Thermite and Its Reaction Kinetics: A Detailed Study
JUN 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Thermite Reaction Background and Objectives
Thermite reactions have been a subject of fascination and study for over a century, dating back to their discovery by German chemist Hans Goldschmidt in 1893. These highly exothermic reactions involve the reduction of a metal oxide by a more reactive metal, typically aluminum, resulting in the production of a more stable oxide and the corresponding reduced metal. The intense heat and light generated during thermite reactions have led to their widespread use in various applications, from welding and metal purification to pyrotechnics and military ordnance.
The primary objective of this study is to conduct a comprehensive investigation into the reaction kinetics of thermite systems. By examining the fundamental mechanisms and rate-determining steps of these reactions, we aim to gain deeper insights into the factors that influence their initiation, propagation, and completion. This understanding is crucial for optimizing existing applications and developing new ones, as well as for enhancing safety protocols in industries that utilize thermite reactions.
One of the key areas of focus in this study is the exploration of different thermite compositions and their impact on reaction kinetics. Traditional thermite mixtures typically consist of aluminum powder and iron oxide, but numerous variations exist, incorporating different metal oxides such as copper oxide, chromium oxide, or even more exotic compounds. By systematically analyzing these diverse compositions, we seek to establish correlations between reactant properties and reaction rates, thermal output, and overall efficiency.
Another critical aspect of this research is the investigation of particle size and morphology effects on thermite reaction kinetics. The surface area-to-volume ratio of reactant particles plays a significant role in determining reaction rates and ignition thresholds. Through careful examination of nano-sized particles and various particle geometries, we aim to elucidate the relationship between reactant physical characteristics and reaction dynamics.
Furthermore, this study will delve into the influence of environmental factors on thermite reactions. Factors such as ambient temperature, pressure, and the presence of inert or reactive gases can significantly impact reaction kinetics. By conducting experiments under controlled conditions, we intend to quantify these effects and develop predictive models for thermite behavior across a range of environmental scenarios.
Ultimately, the goal of this research is to bridge the gap between theoretical understanding and practical applications of thermite reactions. By combining experimental data with advanced modeling techniques, we aim to develop a comprehensive framework for predicting and controlling thermite reaction kinetics. This knowledge will not only contribute to the scientific community but also have far-reaching implications for industries relying on thermite-based technologies, potentially leading to improved safety measures, enhanced performance, and novel applications in fields such as materials science, energy storage, and advanced manufacturing.
The primary objective of this study is to conduct a comprehensive investigation into the reaction kinetics of thermite systems. By examining the fundamental mechanisms and rate-determining steps of these reactions, we aim to gain deeper insights into the factors that influence their initiation, propagation, and completion. This understanding is crucial for optimizing existing applications and developing new ones, as well as for enhancing safety protocols in industries that utilize thermite reactions.
One of the key areas of focus in this study is the exploration of different thermite compositions and their impact on reaction kinetics. Traditional thermite mixtures typically consist of aluminum powder and iron oxide, but numerous variations exist, incorporating different metal oxides such as copper oxide, chromium oxide, or even more exotic compounds. By systematically analyzing these diverse compositions, we seek to establish correlations between reactant properties and reaction rates, thermal output, and overall efficiency.
Another critical aspect of this research is the investigation of particle size and morphology effects on thermite reaction kinetics. The surface area-to-volume ratio of reactant particles plays a significant role in determining reaction rates and ignition thresholds. Through careful examination of nano-sized particles and various particle geometries, we aim to elucidate the relationship between reactant physical characteristics and reaction dynamics.
Furthermore, this study will delve into the influence of environmental factors on thermite reactions. Factors such as ambient temperature, pressure, and the presence of inert or reactive gases can significantly impact reaction kinetics. By conducting experiments under controlled conditions, we intend to quantify these effects and develop predictive models for thermite behavior across a range of environmental scenarios.
Ultimately, the goal of this research is to bridge the gap between theoretical understanding and practical applications of thermite reactions. By combining experimental data with advanced modeling techniques, we aim to develop a comprehensive framework for predicting and controlling thermite reaction kinetics. This knowledge will not only contribute to the scientific community but also have far-reaching implications for industries relying on thermite-based technologies, potentially leading to improved safety measures, enhanced performance, and novel applications in fields such as materials science, energy storage, and advanced manufacturing.
