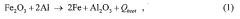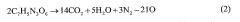Exploring the Chemical Properties of Thermite Compounds
JUN 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Thermite Compounds Background and Objectives
Thermite compounds have been a subject of fascination and study in the field of chemistry for over a century. These highly exothermic mixtures, typically consisting of a metal powder fuel and a metal oxide, have played significant roles in various applications, from industrial welding to pyrotechnics. The history of thermite reactions can be traced back to 1893 when German chemist Hans Goldschmidt discovered the process, initially patenting it for producing pure metals.
The evolution of thermite technology has been driven by the unique properties these compounds exhibit. Characterized by their ability to generate extremely high temperatures, often exceeding 2500°C, thermites have found applications in areas where localized, intense heat is required. This has led to their use in welding railway tracks, incendiary devices, and even in space exploration for satellite separation mechanisms.
As research in this field progresses, the objectives of exploring thermite compounds have expanded beyond their traditional applications. Modern investigations aim to understand the fundamental chemical and physical processes occurring during thermite reactions at a molecular level. This includes studying reaction kinetics, heat transfer mechanisms, and the formation of reaction products under various conditions.
One of the key goals in current thermite research is to develop more efficient and controllable reactions. This involves exploring new combinations of fuels and oxidizers, as well as investigating the effects of particle size, mixing ratios, and additives on reaction characteristics. Researchers are also focusing on enhancing the safety aspects of thermite compounds, given their potential hazards due to their high reactivity and heat generation.
Another significant objective is to expand the application spectrum of thermite compounds. This includes their potential use in advanced materials synthesis, where the high temperatures and rapid cooling rates can lead to the formation of novel materials with unique properties. Additionally, there is growing interest in harnessing the energy released during thermite reactions for power generation or as a heat source in extreme environments.
Environmental considerations have also become a crucial aspect of thermite research. Scientists are exploring ways to make these reactions more environmentally friendly by developing green thermites that produce less toxic byproducts. This aligns with the broader trend in chemistry towards sustainable and eco-friendly processes.
As we look to the future, the exploration of thermite compounds continues to evolve, driven by advancements in analytical techniques and computational modeling. These tools allow for more precise control and understanding of thermite reactions, opening up new possibilities for their application in various fields of science and technology.
The evolution of thermite technology has been driven by the unique properties these compounds exhibit. Characterized by their ability to generate extremely high temperatures, often exceeding 2500°C, thermites have found applications in areas where localized, intense heat is required. This has led to their use in welding railway tracks, incendiary devices, and even in space exploration for satellite separation mechanisms.
As research in this field progresses, the objectives of exploring thermite compounds have expanded beyond their traditional applications. Modern investigations aim to understand the fundamental chemical and physical processes occurring during thermite reactions at a molecular level. This includes studying reaction kinetics, heat transfer mechanisms, and the formation of reaction products under various conditions.
One of the key goals in current thermite research is to develop more efficient and controllable reactions. This involves exploring new combinations of fuels and oxidizers, as well as investigating the effects of particle size, mixing ratios, and additives on reaction characteristics. Researchers are also focusing on enhancing the safety aspects of thermite compounds, given their potential hazards due to their high reactivity and heat generation.
Another significant objective is to expand the application spectrum of thermite compounds. This includes their potential use in advanced materials synthesis, where the high temperatures and rapid cooling rates can lead to the formation of novel materials with unique properties. Additionally, there is growing interest in harnessing the energy released during thermite reactions for power generation or as a heat source in extreme environments.
Environmental considerations have also become a crucial aspect of thermite research. Scientists are exploring ways to make these reactions more environmentally friendly by developing green thermites that produce less toxic byproducts. This aligns with the broader trend in chemistry towards sustainable and eco-friendly processes.
As we look to the future, the exploration of thermite compounds continues to evolve, driven by advancements in analytical techniques and computational modeling. These tools allow for more precise control and understanding of thermite reactions, opening up new possibilities for their application in various fields of science and technology.
