
Existing Solutions for High-throughput Chemical Analysis
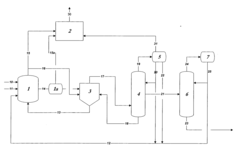
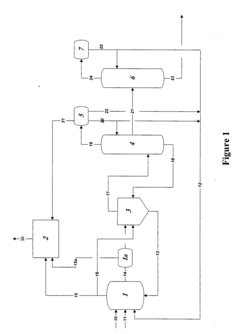
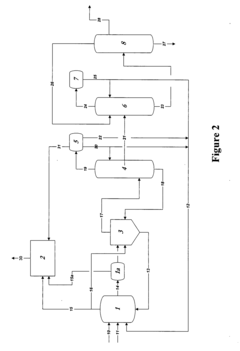
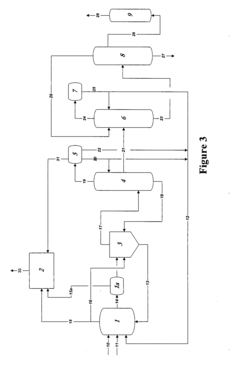
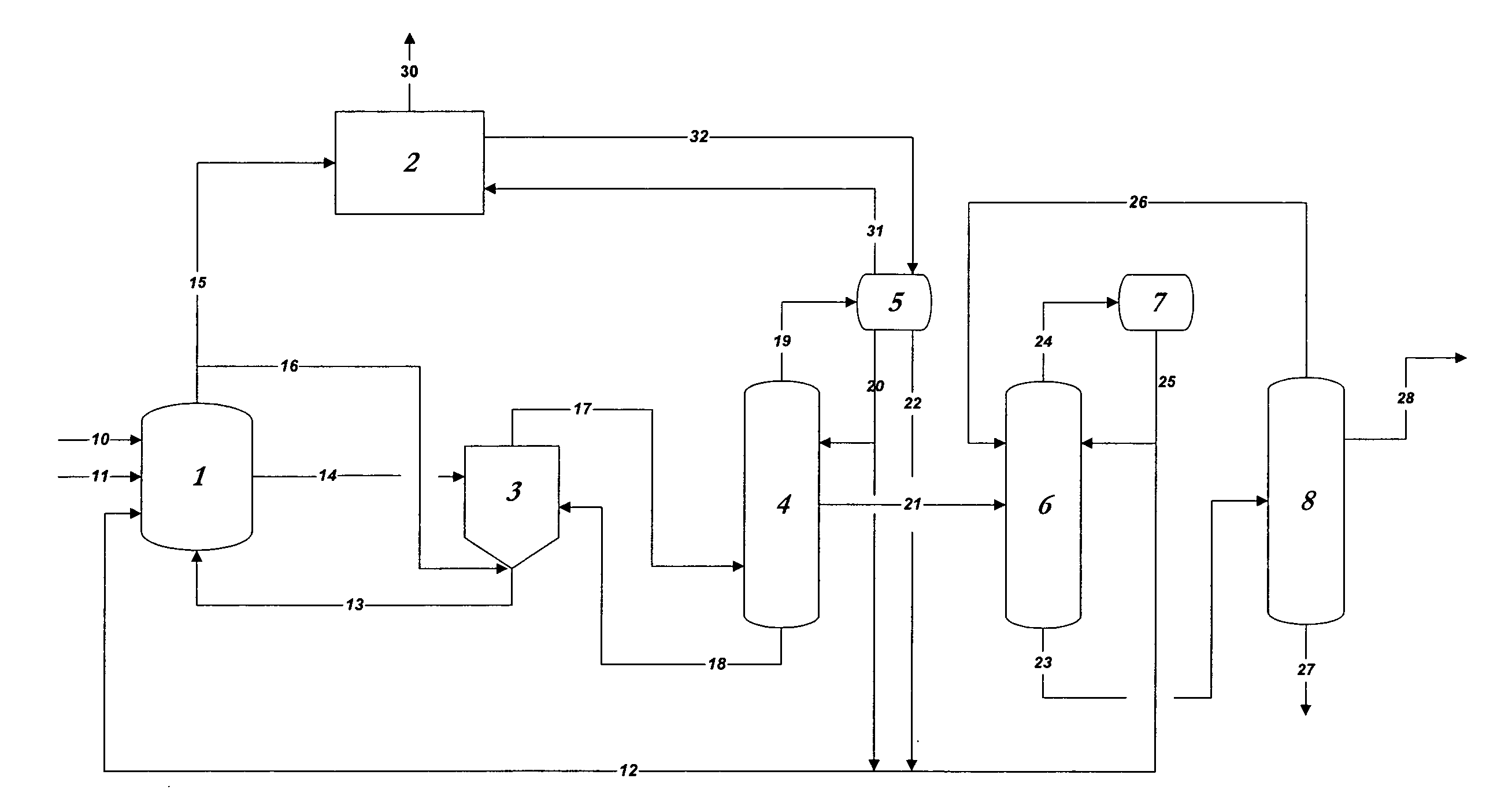
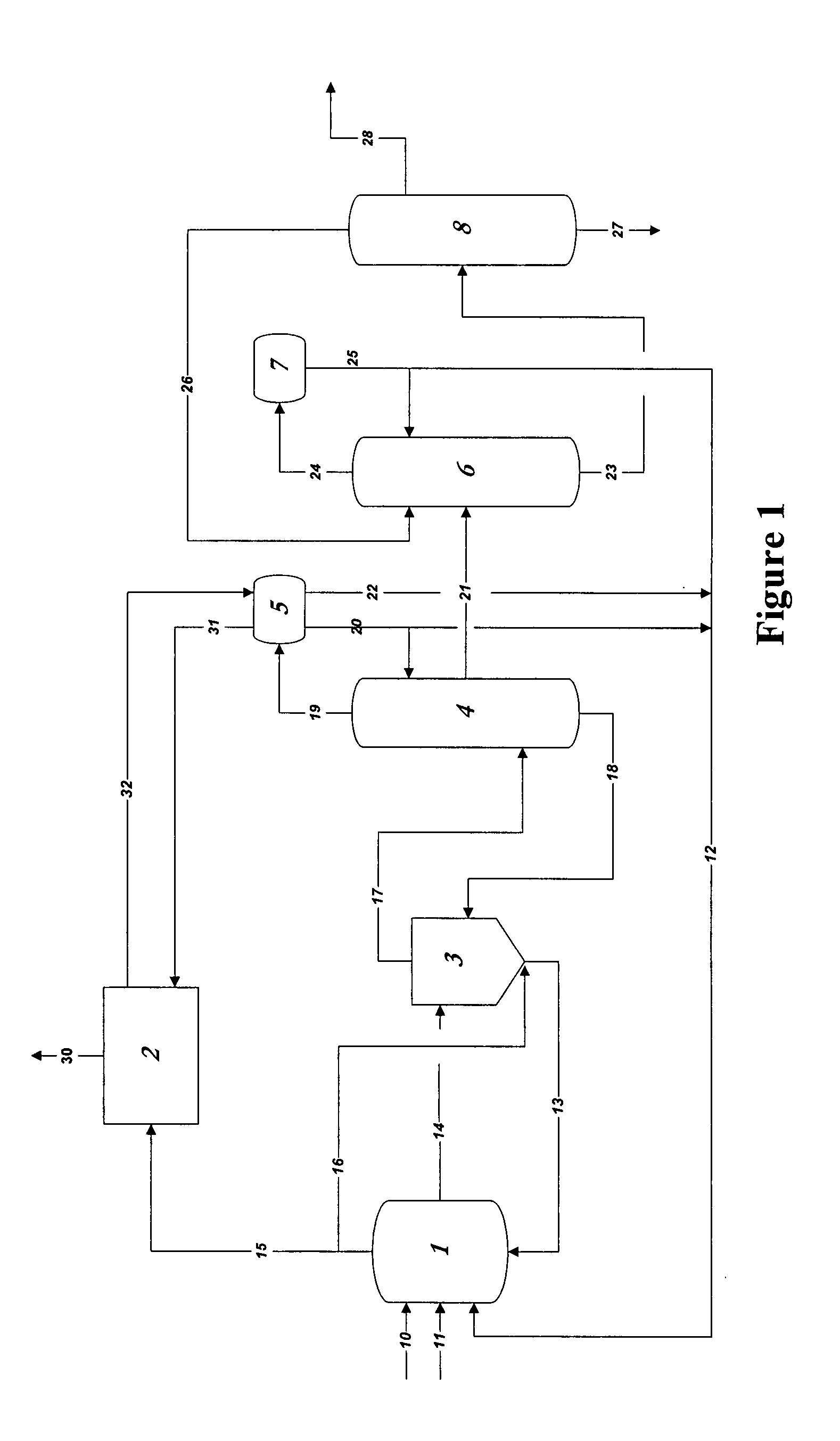
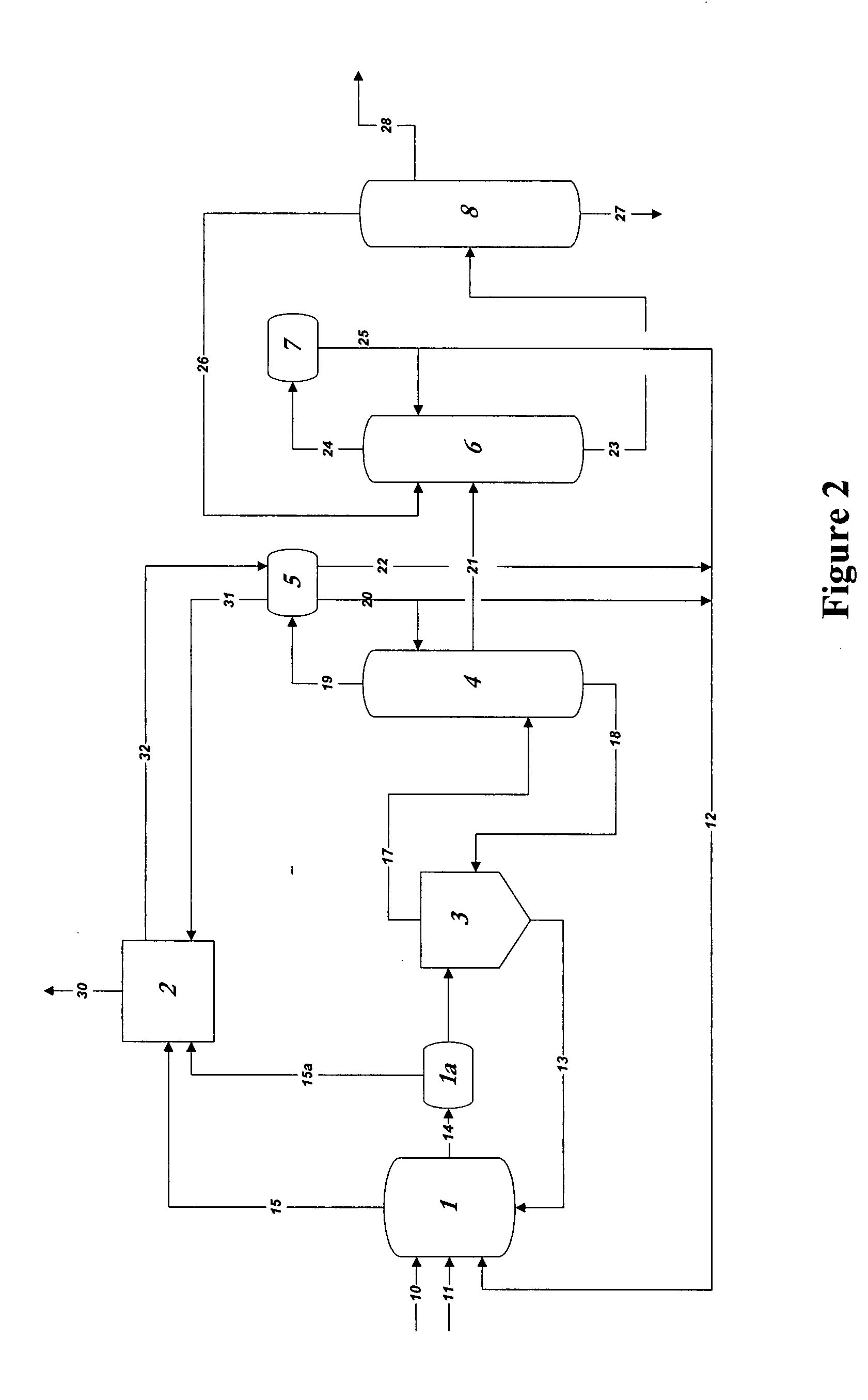
01 Production methods of glacial acetic acid
Various methods are employed to produce glacial acetic acid, including oxidation of acetaldehyde, fermentation processes, and catalytic reactions. These methods often involve specific reaction conditions, catalysts, and purification steps to achieve high purity acetic acid.- Production methods of glacial acetic acid: Various methods are employed to produce glacial acetic acid, including oxidation of acetaldehyde, fermentation processes, and catalytic reactions. These methods often involve specific reaction conditions, catalysts, and purification steps to achieve high purity acetic acid.
