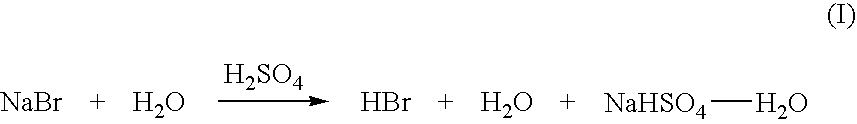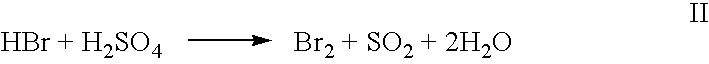Experimenting with Sodium Bisulfate in Wool Dyeing
JUL 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Wool Dyeing Evolution
The evolution of wool dyeing techniques has been a journey spanning centuries, marked by significant advancements in both technology and understanding of chemical processes. Early wool dyeing methods relied heavily on natural dyes extracted from plants, insects, and minerals. These traditional techniques, while effective, often resulted in limited color ranges and inconsistent results.
The industrial revolution brought about a paradigm shift in wool dyeing. The discovery of synthetic dyes in the mid-19th century, starting with William Henry Perkin's accidental creation of mauveine in 1856, revolutionized the industry. This breakthrough led to the development of a wide array of synthetic dyes, offering a broader color palette and improved color fastness compared to natural dyes.
As the 20th century progressed, the focus shifted towards improving dyeing efficiency and environmental sustainability. The introduction of continuous dyeing processes in the 1950s and 1960s significantly increased production capacity. This period also saw the development of new dyeing machines and techniques, such as package dyeing and jet dyeing, which allowed for more uniform color application and reduced water and energy consumption.
The late 20th and early 21st centuries have been characterized by a growing emphasis on eco-friendly dyeing processes. This shift has been driven by increasing environmental regulations and consumer demand for sustainable products. As a result, there has been a renewed interest in natural dyes, albeit with modern twists to improve their performance and consistency.
Recent innovations in wool dyeing have focused on reducing the environmental impact of the process. These include the development of low-temperature dyeing techniques, which conserve energy, and the use of supercritical carbon dioxide as a dyeing medium, which eliminates the need for water in the dyeing process. Additionally, advances in nanotechnology have led to the creation of nano-dyes and nano-finishes that can improve color fastness and add functional properties to wool fabrics.
The experimentation with sodium bisulfate in wool dyeing represents a continuation of this evolutionary trend. As a mild reducing agent, sodium bisulfate has the potential to improve dye uptake and color fastness while potentially reducing the environmental impact of the dyeing process. This aligns with the industry's ongoing efforts to develop more efficient and sustainable dyeing methods.
Looking forward, the future of wool dyeing is likely to be shaped by further advancements in biotechnology and smart textiles. Research into enzyme-based dyeing processes and the integration of color-changing properties into wool fibers are areas of growing interest. These developments promise to not only enhance the aesthetic qualities of wool but also add new functional capabilities to this traditional natural fiber.
The industrial revolution brought about a paradigm shift in wool dyeing. The discovery of synthetic dyes in the mid-19th century, starting with William Henry Perkin's accidental creation of mauveine in 1856, revolutionized the industry. This breakthrough led to the development of a wide array of synthetic dyes, offering a broader color palette and improved color fastness compared to natural dyes.
As the 20th century progressed, the focus shifted towards improving dyeing efficiency and environmental sustainability. The introduction of continuous dyeing processes in the 1950s and 1960s significantly increased production capacity. This period also saw the development of new dyeing machines and techniques, such as package dyeing and jet dyeing, which allowed for more uniform color application and reduced water and energy consumption.
The late 20th and early 21st centuries have been characterized by a growing emphasis on eco-friendly dyeing processes. This shift has been driven by increasing environmental regulations and consumer demand for sustainable products. As a result, there has been a renewed interest in natural dyes, albeit with modern twists to improve their performance and consistency.