Industrial Applications and Market Analysis
Thermite compounds have found extensive applications across various industrial sectors, driven by their unique exothermic properties and ability to generate high temperatures rapidly. The market for thermite-based products and technologies has shown steady growth in recent years, particularly in the metallurgy, welding, and defense industries.
In the metallurgy sector, thermite reactions are widely used for metal purification and alloying processes. The ability of thermite compounds to produce high-purity metals through reduction reactions has made them invaluable in the production of specialty alloys and high-performance materials. This application has seen increased demand in aerospace and automotive industries, where lightweight yet strong materials are crucial.
The welding industry has also embraced thermite technology, particularly for large-scale welding operations such as railway track joining and pipeline repairs. Thermite welding offers advantages in remote locations or where traditional welding equipment is impractical. The market for thermite welding kits and consumables has expanded, driven by infrastructure development projects and the need for efficient, on-site welding solutions.
In the defense sector, thermite compounds play a critical role in incendiary devices and pyrotechnics. The high heat generation and rapid reaction rates make them suitable for various military applications, including armor-piercing munitions and emergency destruction of sensitive equipment. This segment of the market is influenced by geopolitical factors and defense spending trends.
The construction industry has found niche applications for thermite compounds in demolition and controlled building implosions. The precise and powerful nature of thermite reactions allows for targeted structural weakening, offering an alternative to traditional explosive methods in certain scenarios.
Emerging applications in the energy sector are also contributing to market growth. Research into thermite-based thermal batteries and energy storage systems shows promise for high-temperature industrial processes and emergency power systems.
The global market for thermite compounds and related technologies is geographically diverse, with significant demand in North America, Europe, and Asia-Pacific regions. Key market players include established chemical companies and specialized manufacturers focusing on specific industrial applications.
Regulatory considerations and safety concerns play a crucial role in shaping the market landscape. Stringent regulations regarding the transport, storage, and use of thermite compounds have led to innovations in safer formulations and handling procedures, opening new market opportunities for compliant products.
Looking ahead, the market for thermite compounds is expected to continue its growth trajectory, driven by ongoing industrialization in developing economies, infrastructure renewal projects in developed nations, and technological advancements that expand the range of potential applications. The push for more efficient and environmentally friendly industrial processes may also spur further innovations in thermite technology, potentially opening new market segments in the coming years.
In the metallurgy sector, thermite reactions are widely used for metal purification and alloying processes. The ability of thermite compounds to produce high-purity metals through reduction reactions has made them invaluable in the production of specialty alloys and high-performance materials. This application has seen increased demand in aerospace and automotive industries, where lightweight yet strong materials are crucial.
The welding industry has also embraced thermite technology, particularly for large-scale welding operations such as railway track joining and pipeline repairs. Thermite welding offers advantages in remote locations or where traditional welding equipment is impractical. The market for thermite welding kits and consumables has expanded, driven by infrastructure development projects and the need for efficient, on-site welding solutions.
In the defense sector, thermite compounds play a critical role in incendiary devices and pyrotechnics. The high heat generation and rapid reaction rates make them suitable for various military applications, including armor-piercing munitions and emergency destruction of sensitive equipment. This segment of the market is influenced by geopolitical factors and defense spending trends.
The construction industry has found niche applications for thermite compounds in demolition and controlled building implosions. The precise and powerful nature of thermite reactions allows for targeted structural weakening, offering an alternative to traditional explosive methods in certain scenarios.
Emerging applications in the energy sector are also contributing to market growth. Research into thermite-based thermal batteries and energy storage systems shows promise for high-temperature industrial processes and emergency power systems.
The global market for thermite compounds and related technologies is geographically diverse, with significant demand in North America, Europe, and Asia-Pacific regions. Key market players include established chemical companies and specialized manufacturers focusing on specific industrial applications.
Regulatory considerations and safety concerns play a crucial role in shaping the market landscape. Stringent regulations regarding the transport, storage, and use of thermite compounds have led to innovations in safer formulations and handling procedures, opening new market opportunities for compliant products.