- Applications in chemical synthesis: Glacial acetic acid serves as a crucial reagent and solvent in numerous chemical synthesis processes. It is used in the production of various organic compounds, pharmaceuticals, and industrial chemicals, often as an acidifying agent or reaction medium.
- Purification and concentration techniques: Achieving high purity glacial acetic acid requires specialized purification and concentration techniques. These may include distillation, crystallization, and membrane separation processes to remove impurities and increase the acid concentration to near 100%.
- Industrial equipment and processes: Specialized equipment and processes are used in the industrial production and handling of glacial acetic acid. This includes corrosion-resistant materials, safety systems, and specific reactor designs to manage the highly corrosive nature of the acid.
- Safety and environmental considerations: Handling glacial acetic acid requires strict safety measures due to its corrosive nature and potential environmental impact. This includes proper storage, transportation methods, and waste treatment processes to minimize risks and environmental contamination.
02 Applications of glacial acetic acid in chemical synthesis
Glacial acetic acid serves as a versatile reagent and solvent in numerous chemical synthesis processes. It is used in the production of various organic compounds, pharmaceuticals, and industrial chemicals, often acting as an acidifying agent or reaction medium.Expand Specific Solutions03 Purification and concentration techniques
Various techniques are employed to purify and concentrate acetic acid to achieve glacial purity. These may include distillation, crystallization, and membrane separation processes, often involving specialized equipment and precise control of temperature and pressure.Expand Specific Solutions04 Storage and handling of glacial acetic acid
Due to its corrosive nature and specific physical properties, glacial acetic acid requires specialized storage and handling equipment. This includes corrosion-resistant materials, safety measures, and specific designs for tanks, pipes, and transfer systems to ensure safe and efficient handling.Expand Specific Solutions05 Environmental and safety considerations
The production, use, and disposal of glacial acetic acid involve various environmental and safety considerations. This includes emission control, waste treatment, worker protection measures, and emergency response protocols to mitigate risks associated with its corrosive and potentially harmful nature.Expand Specific Solutions
Key Players in Chemical Analysis Industry
The use of glacial acetic acid in high-throughput chemical analysis is in a mature stage of development, with a significant market presence and established applications. The global market for this technology is substantial, driven by increasing demand for efficient analytical methods in pharmaceuticals, biotechnology, and environmental monitoring. Key players like Shimadzu Corp., Waters Technology Corp., and MGI Tech Co., Ltd. have developed advanced systems integrating glacial acetic acid for high-throughput analysis. These companies, along with others such as Wyeth LLC and Novartis AG, are continuously improving the technology's efficiency and accuracy. The competitive landscape is characterized by ongoing innovation and strategic partnerships to enhance analytical capabilities and expand market reach.