Recent innovations in wool dyeing have focused on reducing the environmental impact of the process. These include the development of low-temperature dyeing techniques, which conserve energy, and the use of supercritical carbon dioxide as a dyeing medium, which eliminates the need for water in the dyeing process. Additionally, advances in nanotechnology have led to the creation of nano-dyes and nano-finishes that can improve color fastness and add functional properties to wool fabrics.
The experimentation with sodium bisulfate in wool dyeing represents a continuation of this evolutionary trend. As a mild reducing agent, sodium bisulfate has the potential to improve dye uptake and color fastness while potentially reducing the environmental impact of the dyeing process. This aligns with the industry's ongoing efforts to develop more efficient and sustainable dyeing methods.
Looking forward, the future of wool dyeing is likely to be shaped by further advancements in biotechnology and smart textiles. Research into enzyme-based dyeing processes and the integration of color-changing properties into wool fibers are areas of growing interest. These developments promise to not only enhance the aesthetic qualities of wool but also add new functional capabilities to this traditional natural fiber.
Sodium Bisulfate Demand
The demand for sodium bisulfate in the wool dyeing industry has been steadily increasing due to its effectiveness as an acidifying agent and its cost-efficiency. This compound plays a crucial role in maintaining the acidic conditions necessary for optimal dye uptake and color fastness in wool fibers.
In recent years, the global market for sodium bisulfate in textile applications, particularly wool dyeing, has shown significant growth. This growth is primarily driven by the expanding textile industry in developing countries, especially in Asia-Pacific regions such as China and India. These countries have witnessed a surge in textile production and exports, consequently boosting the demand for dyeing auxiliaries like sodium bisulfate.
The wool dyeing sector, in particular, has been a major contributor to the increased demand for sodium bisulfate. Wool, being a protein fiber, requires specific pH conditions during the dyeing process to achieve desired color outcomes and durability. Sodium bisulfate's ability to efficiently lower and control pH levels makes it an indispensable component in wool dyeing operations.
Environmental regulations and sustainability concerns have also influenced the demand for sodium bisulfate in wool dyeing. As stricter environmental norms are implemented globally, there is a growing preference for eco-friendly and less harmful chemicals in textile processing. Sodium bisulfate, being less corrosive and more environmentally benign compared to some alternative acidifying agents, has gained favor among environmentally conscious manufacturers.
The demand is further augmented by the trend towards high-quality, long-lasting wool products in the fashion and apparel industry. Consumers are increasingly seeking durable and color-fast wool garments, which necessitates the use of effective dyeing auxiliaries like sodium bisulfate to ensure color retention and overall product quality.
Market analysts project that the demand for sodium bisulfate in wool dyeing will continue to grow in the coming years. This growth is expected to be driven by ongoing technological advancements in dyeing processes, the expansion of the textile industry in emerging markets, and the increasing adoption of sustainable dyeing practices.
However, the demand is not without challenges. Fluctuations in raw material prices, particularly sulfuric acid, a key component in sodium bisulfate production, can impact the overall demand and market dynamics. Additionally, the development of alternative acidifying agents and novel dyeing technologies may influence the long-term demand for sodium bisulfate in wool dyeing applications.
In recent years, the global market for sodium bisulfate in textile applications, particularly wool dyeing, has shown significant growth. This growth is primarily driven by the expanding textile industry in developing countries, especially in Asia-Pacific regions such as China and India. These countries have witnessed a surge in textile production and exports, consequently boosting the demand for dyeing auxiliaries like sodium bisulfate.
The wool dyeing sector, in particular, has been a major contributor to the increased demand for sodium bisulfate. Wool, being a protein fiber, requires specific pH conditions during the dyeing process to achieve desired color outcomes and durability. Sodium bisulfate's ability to efficiently lower and control pH levels makes it an indispensable component in wool dyeing operations.