Market Applications of Thermite Reactions
Thermite reactions have found diverse applications across various industries due to their unique properties and high energy output. In the metallurgical sector, thermite reactions are extensively used for welding railway tracks, repairing heavy machinery, and joining large metal components. The intense heat generated by these reactions allows for rapid and efficient welding of thick metal sections, making it particularly valuable in infrastructure maintenance and construction.
The aerospace and defense industries also leverage thermite reactions for specialized applications. Thermite mixtures are utilized in incendiary devices, pyrotechnics, and as a component in certain types of rocket propellants. The high temperature and rapid reaction rate of thermite make it suitable for emergency destruction of sensitive equipment or documents, a critical feature in military and intelligence operations.
In the field of materials science, thermite reactions play a crucial role in the production of high-purity metals and alloys. The aluminothermic process, a type of thermite reaction, is employed to produce metals like chromium, manganese, and vanadium from their oxides. This method allows for the creation of ultra-pure metals that are essential in advanced manufacturing and electronics industries.
The automotive industry has found applications for thermite reactions in airbag deployment systems. The rapid and controlled combustion of a small thermite charge provides the necessary force to inflate airbags in milliseconds during a collision, significantly enhancing vehicle safety.
In the realm of firefighting and rescue operations, thermite-based devices are used for breaching obstacles and creating emergency exits. The intense heat and focused nature of the reaction allow for quick penetration of metal barriers, aiding in rescue efforts during disasters or hostage situations.
The mining and demolition sectors utilize thermite reactions for specialized cutting and perforation tasks. Thermite lances can cut through reinforced concrete and steel structures, facilitating controlled demolitions and mining operations in challenging environments.
Emerging applications of thermite reactions are being explored in the field of renewable energy. Research is ongoing to harness the high-temperature capabilities of thermite reactions for solar thermal energy storage and conversion systems, potentially offering new solutions for sustainable energy production and storage.
As industries continue to evolve, the unique properties of thermite reactions present opportunities for innovation across various sectors. From traditional metallurgy to cutting-edge energy solutions, the market applications of thermite reactions demonstrate their versatility and ongoing relevance in modern industrial processes.
The aerospace and defense industries also leverage thermite reactions for specialized applications. Thermite mixtures are utilized in incendiary devices, pyrotechnics, and as a component in certain types of rocket propellants. The high temperature and rapid reaction rate of thermite make it suitable for emergency destruction of sensitive equipment or documents, a critical feature in military and intelligence operations.
In the field of materials science, thermite reactions play a crucial role in the production of high-purity metals and alloys. The aluminothermic process, a type of thermite reaction, is employed to produce metals like chromium, manganese, and vanadium from their oxides. This method allows for the creation of ultra-pure metals that are essential in advanced manufacturing and electronics industries.
The automotive industry has found applications for thermite reactions in airbag deployment systems. The rapid and controlled combustion of a small thermite charge provides the necessary force to inflate airbags in milliseconds during a collision, significantly enhancing vehicle safety.
In the realm of firefighting and rescue operations, thermite-based devices are used for breaching obstacles and creating emergency exits. The intense heat and focused nature of the reaction allow for quick penetration of metal barriers, aiding in rescue efforts during disasters or hostage situations.
The mining and demolition sectors utilize thermite reactions for specialized cutting and perforation tasks. Thermite lances can cut through reinforced concrete and steel structures, facilitating controlled demolitions and mining operations in challenging environments.
Emerging applications of thermite reactions are being explored in the field of renewable energy. Research is ongoing to harness the high-temperature capabilities of thermite reactions for solar thermal energy storage and conversion systems, potentially offering new solutions for sustainable energy production and storage.
As industries continue to evolve, the unique properties of thermite reactions present opportunities for innovation across various sectors. From traditional metallurgy to cutting-edge energy solutions, the market applications of thermite reactions demonstrate their versatility and ongoing relevance in modern industrial processes.
Current Challenges in Thermite Reaction Control
Controlling thermite reactions presents several significant challenges that researchers and engineers are actively working to address. One of the primary difficulties lies in managing the rapid and highly exothermic nature of these reactions. Thermite reactions, once initiated, proceed at an extremely fast rate, making it challenging to control the reaction progression and energy release.