Waters Technology Corp.
Technical Solution: Waters Technology Corp. has developed advanced high-throughput liquid chromatography systems that utilize glacial acetic acid as a key component in their mobile phases. Their ACQUITY UPLC system, coupled with mass spectrometry, enables rapid and efficient chemical analysis in pharmaceutical and environmental applications. The company has optimized the use of glacial acetic acid in their eluent systems to enhance peak resolution and sensitivity, particularly for the analysis of polar compounds[1]. They have also introduced automated sample preparation techniques that incorporate glacial acetic acid for improved extraction efficiency and sample cleanup[2]. Waters' technology allows for the analysis of hundreds of samples per day, significantly reducing analysis time and increasing laboratory productivity[3].
Strengths: High-throughput capability, improved sensitivity for polar compounds, and automated sample preparation. Weaknesses: Potential for equipment corrosion due to acetic acid use, and the need for specialized training for system operation.
Shimadzu Corp.
Technical Solution: Shimadzu Corp. has integrated glacial acetic acid into their high-throughput chemical analysis platforms, particularly in their liquid chromatography-mass spectrometry (LC-MS) systems. Their Nexera series UHPLC systems, when used with acetic acid-containing mobile phases, offer exceptional peak resolution and rapid analysis times for complex mixtures[4]. Shimadzu has also developed specialized columns that are compatible with acetic acid-based eluents, enhancing the separation of challenging analytes. Their LabSolutions software suite includes features for optimizing mobile phase composition, including acetic acid concentration, to improve method development efficiency[5]. Additionally, Shimadzu has introduced automated sample preparation modules that incorporate glacial acetic acid for protein precipitation and extraction in bioanalytical applications, further streamlining the high-throughput workflow[6].
Strengths: Comprehensive integrated systems from sample prep to analysis, robust hardware for acetic acid compatibility. Weaknesses: Higher initial investment cost, potential complexity for non-expert users.
Core Innovations in Glacial Acetic Acid Utilization
Control of impurities in product glacial acetic acid of rhodium-catalyzed methanol carbonylation
PatentInactiveUS20090209786A1
Innovation
- A method involving a rhodium-catalyzed methanol carbonylation process with controlled water concentration (0.5-14 wt%), iodide salt, and methyl iodide/methyl acetate levels, followed by treatment with a macroreticular strong-acid cation exchange resin or silver/mercury exchanged cation exchange substrate to reduce impurities in the acetic acid product.
Control of impurities in reaction product of rhodium-catalyzed methanol carbonylation
PatentInactiveUS20080293966A1
Innovation
- A method involving the reaction of methanol, methyl acetate, or dimethyl ether with carbon monoxide in the presence of a rhodium catalyst, maintaining a water concentration of 0.5 to 14 weight percent to control formic acid content in the product acetic acid to 15-160 ppm, and using a silver exchanged cationic ion exchange resin to reduce sulfur levels below 1 ppm.
Safety and Handling Protocols for Glacial Acetic Acid
Glacial acetic acid is a highly corrosive and flammable substance that requires careful handling in high-throughput chemical analysis settings. Proper safety protocols are essential to protect laboratory personnel and maintain the integrity of analytical processes.
Personal protective equipment (PPE) is crucial when working with glacial acetic acid. Laboratory staff must wear chemical-resistant gloves, safety goggles, and a lab coat or chemical-resistant apron. In cases where vapors may be present, a fume hood or respiratory protection should be used.