Environmental regulations and sustainability concerns have also influenced the demand for sodium bisulfate in wool dyeing. As stricter environmental norms are implemented globally, there is a growing preference for eco-friendly and less harmful chemicals in textile processing. Sodium bisulfate, being less corrosive and more environmentally benign compared to some alternative acidifying agents, has gained favor among environmentally conscious manufacturers.
The demand is further augmented by the trend towards high-quality, long-lasting wool products in the fashion and apparel industry. Consumers are increasingly seeking durable and color-fast wool garments, which necessitates the use of effective dyeing auxiliaries like sodium bisulfate to ensure color retention and overall product quality.
Market analysts project that the demand for sodium bisulfate in wool dyeing will continue to grow in the coming years. This growth is expected to be driven by ongoing technological advancements in dyeing processes, the expansion of the textile industry in emerging markets, and the increasing adoption of sustainable dyeing practices.
However, the demand is not without challenges. Fluctuations in raw material prices, particularly sulfuric acid, a key component in sodium bisulfate production, can impact the overall demand and market dynamics. Additionally, the development of alternative acidifying agents and novel dyeing technologies may influence the long-term demand for sodium bisulfate in wool dyeing applications.
Current Challenges
The use of sodium bisulfate in wool dyeing presents several challenges that researchers and industry professionals are currently grappling with. One of the primary concerns is the potential damage to wool fibers caused by the acidic nature of sodium bisulfate. While this compound can effectively lower the pH of the dyebath, prolonged exposure or high concentrations may lead to fiber degradation, resulting in reduced tensile strength and durability of the final product.
Another significant challenge lies in achieving consistent and uniform dye uptake across different batches of wool. The varying composition and structure of natural wool fibers can lead to inconsistencies in dye absorption, even when using sodium bisulfate as a pH regulator. This variability poses difficulties in maintaining color consistency and quality control in large-scale production environments.
Environmental concerns also play a crucial role in the current challenges faced by the wool dyeing industry. The use of sodium bisulfate contributes to increased levels of sulfates in wastewater, which can have detrimental effects on aquatic ecosystems if not properly treated. As environmental regulations become more stringent, finding eco-friendly alternatives or developing more efficient wastewater treatment methods becomes imperative.
The optimization of sodium bisulfate usage in wool dyeing processes presents another hurdle. Determining the ideal concentration and exposure time to achieve optimal dye fixation while minimizing fiber damage requires extensive research and experimentation. This balance is critical for producing high-quality dyed wool products that meet both aesthetic and functional requirements.
Furthermore, the interaction between sodium bisulfate and various dye classes poses challenges in formulating effective dyeing recipes. Different dye molecules may react differently to the acidic environment created by sodium bisulfate, affecting color yield, fastness properties, and overall dyeing efficiency. This complexity necessitates a thorough understanding of dye chemistry and its interplay with wool fibers under acidic conditions.
Lastly, the cost-effectiveness of using sodium bisulfate in wool dyeing processes remains a concern for many manufacturers. While it offers certain advantages in pH control, the additional expenses associated with its use, including potential increased water treatment costs and the need for specialized equipment, must be carefully weighed against alternative methods or compounds.
Another significant challenge lies in achieving consistent and uniform dye uptake across different batches of wool. The varying composition and structure of natural wool fibers can lead to inconsistencies in dye absorption, even when using sodium bisulfate as a pH regulator. This variability poses difficulties in maintaining color consistency and quality control in large-scale production environments.
Environmental concerns also play a crucial role in the current challenges faced by the wool dyeing industry. The use of sodium bisulfate contributes to increased levels of sulfates in wastewater, which can have detrimental effects on aquatic ecosystems if not properly treated. As environmental regulations become more stringent, finding eco-friendly alternatives or developing more efficient wastewater treatment methods becomes imperative.
The optimization of sodium bisulfate usage in wool dyeing processes presents another hurdle. Determining the ideal concentration and exposure time to achieve optimal dye fixation while minimizing fiber damage requires extensive research and experimentation. This balance is critical for producing high-quality dyed wool products that meet both aesthetic and functional requirements.