Looking ahead, the market for thermite compounds is expected to continue its growth trajectory, driven by ongoing industrialization in developing economies, infrastructure renewal projects in developed nations, and technological advancements that expand the range of potential applications. The push for more efficient and environmentally friendly industrial processes may also spur further innovations in thermite technology, potentially opening new market segments in the coming years.
Current State and Challenges in Thermite Research
Thermite research has made significant strides in recent years, with advancements in both understanding and application of these highly exothermic compounds. The current state of thermite research is characterized by a focus on enhancing performance, improving safety, and expanding potential applications across various industries.
One of the primary areas of progress is in the development of nanoscale thermite materials. These nanocomposites have shown superior reactivity and energy release compared to their microscale counterparts. Researchers have successfully synthesized and characterized a wide range of nanothermites, including Al/CuO, Al/Fe2O3, and Al/MoO3 systems. These materials exhibit faster reaction rates, lower ignition temperatures, and higher energy densities, making them promising candidates for energetic applications.
Despite these advancements, several challenges persist in thermite research. One major hurdle is controlling the reactivity and stability of nanothermites. The high surface area and reactivity of nanoparticles can lead to unintended ignition or degradation during storage and handling. Researchers are actively exploring methods to passivate nanoparticles and develop stable formulations without compromising performance.
Another significant challenge lies in scaling up the production of nanothermites for practical applications. While laboratory-scale synthesis has been successful, translating these processes to industrial-scale production while maintaining consistent quality and performance remains difficult. Issues such as agglomeration, oxidation, and uniformity of particle size distribution need to be addressed for large-scale manufacturing.
The environmental impact of thermite reactions is also a growing concern. The production of toxic byproducts and the potential for contamination in field applications have led researchers to investigate more environmentally friendly alternatives. This includes exploring green oxidizers and developing methods for capturing and neutralizing harmful reaction products.
In the realm of characterization and testing, researchers face challenges in accurately measuring the ultra-fast reaction kinetics of thermites. Conventional diagnostic techniques often lack the temporal and spatial resolution required to capture the rapid energy release and propagation of these reactions. Advanced high-speed imaging, spectroscopy, and pressure measurement techniques are being developed to overcome these limitations.
Lastly, the multidisciplinary nature of thermite research presents both opportunities and challenges. Collaboration between chemists, materials scientists, physicists, and engineers is crucial for addressing complex problems and driving innovation. However, integrating diverse expertise and methodologies can be challenging, requiring effective communication and coordination among research teams.
As thermite research continues to evolve, addressing these challenges will be critical for realizing the full potential of these energetic materials in applications ranging from propulsion systems and welding to pyrotechnics and materials synthesis.
One of the primary areas of progress is in the development of nanoscale thermite materials. These nanocomposites have shown superior reactivity and energy release compared to their microscale counterparts. Researchers have successfully synthesized and characterized a wide range of nanothermites, including Al/CuO, Al/Fe2O3, and Al/MoO3 systems. These materials exhibit faster reaction rates, lower ignition temperatures, and higher energy densities, making them promising candidates for energetic applications.
Despite these advancements, several challenges persist in thermite research. One major hurdle is controlling the reactivity and stability of nanothermites. The high surface area and reactivity of nanoparticles can lead to unintended ignition or degradation during storage and handling. Researchers are actively exploring methods to passivate nanoparticles and develop stable formulations without compromising performance.
Another significant challenge lies in scaling up the production of nanothermites for practical applications. While laboratory-scale synthesis has been successful, translating these processes to industrial-scale production while maintaining consistent quality and performance remains difficult. Issues such as agglomeration, oxidation, and uniformity of particle size distribution need to be addressed for large-scale manufacturing.
The environmental impact of thermite reactions is also a growing concern. The production of toxic byproducts and the potential for contamination in field applications have led researchers to investigate more environmentally friendly alternatives. This includes exploring green oxidizers and developing methods for capturing and neutralizing harmful reaction products.