The high temperatures generated during thermite reactions, often exceeding 2500°C, pose substantial safety risks and material constraints. These extreme temperatures can lead to unintended ignition of surrounding materials and make it difficult to contain the reaction within desired boundaries. Additionally, the intense heat can cause rapid degradation of reaction vessels and containment systems, limiting the ability to study and harness the reaction in controlled environments.
Another critical challenge is achieving precise control over the reaction kinetics. The rate of energy release and the propagation of the reaction front are highly sensitive to factors such as particle size, composition ratios, and environmental conditions. This sensitivity makes it challenging to predict and consistently reproduce reaction outcomes, especially when scaling up from laboratory experiments to practical applications.
The heterogeneous nature of thermite mixtures further complicates control efforts. Ensuring uniform mixing and distribution of reactants is crucial for consistent performance, but achieving this uniformity becomes increasingly difficult as the scale of the reaction increases. Variations in particle size distribution and local composition can lead to hotspots and uneven reaction propagation, compromising the overall control and efficiency of the process.
Researchers are also grappling with the challenge of tailoring thermite reactions for specific applications. While the high energy density of thermites is advantageous in many scenarios, fine-tuning the reaction to release energy at a controlled rate or to produce specific reaction products remains a significant hurdle. This is particularly relevant in applications such as materials synthesis, where precise control over reaction conditions is essential for obtaining desired product characteristics.
The development of safe and effective ignition methods for thermite reactions is another area of ongoing research. Current ignition techniques often lack the precision required for controlled initiation, especially in large-scale applications. Improving ignition control is crucial for enhancing the overall safety and reliability of thermite-based systems.
The high temperatures generated during thermite reactions, often exceeding 2500°C, pose substantial safety risks and material constraints. These extreme temperatures can lead to unintended ignition of surrounding materials and make it difficult to contain the reaction within desired boundaries. Additionally, the intense heat can cause rapid degradation of reaction vessels and containment systems, limiting the ability to study and harness the reaction in controlled environments.
Another critical challenge is achieving precise control over the reaction kinetics. The rate of energy release and the propagation of the reaction front are highly sensitive to factors such as particle size, composition ratios, and environmental conditions. This sensitivity makes it challenging to predict and consistently reproduce reaction outcomes, especially when scaling up from laboratory experiments to practical applications.
The heterogeneous nature of thermite mixtures further complicates control efforts. Ensuring uniform mixing and distribution of reactants is crucial for consistent performance, but achieving this uniformity becomes increasingly difficult as the scale of the reaction increases. Variations in particle size distribution and local composition can lead to hotspots and uneven reaction propagation, compromising the overall control and efficiency of the process.
Researchers are also grappling with the challenge of tailoring thermite reactions for specific applications. While the high energy density of thermites is advantageous in many scenarios, fine-tuning the reaction to release energy at a controlled rate or to produce specific reaction products remains a significant hurdle. This is particularly relevant in applications such as materials synthesis, where precise control over reaction conditions is essential for obtaining desired product characteristics.
The development of safe and effective ignition methods for thermite reactions is another area of ongoing research. Current ignition techniques often lack the precision required for controlled initiation, especially in large-scale applications. Improving ignition control is crucial for enhancing the overall safety and reliability of thermite-based systems.
State-of-the-Art Thermite Reaction Mechanisms
01 Reaction rate control in thermite reactions
Controlling the reaction rate in thermite reactions is crucial for various applications. This can be achieved through methods such as adjusting particle size, using additives, or modifying the composition of reactants. These techniques allow for tailoring the reaction kinetics to suit specific needs, from rapid energy release to controlled, sustained reactions.- Reaction kinetics modeling and simulation: Advanced modeling and simulation techniques are employed to study the kinetics of thermite reactions. These methods help in understanding the reaction rates, energy release, and propagation characteristics of thermite mixtures under various conditions. Computer-aided simulations allow for the optimization of reaction parameters and prediction of performance in different applications.
- Particle size and composition effects: The kinetics of thermite reactions are significantly influenced by the particle size and composition of the reactants. Research focuses on how varying the size distribution and ratios of fuel and oxidizer particles affects reaction rates, ignition temperatures, and energy release. Optimizing these parameters can lead to improved performance in applications such as welding, metal purification, and pyrotechnics.
- Ignition and propagation mechanisms: Studies on the ignition and propagation mechanisms of thermite reactions are crucial for understanding their kinetics. Research in this area includes investigating different ignition methods, such as electrical, laser, or chemical initiation, and analyzing how the reaction front propagates through the mixture. This knowledge is essential for designing safer and more efficient thermite-based systems.