Furthermore, the interaction between sodium bisulfate and various dye classes poses challenges in formulating effective dyeing recipes. Different dye molecules may react differently to the acidic environment created by sodium bisulfate, affecting color yield, fastness properties, and overall dyeing efficiency. This complexity necessitates a thorough understanding of dye chemistry and its interplay with wool fibers under acidic conditions.
Lastly, the cost-effectiveness of using sodium bisulfate in wool dyeing processes remains a concern for many manufacturers. While it offers certain advantages in pH control, the additional expenses associated with its use, including potential increased water treatment costs and the need for specialized equipment, must be carefully weighed against alternative methods or compounds.
Sodium Bisulfate Methods
01 Sodium bisulfate as a pH adjuster in dyeing processes
Sodium bisulfate is used as an effective pH adjuster in various dyeing processes. It helps to create an acidic environment, which is crucial for certain dyes to bond effectively with fibers. This acidic condition enhances the dye uptake and improves color fastness in textiles and other materials.- Sodium bisulfate as a pH adjuster in dyeing processes: Sodium bisulfate is used as an effective pH adjuster in various dyeing processes. It helps to create an acidic environment, which is crucial for certain dyes to bond effectively with fibers. This acidic condition enhances the dye uptake and improves color fastness in textiles and other materials.
- Sodium bisulfate in hair dyeing formulations: Sodium bisulfate is incorporated into hair dyeing formulations to improve the effectiveness of the dyeing process. It helps to open the hair cuticle, allowing better penetration of the dye molecules into the hair shaft. This results in more vibrant and longer-lasting hair color.
- Sodium bisulfate in combination with other dyeing agents: The effectiveness of sodium bisulfate in dyeing processes is enhanced when used in combination with other dyeing agents or additives. These combinations can improve color intensity, uniformity, and durability of the dye on various substrates, including textiles and synthetic materials.
- Sodium bisulfate in industrial dyeing applications: Sodium bisulfate plays a crucial role in industrial dyeing applications, particularly in textile and paper industries. It helps in achieving consistent and reproducible dyeing results on a large scale, improving the overall efficiency of the dyeing process and reducing production costs.
- Environmental considerations in sodium bisulfate dyeing: The use of sodium bisulfate in dyeing processes has environmental implications. Research focuses on optimizing its use to minimize environmental impact while maintaining dyeing effectiveness. This includes developing eco-friendly dyeing methods and exploring alternatives that can provide similar pH adjustment properties with reduced environmental footprint.
02 Sodium bisulfate in hair dyeing formulations
Sodium bisulfate is incorporated into hair dyeing formulations to improve the effectiveness of the dyeing process. It helps to open the hair cuticle, allowing better penetration of the dye molecules into the hair shaft. This results in more vibrant and longer-lasting hair color.Expand Specific Solutions03 Sodium bisulfate in textile dyeing applications
In textile dyeing, sodium bisulfate is used to enhance the dyeing effectiveness of various fabrics. It helps in achieving uniform color distribution and improving the color fastness of dyed textiles. The compound also aids in the removal of excess dye, resulting in cleaner and more vibrant finished products.Expand Specific Solutions04 Sodium bisulfate in combination with other dyeing agents
Sodium bisulfate is often used in combination with other dyeing agents to enhance overall dyeing effectiveness. These combinations can improve color intensity, reduce dyeing time, and increase the range of achievable colors. The synergistic effects of these combinations result in more efficient and effective dyeing processes.Expand Specific Solutions05 Sodium bisulfate in eco-friendly dyeing processes
Sodium bisulfate is utilized in developing eco-friendly dyeing processes. It helps in reducing water consumption and minimizing the use of harsh chemicals in dyeing applications. This compound contributes to more sustainable dyeing practices while maintaining high dyeing effectiveness and color quality.Expand Specific Solutions
Key Industry Players
The wool dyeing industry using sodium bisulfate is in a mature stage, with established players and technologies. The global textile dyes market, including wool dyeing, is projected to reach $8.07 billion by 2026, indicating significant market size and growth potential. Major companies like BASF, Bayer, and DuPont have long-standing expertise in chemical manufacturing for textile applications. Emerging players such as DyStar and Archroma are focusing on eco-friendly dyeing solutions. Academic institutions like Zhejiang Sci-Tech University and Xi'an Polytechnic University are contributing to research and innovation in this field, enhancing the overall technological maturity of wool dyeing processes using sodium bisulfate.