Storage of glacial acetic acid requires special consideration. It should be kept in tightly sealed containers made of compatible materials such as stainless steel or glass. Storage areas must be well-ventilated and away from sources of heat or ignition. Segregation from incompatible substances, such as oxidizing agents and strong bases, is essential to prevent hazardous reactions.
Handling procedures for glacial acetic acid in high-throughput analysis involve careful transfer techniques. Use of closed systems and automated dispensing equipment can minimize exposure risks. When manual transfers are necessary, proper pouring techniques and the use of secondary containment should be employed to prevent spills.
Emergency response protocols must be in place for accidental spills or exposures. This includes readily accessible eyewash stations and safety showers, as well as spill control materials compatible with acetic acid. Staff should be trained in proper spill cleanup procedures and the use of neutralizing agents.
Waste disposal of glacial acetic acid and its solutions requires adherence to local regulations. Neutralization may be necessary before disposal, and proper documentation of waste streams is essential for regulatory compliance.
Regular safety training and refresher courses should be provided to all personnel working with glacial acetic acid. This training should cover proper handling techniques, PPE use, emergency procedures, and the specific hazards associated with the substance in high-throughput analysis contexts.
Implementing a robust safety culture is critical when using glacial acetic acid. This includes regular safety audits, clear labeling of containers and work areas, and encouraging open communication about safety concerns among laboratory staff.
By adhering to these comprehensive safety and handling protocols, laboratories can effectively mitigate the risks associated with glacial acetic acid use in high-throughput chemical analysis, ensuring both personnel safety and analytical reliability.
Personal protective equipment (PPE) is crucial when working with glacial acetic acid. Laboratory staff must wear chemical-resistant gloves, safety goggles, and a lab coat or chemical-resistant apron. In cases where vapors may be present, a fume hood or respiratory protection should be used.
Storage of glacial acetic acid requires special consideration. It should be kept in tightly sealed containers made of compatible materials such as stainless steel or glass. Storage areas must be well-ventilated and away from sources of heat or ignition. Segregation from incompatible substances, such as oxidizing agents and strong bases, is essential to prevent hazardous reactions.
Handling procedures for glacial acetic acid in high-throughput analysis involve careful transfer techniques. Use of closed systems and automated dispensing equipment can minimize exposure risks. When manual transfers are necessary, proper pouring techniques and the use of secondary containment should be employed to prevent spills.
Emergency response protocols must be in place for accidental spills or exposures. This includes readily accessible eyewash stations and safety showers, as well as spill control materials compatible with acetic acid. Staff should be trained in proper spill cleanup procedures and the use of neutralizing agents.
Waste disposal of glacial acetic acid and its solutions requires adherence to local regulations. Neutralization may be necessary before disposal, and proper documentation of waste streams is essential for regulatory compliance.
Regular safety training and refresher courses should be provided to all personnel working with glacial acetic acid. This training should cover proper handling techniques, PPE use, emergency procedures, and the specific hazards associated with the substance in high-throughput analysis contexts.
Implementing a robust safety culture is critical when using glacial acetic acid. This includes regular safety audits, clear labeling of containers and work areas, and encouraging open communication about safety concerns among laboratory staff.
By adhering to these comprehensive safety and handling protocols, laboratories can effectively mitigate the risks associated with glacial acetic acid use in high-throughput chemical analysis, ensuring both personnel safety and analytical reliability.
Environmental Impact and Sustainability Considerations
The use of glacial acetic acid in high-throughput chemical analysis raises significant environmental and sustainability concerns that warrant careful consideration. As a widely used solvent and reagent, glacial acetic acid's production, handling, and disposal have far-reaching implications for ecosystems and human health.
From a production standpoint, the manufacturing of glacial acetic acid primarily relies on petrochemical processes, contributing to fossil fuel depletion and greenhouse gas emissions. The energy-intensive nature of these processes further exacerbates their environmental footprint. Efforts to develop more sustainable production methods, such as bio-based acetic acid from renewable resources, are ongoing but have yet to achieve widespread commercial adoption.