In the realm of characterization and testing, researchers face challenges in accurately measuring the ultra-fast reaction kinetics of thermites. Conventional diagnostic techniques often lack the temporal and spatial resolution required to capture the rapid energy release and propagation of these reactions. Advanced high-speed imaging, spectroscopy, and pressure measurement techniques are being developed to overcome these limitations.
Lastly, the multidisciplinary nature of thermite research presents both opportunities and challenges. Collaboration between chemists, materials scientists, physicists, and engineers is crucial for addressing complex problems and driving innovation. However, integrating diverse expertise and methodologies can be challenging, requiring effective communication and coordination among research teams.
As thermite research continues to evolve, addressing these challenges will be critical for realizing the full potential of these energetic materials in applications ranging from propulsion systems and welding to pyrotechnics and materials synthesis.
Existing Thermite Compound Formulations
01 Composition of thermite compounds
Thermite compounds typically consist of a metal oxide and a more reactive metal, usually aluminum. The chemical properties of these compounds are characterized by their highly exothermic reaction when ignited, producing intense heat and molten metal. This reaction is self-sustaining and does not require external oxygen, making thermite compounds useful in various applications such as welding and incendiary devices.- Composition of thermite compounds: Thermite compounds typically consist of a metal oxide and a more reactive metal, usually aluminum. The chemical properties of these compounds are characterized by their highly exothermic reaction when ignited, producing intense heat and molten metal. This reaction is self-sustaining and does not require external oxygen, making thermite compounds useful in various applications such as welding and incendiary devices.
- Ignition and reaction mechanisms: The chemical properties of thermite compounds include their ignition mechanisms and reaction kinetics. These compounds require a high activation energy to initiate the reaction, often achieved through high temperatures or specific initiators. Once ignited, the reaction proceeds rapidly, with the reduction of the metal oxide by the more reactive metal. The speed and intensity of the reaction can be controlled by adjusting the composition and particle size of the reactants.
- Heat generation and temperature characteristics: A key chemical property of thermite compounds is their ability to generate extremely high temperatures, often exceeding 2500°C. This intense heat production is due to the large negative enthalpy of reaction. The temperature achieved can be influenced by the specific metals used in the thermite mixture, with some combinations capable of reaching temperatures high enough to melt through metal containers or weld large metal parts together.
- Byproducts and environmental considerations: The chemical properties of thermite compounds also include the nature of their reaction byproducts. Typically, these reactions produce a metal oxide slag and pure metal. The environmental impact of these byproducts can vary depending on the specific metals used. Some thermite reactions may produce toxic fumes or particulates, necessitating careful handling and disposal procedures. The stability of the reaction products and their potential for further chemical interactions are important considerations in the use and management of thermite compounds.
- Modifications and enhancements: The chemical properties of thermite compounds can be modified and enhanced through various means. This includes the addition of binders or catalysts to control reaction rates, the use of nano-sized particles to increase reactivity, or the incorporation of additives to produce specific effects such as gas generation or color changes during reaction. These modifications can tailor the thermite compounds for specific applications, altering their ignition sensitivity, reaction speed, or the properties of the reaction products.