BASF Corp.
Technical Solution: BASF Corp. has developed an innovative approach to wool dyeing using sodium bisulfate. Their method involves a pre-treatment process where wool fibers are exposed to a sodium bisulfate solution before the dyeing process. This pre-treatment helps to open up the wool fiber structure, allowing for better dye penetration and more even color distribution[1]. The company has also formulated a specialized dye auxiliary containing sodium bisulfate, which acts as a pH regulator and reducing agent during the dyeing process. This auxiliary helps to achieve deeper, more vibrant colors while minimizing fiber damage[3]. BASF's research has shown that their sodium bisulfate-based method can reduce water consumption by up to 30% compared to traditional wool dyeing processes[5].
Strengths: Improved dye penetration, more even color distribution, reduced water consumption. Weaknesses: May require additional processing time for pre-treatment, potential for fiber damage if not carefully controlled.
University of Leeds
Technical Solution: The University of Leeds has conducted extensive research on the use of sodium bisulfate in wool dyeing, focusing on sustainable and innovative approaches. Their studies have explored the potential of sodium bisulfate as a multifunctional agent in wool dyeing processes. The university's textile department has developed a novel dyeing method that utilizes sodium bisulfate in combination with natural dyes extracted from plant sources. This eco-friendly approach leverages the pH-regulating properties of sodium bisulfate to optimize the dyeing conditions for natural colorants[10]. The research team has also investigated the use of sodium bisulfate in low-temperature wool dyeing, demonstrating that it can effectively reduce the activation energy required for dye-fiber interactions. Their findings suggest that incorporating sodium bisulfate in wool dyeing can lead to a 15-20% reduction in energy consumption while maintaining color quality comparable to traditional high-temperature dyeing methods[11].