In laboratory settings, the handling and storage of glacial acetic acid pose potential risks of spills and vapor emissions. These incidents can lead to localized environmental contamination and air quality issues. Proper containment, ventilation systems, and personal protective equipment are essential to mitigate these risks, but they also increase the resource intensity of analytical processes.
The disposal of glacial acetic acid and its waste products presents another significant environmental challenge. Improper disposal can lead to soil and water contamination, disrupting aquatic ecosystems and potentially entering the food chain. Neutralization and dilution are common treatment methods, but they require additional chemicals and water resources, contributing to the overall environmental impact.
From a sustainability perspective, the high-throughput nature of chemical analysis using glacial acetic acid amplifies these concerns. The increased volume of reagent usage and waste generation in large-scale operations necessitates more robust waste management protocols and potentially greater environmental risks.
To address these issues, researchers and industry professionals are exploring alternative solvents and analytical methods that offer comparable performance with reduced environmental impact. Green chemistry principles, such as the use of less hazardous chemicals, increased energy efficiency, and waste reduction, are being applied to develop more sustainable high-throughput analysis techniques.
Additionally, lifecycle assessment studies are being conducted to quantify the environmental impacts of glacial acetic acid use in chemical analysis. These assessments consider factors such as resource depletion, carbon footprint, and ecotoxicity, providing valuable insights for decision-makers and researchers seeking to optimize their analytical processes for sustainability.
As environmental regulations become more stringent, there is a growing impetus for the chemical analysis industry to adopt more sustainable practices. This includes not only the substitution of hazardous substances but also the implementation of closed-loop systems, recycling initiatives, and improved waste treatment technologies to minimize the environmental footprint of high-throughput chemical analysis operations.
From a production standpoint, the manufacturing of glacial acetic acid primarily relies on petrochemical processes, contributing to fossil fuel depletion and greenhouse gas emissions. The energy-intensive nature of these processes further exacerbates their environmental footprint. Efforts to develop more sustainable production methods, such as bio-based acetic acid from renewable resources, are ongoing but have yet to achieve widespread commercial adoption.
In laboratory settings, the handling and storage of glacial acetic acid pose potential risks of spills and vapor emissions. These incidents can lead to localized environmental contamination and air quality issues. Proper containment, ventilation systems, and personal protective equipment are essential to mitigate these risks, but they also increase the resource intensity of analytical processes.
The disposal of glacial acetic acid and its waste products presents another significant environmental challenge. Improper disposal can lead to soil and water contamination, disrupting aquatic ecosystems and potentially entering the food chain. Neutralization and dilution are common treatment methods, but they require additional chemicals and water resources, contributing to the overall environmental impact.
From a sustainability perspective, the high-throughput nature of chemical analysis using glacial acetic acid amplifies these concerns. The increased volume of reagent usage and waste generation in large-scale operations necessitates more robust waste management protocols and potentially greater environmental risks.
To address these issues, researchers and industry professionals are exploring alternative solvents and analytical methods that offer comparable performance with reduced environmental impact. Green chemistry principles, such as the use of less hazardous chemicals, increased energy efficiency, and waste reduction, are being applied to develop more sustainable high-throughput analysis techniques.
Additionally, lifecycle assessment studies are being conducted to quantify the environmental impacts of glacial acetic acid use in chemical analysis. These assessments consider factors such as resource depletion, carbon footprint, and ecotoxicity, providing valuable insights for decision-makers and researchers seeking to optimize their analytical processes for sustainability.
As environmental regulations become more stringent, there is a growing impetus for the chemical analysis industry to adopt more sustainable practices. This includes not only the substitution of hazardous substances but also the implementation of closed-loop systems, recycling initiatives, and improved waste treatment technologies to minimize the environmental footprint of high-throughput chemical analysis operations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!