02 Ignition and reaction mechanisms
The chemical properties of thermite compounds involve specific ignition and reaction mechanisms. These compounds require a high activation energy to initiate the reaction, often achieved through high temperatures or specific initiators. Once ignited, the reaction proceeds rapidly, with the more reactive metal reducing the metal oxide and releasing substantial heat. Understanding these mechanisms is crucial for controlling and optimizing thermite reactions in various applications.Expand Specific Solutions03 Thermal and energetic properties
Thermite compounds exhibit unique thermal and energetic properties. They are known for their extremely high reaction temperatures, often exceeding 2500°C. The energy density of these compounds is significant, releasing large amounts of heat per unit mass. These properties make thermite compounds valuable in applications requiring intense, localized heat sources, such as metal purification and welding of railway tracks.Expand Specific Solutions04 Variations in composition and their effects
The chemical properties of thermite compounds can be modified by varying their composition. Different metal oxides and reactive metals can be used to create thermite mixtures with specific characteristics. For instance, using copper oxide instead of iron oxide can result in different reaction temperatures and products. Additionally, the particle size and ratio of components can significantly affect the reaction rate and efficiency, allowing for tailored thermite compounds for specific applications.Expand Specific Solutions05 Safety and handling considerations
Due to their highly reactive nature, thermite compounds require specific safety and handling considerations. These materials are sensitive to friction, impact, and electrostatic discharge, necessitating careful storage and transport protocols. The chemical properties of thermite compounds also make them potentially hazardous in uncontrolled environments, requiring specialized knowledge and equipment for safe handling and use in industrial or research settings.Expand Specific Solutions
Key Players in Thermite Compound Industry
The exploration of thermite compounds' chemical properties is in a mature stage, with significant market potential in various industries. The global market for thermite-related applications is steadily growing, driven by demand in sectors such as defense, aerospace, and metallurgy. Technologically, the field is well-established, with ongoing research focused on enhancing efficiency and safety. Key players like Lockheed Martin Corp., Naval Research Laboratory, and General Atomics lead in defense applications, while companies such as BASF Corp. and DuPont de Nemours, Inc. contribute to industrial uses. Academic institutions like Northwestern University and Nanjing University of Science & Technology continue to advance fundamental research, ensuring a pipeline of innovations in thermite technology.
Lockheed Martin Corp.
Technical Solution: Lockheed Martin has developed advanced thermite-based propulsion systems for aerospace applications. Their technology utilizes precisely engineered thermite compounds to create controlled, high-energy reactions. These systems incorporate nano-scale aluminum particles and metal oxide oxidizers, optimized for maximum energy density and reaction rate control. The company has also explored the use of thermite reactions for welding and metal joining in extreme environments, such as space or underwater applications.
Strengths: Highly advanced and precise control of thermite reactions; Extensive experience in aerospace applications. Weaknesses: High costs associated with research and development; Limited to specialized applications.
Naval Research Laboratory
Technical Solution: The Naval Research Laboratory has conducted extensive research on thermite compounds for military applications. Their work focuses on developing novel thermite compositions with enhanced performance characteristics, such as increased energy output and improved stability. They have explored the use of various metal fuels and oxidizers, including rare earth elements, to create thermite mixtures with tailored properties. The NRL has also investigated the use of thermite reactions for underwater welding and cutting operations, as well as for the disposal of hazardous materials.
Strengths: Cutting-edge research in novel thermite compositions; Diverse range of potential military applications. Weaknesses: Some research may be classified and not publicly available; Focus primarily on military applications may limit commercial potential.
Core Innovations in Thermite Chemistry
Thermite compositions, articles and low temperature impact milling processes for forming the same
PatentInactiveUS8333854B2
Innovation
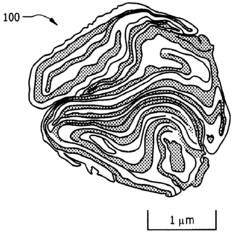
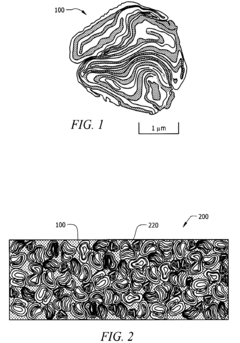
- A process involving cryogenic milling at temperatures below −50° C. to create a convoluted lamellar structure of alternating metal and metal oxide layers, which enhances the interface area and prevents premature reaction initiation, allowing for better control over ignition and propagation phases.
Thermite ignition and rusty iron regeneration by localized microwaves
PatentWO2012120412A1
Innovation
- Localized application of microwave radiation to generate a hot spot within the thermite mixture, reducing the need for intermediate additives and enabling ignition with low-power microwave sources, allowing for efficient ignition of both small and large quantities of thermite.
Safety Regulations for Thermite Handling
The handling of thermite compounds requires strict adherence to safety regulations due to their highly reactive nature and potential for causing severe burns and fires. Proper safety measures are essential to protect personnel, facilities, and the environment from the risks associated with thermite reactions.