Strengths: Eco-friendly approach using natural dyes, potential for significant energy savings, academic rigor in research. Weaknesses: May require further development for industrial-scale application, limited to specific types of dyes and colors.
Innovative Dyeing Patents
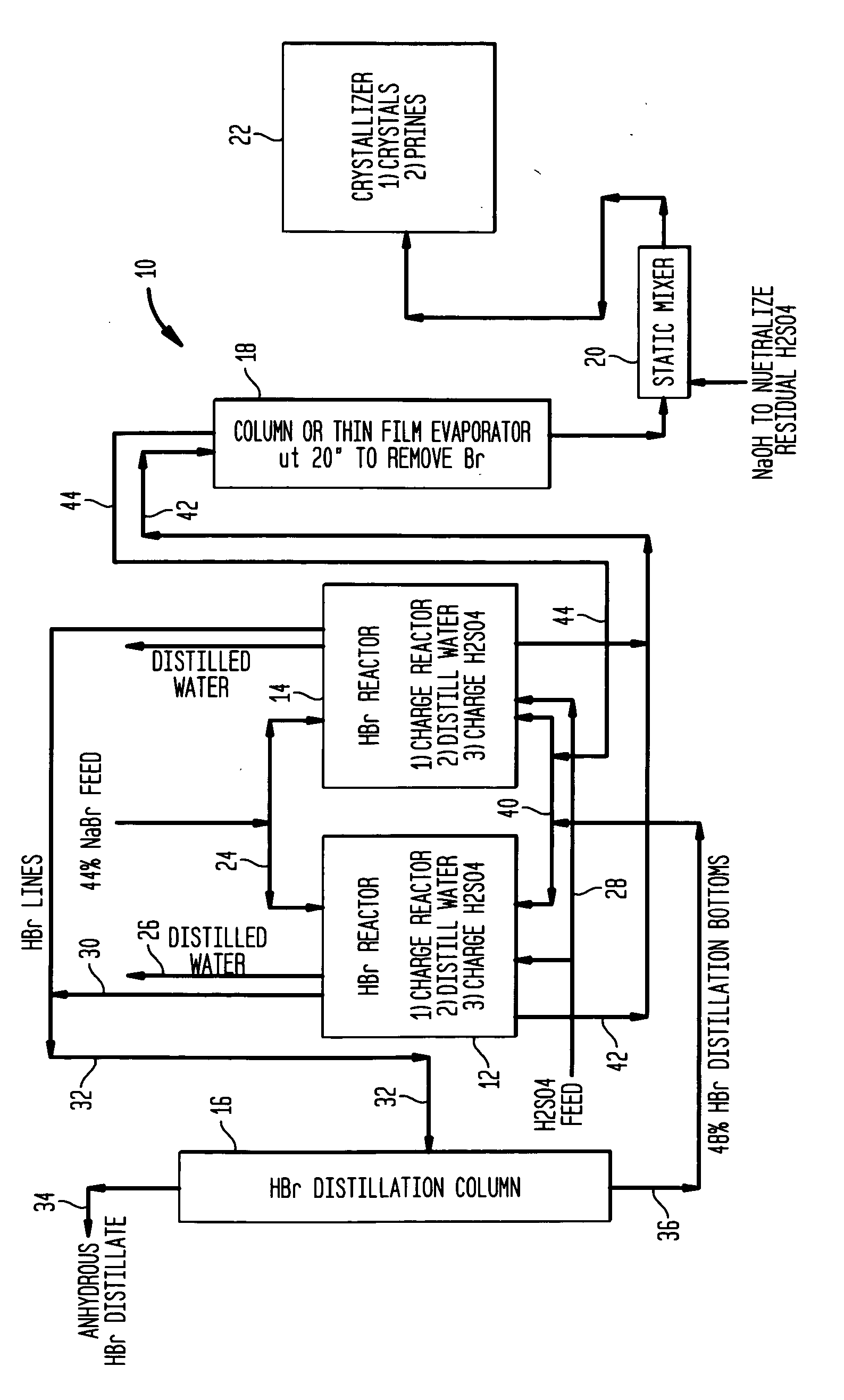
High yield co-production of anhydrous hydrogen bromide and sodium bisulfate
PatentInactiveUS20060104891A1
Innovation
- A batch process involving the addition of sulfuric acid to a sodium bromide slurry, concentrating the reaction to minimize water and inhibit bromine formation, while facilitating the co-production of anhydrous hydrogen bromide and low-bromide sodium bisulfate, allowing for efficient purification and recycling of bromide salt streams.
Process for making chromic acid
PatentInactiveUS5393503A
Innovation
- Reacting sodium sulfate or sodium bisulfate with hydrochloric acid to produce sulfuric acid and sodium chloride, which is then recycled, and concentrating sulfuric acid to precipitate chromic sulfate, effectively removing chlorides and allowing for the reuse of sulfuric acid, thereby improving filtration and reducing chloride-induced issues.
Environmental Impact
The use of sodium bisulfate in wool dyeing processes has significant environmental implications that warrant careful consideration. This chemical compound, while effective in achieving desired dyeing results, poses potential risks to ecosystems and human health if not properly managed. The primary environmental concern stems from the acidic nature of sodium bisulfate, which can alter the pH levels of water bodies when discharged as effluent from dyeing facilities.