- Environmental and pressure effects: The kinetics of thermite reactions can be significantly affected by environmental conditions and pressure. Research examines how factors such as ambient temperature, humidity, and atmospheric or applied pressure influence reaction rates and completeness. Understanding these effects is crucial for predicting performance in various applications and ensuring safety in different operating environments.
- Novel thermite compositions and additives: Ongoing research explores novel thermite compositions and additives to enhance reaction kinetics. This includes investigating new fuel-oxidizer combinations, nanomaterials, and catalysts that can improve ignition sensitivity, reaction rates, and energy output. These advancements aim to develop more efficient and controllable thermite reactions for various industrial and technological applications.
02 Thermite composition optimization
Optimizing thermite compositions involves carefully selecting and combining metal fuels and metal oxide oxidizers. Factors such as stoichiometry, particle size distribution, and the inclusion of additives can significantly affect reaction kinetics. Advanced compositions may incorporate nanomaterials or novel fuel-oxidizer pairs to enhance performance or achieve specific reaction characteristics.Expand Specific Solutions03 Modeling and simulation of thermite reactions
Computational modeling and simulation play a crucial role in understanding and predicting thermite reaction kinetics. These tools can account for complex factors such as heat transfer, mass transport, and chemical kinetics. Advanced models may incorporate multiscale approaches, from molecular dynamics to continuum-level simulations, providing insights into reaction mechanisms and helping optimize reaction conditions.Expand Specific Solutions04 Ignition and propagation mechanisms in thermite reactions
Understanding the ignition and propagation mechanisms in thermite reactions is essential for controlling and utilizing these reactions effectively. Factors such as ignition temperature, heat release rate, and reaction front propagation velocity are critical aspects of reaction kinetics. Various ignition methods and their effects on subsequent reaction progression are studied to optimize performance for different applications.Expand Specific Solutions05 Environmental and safety considerations in thermite reactions
The study of thermite reaction kinetics also involves addressing environmental and safety concerns. This includes developing methods to control and contain the high-temperature reactions, managing byproducts, and ensuring safe handling and storage of reactive materials. Research in this area focuses on mitigating risks while maintaining the desired reaction characteristics for various industrial and technological applications.Expand Specific Solutions
Key Players in Thermite Research and Industry
The thermite reaction and its kinetics represent a mature field with ongoing research and development. The market for thermite applications spans various industries, including defense, aerospace, and manufacturing. While the technology is well-established, there is continuous innovation in reaction control and efficiency. Key players like Naval Research Laboratory, Lockheed Martin Corp., and Battelle Memorial Institute lead in defense applications, while academic institutions such as Nanjing University of Science & Technology and Chongqing University contribute to fundamental research. Companies like Elektro-Thermit GmbH & Co. KG focus on industrial applications. The competitive landscape is characterized by a mix of established corporations, specialized firms, and research institutions, each contributing to the advancement of thermite technology in their respective domains.
Naval Research Laboratory
Technical Solution: The Naval Research Laboratory has developed advanced thermite compositions with enhanced reaction kinetics. Their research focuses on tailoring the particle size and morphology of metal fuels and oxidizers to optimize the energy release rate and combustion efficiency. They have also explored the use of nanoscale additives to further enhance reactivity and control the reaction propagation. The NRL's approach involves a combination of experimental studies and computational modeling to understand the fundamental mechanisms of thermite reactions and predict performance under various conditions.
Strengths: Cutting-edge research capabilities, access to advanced characterization techniques, and ability to develop tailored thermite compositions for specific applications. Weaknesses: Potential limitations in scaling up laboratory-scale formulations for practical applications.
Nanjing University of Science & Technology
Technical Solution: Nanjing University of Science & Technology has conducted extensive research on thermite reaction kinetics, focusing on the development of novel thermite compositions with improved performance. Their approach involves the synthesis of nanostructured metal oxides and fuel particles to enhance reactivity and energy release. They have also investigated the effects of various additives on reaction rates and combustion temperatures. The university's research team has developed advanced diagnostic techniques to study the reaction mechanisms in real-time, including high-speed imaging and spectroscopic methods. Their work has led to the development of thermite formulations with tailored properties for applications in materials processing and energetic systems.