Personal protective equipment (PPE) is crucial when working with thermite. This includes fire-resistant clothing, heat-resistant gloves, safety goggles, and face shields. Respiratory protection may also be necessary to prevent inhalation of fine metal powders or reaction products.
Storage of thermite components must be carefully managed. Aluminum powder and metal oxides should be stored separately in sealed, moisture-proof containers in a cool, dry area away from potential ignition sources. Proper labeling and inventory control are essential to prevent accidental mixing or misuse.
Workplace design and engineering controls play a significant role in thermite safety. Dedicated work areas should be equipped with proper ventilation systems, fire suppression equipment, and emergency showers. Conductive flooring and grounding systems help prevent static electricity buildup, which could potentially trigger unintended reactions.
Training and education are paramount for personnel working with thermite. Comprehensive safety protocols must be established and regularly reviewed. This includes proper handling techniques, emergency response procedures, and understanding the chemical properties and potential hazards of thermite compounds.
When conducting thermite reactions, strict protocols must be followed. This includes using appropriate reaction vessels, controlling the reaction environment, and implementing proper ignition methods. Remote ignition systems and blast shields may be necessary for larger-scale reactions.
Waste management and disposal of thermite materials require special consideration. Unreacted materials and reaction products must be disposed of according to local regulations for hazardous waste. Proper neutralization and containment procedures should be in place to handle any spills or residues.
Regular safety audits and inspections are essential to ensure compliance with established protocols and identify potential hazards. This includes checking equipment integrity, verifying proper storage conditions, and reviewing safety documentation.
Emergency response planning is critical when working with thermite. This includes having clearly defined evacuation procedures, readily accessible fire-fighting equipment, and trained personnel capable of responding to thermite-related incidents.
Personal protective equipment (PPE) is crucial when working with thermite. This includes fire-resistant clothing, heat-resistant gloves, safety goggles, and face shields. Respiratory protection may also be necessary to prevent inhalation of fine metal powders or reaction products.
Storage of thermite components must be carefully managed. Aluminum powder and metal oxides should be stored separately in sealed, moisture-proof containers in a cool, dry area away from potential ignition sources. Proper labeling and inventory control are essential to prevent accidental mixing or misuse.
Workplace design and engineering controls play a significant role in thermite safety. Dedicated work areas should be equipped with proper ventilation systems, fire suppression equipment, and emergency showers. Conductive flooring and grounding systems help prevent static electricity buildup, which could potentially trigger unintended reactions.
Training and education are paramount for personnel working with thermite. Comprehensive safety protocols must be established and regularly reviewed. This includes proper handling techniques, emergency response procedures, and understanding the chemical properties and potential hazards of thermite compounds.
When conducting thermite reactions, strict protocols must be followed. This includes using appropriate reaction vessels, controlling the reaction environment, and implementing proper ignition methods. Remote ignition systems and blast shields may be necessary for larger-scale reactions.
Waste management and disposal of thermite materials require special consideration. Unreacted materials and reaction products must be disposed of according to local regulations for hazardous waste. Proper neutralization and containment procedures should be in place to handle any spills or residues.
Regular safety audits and inspections are essential to ensure compliance with established protocols and identify potential hazards. This includes checking equipment integrity, verifying proper storage conditions, and reviewing safety documentation.
Emergency response planning is critical when working with thermite. This includes having clearly defined evacuation procedures, readily accessible fire-fighting equipment, and trained personnel capable of responding to thermite-related incidents.
Environmental Impact of Thermite Reactions
Thermite reactions, while highly effective for their intended purposes, can have significant environmental impacts that warrant careful consideration. The primary environmental concerns associated with thermite reactions stem from the release of various byproducts and the potential for unintended consequences in surrounding ecosystems.
One of the most immediate environmental impacts of thermite reactions is the release of metal oxides, particularly aluminum oxide, into the atmosphere. These fine particulates can contribute to air pollution and may pose respiratory risks to both humans and wildlife in the vicinity of the reaction. The dispersion of these particles can extend well beyond the immediate area of the thermite reaction, potentially affecting air quality over a broader region.