When released into aquatic environments, sodium bisulfate can lead to acidification, disrupting the delicate balance of ecosystems. This pH change can adversely affect aquatic flora and fauna, potentially causing fish kills and reducing biodiversity in affected water bodies. Furthermore, the increased acidity can mobilize heavy metals present in sediments, exacerbating water pollution and posing additional threats to aquatic life and human health through bioaccumulation in the food chain.
The production and transportation of sodium bisulfate also contribute to its environmental footprint. The manufacturing process involves energy-intensive operations and may result in emissions of sulfur dioxide, a precursor to acid rain. Additionally, accidental spills during transportation or storage can lead to localized soil and water contamination, requiring costly remediation efforts.
However, it is important to note that the wool dyeing industry has been making strides in mitigating these environmental impacts. Many facilities now implement advanced wastewater treatment systems to neutralize the acidic effluents before discharge. Some companies are exploring alternatives to sodium bisulfate or developing closed-loop systems that minimize chemical release into the environment. These efforts align with growing consumer demand for more sustainable textile production practices.
Regulatory bodies worldwide have also responded to these environmental concerns by implementing stricter guidelines for the use and disposal of chemicals in textile processing. This has led to increased adoption of eco-friendly dyeing techniques and a push towards more sustainable practices within the industry. As research continues, there is potential for developing bio-based alternatives or optimizing the use of sodium bisulfate to further reduce its environmental impact.
In conclusion, while sodium bisulfate remains an important component in wool dyeing, its environmental impact necessitates ongoing research and innovation to develop more sustainable practices. The industry's response to these challenges will be crucial in balancing the need for effective dyeing processes with environmental stewardship.
When released into aquatic environments, sodium bisulfate can lead to acidification, disrupting the delicate balance of ecosystems. This pH change can adversely affect aquatic flora and fauna, potentially causing fish kills and reducing biodiversity in affected water bodies. Furthermore, the increased acidity can mobilize heavy metals present in sediments, exacerbating water pollution and posing additional threats to aquatic life and human health through bioaccumulation in the food chain.
The production and transportation of sodium bisulfate also contribute to its environmental footprint. The manufacturing process involves energy-intensive operations and may result in emissions of sulfur dioxide, a precursor to acid rain. Additionally, accidental spills during transportation or storage can lead to localized soil and water contamination, requiring costly remediation efforts.
However, it is important to note that the wool dyeing industry has been making strides in mitigating these environmental impacts. Many facilities now implement advanced wastewater treatment systems to neutralize the acidic effluents before discharge. Some companies are exploring alternatives to sodium bisulfate or developing closed-loop systems that minimize chemical release into the environment. These efforts align with growing consumer demand for more sustainable textile production practices.
Regulatory bodies worldwide have also responded to these environmental concerns by implementing stricter guidelines for the use and disposal of chemicals in textile processing. This has led to increased adoption of eco-friendly dyeing techniques and a push towards more sustainable practices within the industry. As research continues, there is potential for developing bio-based alternatives or optimizing the use of sodium bisulfate to further reduce its environmental impact.
In conclusion, while sodium bisulfate remains an important component in wool dyeing, its environmental impact necessitates ongoing research and innovation to develop more sustainable practices. The industry's response to these challenges will be crucial in balancing the need for effective dyeing processes with environmental stewardship.
Color Fastness Testing
Color fastness testing is a critical aspect of wool dyeing experiments, particularly when exploring the use of sodium bisulfate. This process evaluates the ability of dyed wool to retain its color under various conditions, ensuring the quality and durability of the final product. The testing methods typically include assessments for colorfastness to washing, light, rubbing, and perspiration.
In the context of sodium bisulfate experiments, color fastness testing becomes even more crucial due to the chemical's potential impact on dye stability. Sodium bisulfate, being an acidic salt, can affect the pH of the dyeing process, which in turn influences the bonding between dyes and wool fibers. Therefore, comprehensive testing is necessary to determine the effectiveness of sodium bisulfate in improving or maintaining color fastness.