Strengths: Strong expertise in nanostructured materials synthesis and characterization, comprehensive understanding of reaction kinetics. Weaknesses: Potential challenges in translating laboratory-scale results to industrial applications.
Cutting-Edge Thermite Composition Innovations
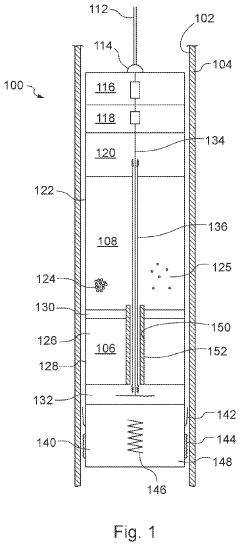
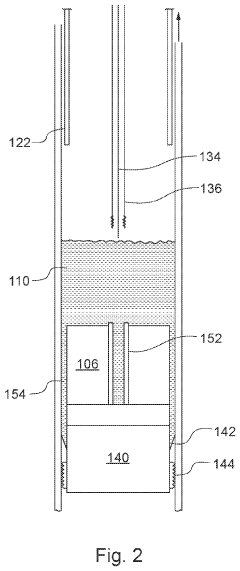
Downhole apparatus and method
PatentActiveUS20240011367A1
Innovation
- A downhole apparatus and method utilizing compressed thermite within a container, which reduces porosity and increases density, facilitating efficient heat transfer and reaction, and is combined with a low-melt-point alloy to form a solid plug, allowing for effective sealing without the need for binding agents and maintaining structural strength under pressure.
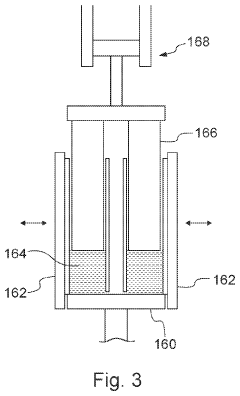
Solid-state thermite composition based heating device
PatentWO2010117857A2
Innovation
- A solid-state thermite reaction composition comprising a fuel component, primary oxidizer, initiating oxidizers, thermal diluent, and fluxing agents, integrated with a heating device featuring a reaction chamber and actuable trigger mechanism, allowing controlled thermite reactions for precise heat generation, with activation mechanisms like piezoelectric spark ignitors or exothermic couples to initiate the reaction safely.
Safety Protocols in Thermite Handling and Use
Thermite handling and use require stringent safety protocols due to the highly exothermic nature of the reaction and the potential for severe burns or fires. Proper personal protective equipment (PPE) is essential, including fire-resistant clothing, heat-resistant gloves, and face shields or goggles. Respiratory protection may also be necessary to prevent inhalation of metal oxide fumes produced during the reaction.
The work area must be carefully prepared before any thermite operations. This includes clearing the surrounding area of flammable materials, ensuring adequate ventilation, and having appropriate fire suppression equipment readily available. A designated safety perimeter should be established to keep unauthorized personnel at a safe distance.
Proper storage and transportation of thermite components are critical. The aluminum powder and metal oxide should be stored separately in sealed, moisture-proof containers to prevent accidental ignition. When transporting these materials, they should be kept in separate, clearly labeled containers and secured to prevent spills or mixing.
Ignition of thermite should only be performed by trained personnel using approved methods. Remote ignition systems are preferred to minimize risk to operators. Once ignited, thermite reactions cannot be easily extinguished, so proper containment and controlled burn-off procedures must be in place.
Disposal of thermite residues requires special consideration. Spent thermite may still contain unreacted materials or hot slag, which can reignite if disturbed. Proper cooling and containment procedures must be followed before disposal, and residues should be treated as hazardous waste.
Emergency response plans must be developed and regularly reviewed. These should include procedures for dealing with accidental ignitions, spills, and potential injuries. All personnel working with or near thermite operations should be trained in these procedures and familiar with the location of emergency equipment.
Regular safety audits and inspections of thermite handling areas and procedures are essential to maintain a safe working environment. This includes checking the integrity of storage containers, verifying the functionality of safety equipment, and ensuring that all personnel are up to date on safety training and protocols.
The work area must be carefully prepared before any thermite operations. This includes clearing the surrounding area of flammable materials, ensuring adequate ventilation, and having appropriate fire suppression equipment readily available. A designated safety perimeter should be established to keep unauthorized personnel at a safe distance.