The intense heat generated by thermite reactions can also lead to localized thermal pollution. This sudden and extreme temperature increase can have detrimental effects on soil microorganisms, plant life, and small animals in the immediate vicinity. The heat can alter soil chemistry and structure, potentially rendering the affected area inhospitable for vegetation regrowth for an extended period.
Water contamination is another significant concern, especially if thermite reactions occur near water bodies or in areas with high groundwater levels. The metal oxides and other byproducts can leach into water systems, altering pH levels and introducing potentially toxic compounds. This can have far-reaching effects on aquatic ecosystems, impacting fish, amphibians, and other water-dependent organisms.
The production and disposal of thermite compounds also present environmental challenges. The manufacturing process of thermite materials often involves energy-intensive procedures and the use of potentially hazardous chemicals. Proper disposal of unused thermite and reaction residues is crucial to prevent soil and water contamination.
In urban or industrial settings, the use of thermite reactions can contribute to the heat island effect, exacerbating local climate impacts. The intense heat and light produced during the reaction can disrupt nocturnal wildlife and potentially interfere with migratory patterns of birds and insects.
Long-term ecological impacts of thermite reactions are still being studied. There is concern about the potential bioaccumulation of metal compounds in food chains and the long-term effects on ecosystem biodiversity. The alteration of soil chemistry may lead to changes in local plant communities, potentially favoring invasive species that are more tolerant of disturbed environments.
Given these environmental considerations, it is crucial to implement strict safety protocols and environmental management strategies when using thermite compounds. This includes careful site selection, containment measures, and post-reaction cleanup procedures to minimize ecological disturbance. Ongoing research into more environmentally friendly alternatives and improved reaction control methods is essential for mitigating the environmental impact of thermite reactions in various applications.
One of the most immediate environmental impacts of thermite reactions is the release of metal oxides, particularly aluminum oxide, into the atmosphere. These fine particulates can contribute to air pollution and may pose respiratory risks to both humans and wildlife in the vicinity of the reaction. The dispersion of these particles can extend well beyond the immediate area of the thermite reaction, potentially affecting air quality over a broader region.
The intense heat generated by thermite reactions can also lead to localized thermal pollution. This sudden and extreme temperature increase can have detrimental effects on soil microorganisms, plant life, and small animals in the immediate vicinity. The heat can alter soil chemistry and structure, potentially rendering the affected area inhospitable for vegetation regrowth for an extended period.
Water contamination is another significant concern, especially if thermite reactions occur near water bodies or in areas with high groundwater levels. The metal oxides and other byproducts can leach into water systems, altering pH levels and introducing potentially toxic compounds. This can have far-reaching effects on aquatic ecosystems, impacting fish, amphibians, and other water-dependent organisms.
The production and disposal of thermite compounds also present environmental challenges. The manufacturing process of thermite materials often involves energy-intensive procedures and the use of potentially hazardous chemicals. Proper disposal of unused thermite and reaction residues is crucial to prevent soil and water contamination.
In urban or industrial settings, the use of thermite reactions can contribute to the heat island effect, exacerbating local climate impacts. The intense heat and light produced during the reaction can disrupt nocturnal wildlife and potentially interfere with migratory patterns of birds and insects.
Long-term ecological impacts of thermite reactions are still being studied. There is concern about the potential bioaccumulation of metal compounds in food chains and the long-term effects on ecosystem biodiversity. The alteration of soil chemistry may lead to changes in local plant communities, potentially favoring invasive species that are more tolerant of disturbed environments.
Given these environmental considerations, it is crucial to implement strict safety protocols and environmental management strategies when using thermite compounds. This includes careful site selection, containment measures, and post-reaction cleanup procedures to minimize ecological disturbance. Ongoing research into more environmentally friendly alternatives and improved reaction control methods is essential for mitigating the environmental impact of thermite reactions in various applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!