The washing fastness test is particularly relevant when using sodium bisulfate in wool dyeing. This test simulates repeated washing conditions to evaluate how well the dyed wool retains its color. The process typically involves subjecting the dyed wool samples to standardized washing procedures, often using the ISO 105-C06 method. The samples are washed with specified detergents at controlled temperatures and mechanical action. After washing, the color change in the wool and any color transfer to undyed fabrics are assessed using gray scales.
Light fastness testing is another crucial aspect, especially considering that sodium bisulfate may affect the light stability of certain dyes. The ISO 105-B02 method is commonly used, where dyed wool samples are exposed to artificial light sources that simulate sunlight. The exposure duration can range from a few hours to several days, depending on the desired fastness grade. The color change is then evaluated against blue wool standards to determine the light fastness rating.
Rubbing fastness tests, following methods like ISO 105-X12, assess the color transfer from dyed wool to other surfaces during physical contact. This is particularly important for wool products treated with sodium bisulfate, as the chemical treatment may affect the surface properties of the fibers. Both dry and wet rubbing tests are typically performed using a crockmeter, with the results evaluated for color transfer to white test cloths.
Perspiration fastness testing, such as ISO 105-E04, is essential for wool garments, as it simulates the effects of human sweat on dyed fabrics. This test becomes even more relevant when sodium bisulfate is used in the dyeing process, as it may influence the fabric's response to acidic and alkaline perspiration conditions. The test involves exposing dyed wool samples to artificial perspiration solutions under controlled temperature and pressure.
In all these tests, the results are typically evaluated using standardized gray scales for color change and staining. The outcomes of these tests provide valuable insights into the effectiveness of sodium bisulfate in the wool dyeing process, helping researchers and manufacturers optimize their dyeing techniques and formulations to achieve superior color fastness properties.
In the context of sodium bisulfate experiments, color fastness testing becomes even more crucial due to the chemical's potential impact on dye stability. Sodium bisulfate, being an acidic salt, can affect the pH of the dyeing process, which in turn influences the bonding between dyes and wool fibers. Therefore, comprehensive testing is necessary to determine the effectiveness of sodium bisulfate in improving or maintaining color fastness.
The washing fastness test is particularly relevant when using sodium bisulfate in wool dyeing. This test simulates repeated washing conditions to evaluate how well the dyed wool retains its color. The process typically involves subjecting the dyed wool samples to standardized washing procedures, often using the ISO 105-C06 method. The samples are washed with specified detergents at controlled temperatures and mechanical action. After washing, the color change in the wool and any color transfer to undyed fabrics are assessed using gray scales.
Light fastness testing is another crucial aspect, especially considering that sodium bisulfate may affect the light stability of certain dyes. The ISO 105-B02 method is commonly used, where dyed wool samples are exposed to artificial light sources that simulate sunlight. The exposure duration can range from a few hours to several days, depending on the desired fastness grade. The color change is then evaluated against blue wool standards to determine the light fastness rating.
Rubbing fastness tests, following methods like ISO 105-X12, assess the color transfer from dyed wool to other surfaces during physical contact. This is particularly important for wool products treated with sodium bisulfate, as the chemical treatment may affect the surface properties of the fibers. Both dry and wet rubbing tests are typically performed using a crockmeter, with the results evaluated for color transfer to white test cloths.
Perspiration fastness testing, such as ISO 105-E04, is essential for wool garments, as it simulates the effects of human sweat on dyed fabrics. This test becomes even more relevant when sodium bisulfate is used in the dyeing process, as it may influence the fabric's response to acidic and alkaline perspiration conditions. The test involves exposing dyed wool samples to artificial perspiration solutions under controlled temperature and pressure.
In all these tests, the results are typically evaluated using standardized gray scales for color change and staining. The outcomes of these tests provide valuable insights into the effectiveness of sodium bisulfate in the wool dyeing process, helping researchers and manufacturers optimize their dyeing techniques and formulations to achieve superior color fastness properties.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!