Proper storage and transportation of thermite components are critical. The aluminum powder and metal oxide should be stored separately in sealed, moisture-proof containers to prevent accidental ignition. When transporting these materials, they should be kept in separate, clearly labeled containers and secured to prevent spills or mixing.
Ignition of thermite should only be performed by trained personnel using approved methods. Remote ignition systems are preferred to minimize risk to operators. Once ignited, thermite reactions cannot be easily extinguished, so proper containment and controlled burn-off procedures must be in place.
Disposal of thermite residues requires special consideration. Spent thermite may still contain unreacted materials or hot slag, which can reignite if disturbed. Proper cooling and containment procedures must be followed before disposal, and residues should be treated as hazardous waste.
Emergency response plans must be developed and regularly reviewed. These should include procedures for dealing with accidental ignitions, spills, and potential injuries. All personnel working with or near thermite operations should be trained in these procedures and familiar with the location of emergency equipment.
Regular safety audits and inspections of thermite handling areas and procedures are essential to maintain a safe working environment. This includes checking the integrity of storage containers, verifying the functionality of safety equipment, and ensuring that all personnel are up to date on safety training and protocols.
Environmental Impact of Thermite Reactions
Thermite reactions, while highly effective for their intended purposes, can have significant environmental impacts that warrant careful consideration. The primary environmental concerns associated with thermite reactions stem from the release of various byproducts and the potential for unintended consequences in surrounding ecosystems.
One of the most immediate environmental impacts of thermite reactions is the release of metal oxides, particularly aluminum oxide, into the atmosphere. These fine particulates can contribute to air pollution and may pose respiratory risks to both humans and wildlife in the vicinity of the reaction. The dispersion of these particles can extend well beyond the immediate area of the thermite reaction, potentially affecting air quality over a broader region.
The intense heat generated by thermite reactions can also lead to localized thermal pollution. This sudden and extreme temperature increase can have detrimental effects on soil microorganisms, plant life, and small animals in the immediate vicinity. The altered soil chemistry and structure resulting from such intense heat may persist long after the reaction has concluded, potentially impacting local ecosystem dynamics and biodiversity.
Furthermore, the residual materials left behind after a thermite reaction can introduce chemical contaminants into the soil and nearby water sources. Depending on the specific composition of the thermite mixture, these contaminants may include heavy metals or other potentially toxic substances. The leaching of these materials into groundwater or surface water bodies could have far-reaching consequences for aquatic ecosystems and potentially impact drinking water sources.
The use of thermite in industrial applications, such as welding or metal purification, also raises concerns about the proper disposal of reaction byproducts. Improper handling or disposal of these materials can lead to long-term environmental contamination and pose risks to human health. This necessitates the development and implementation of strict protocols for the management of thermite-related waste.
In addition to direct environmental impacts, the production and transportation of thermite components contribute to the overall environmental footprint of its use. The mining and processing of raw materials, particularly aluminum, can have significant environmental implications, including habitat destruction, energy consumption, and greenhouse gas emissions.
To mitigate these environmental impacts, ongoing research is focused on developing more environmentally friendly thermite compositions and reaction processes. This includes exploring alternative materials that produce fewer harmful byproducts and investigating methods to contain and control the reaction more effectively. Additionally, efforts are being made to improve the recycling and safe disposal of thermite reaction residues, aiming to minimize long-term environmental contamination.
One of the most immediate environmental impacts of thermite reactions is the release of metal oxides, particularly aluminum oxide, into the atmosphere. These fine particulates can contribute to air pollution and may pose respiratory risks to both humans and wildlife in the vicinity of the reaction. The dispersion of these particles can extend well beyond the immediate area of the thermite reaction, potentially affecting air quality over a broader region.
The intense heat generated by thermite reactions can also lead to localized thermal pollution. This sudden and extreme temperature increase can have detrimental effects on soil microorganisms, plant life, and small animals in the immediate vicinity. The altered soil chemistry and structure resulting from such intense heat may persist long after the reaction has concluded, potentially impacting local ecosystem dynamics and biodiversity.
Furthermore, the residual materials left behind after a thermite reaction can introduce chemical contaminants into the soil and nearby water sources. Depending on the specific composition of the thermite mixture, these contaminants may include heavy metals or other potentially toxic substances. The leaching of these materials into groundwater or surface water bodies could have far-reaching consequences for aquatic ecosystems and potentially impact drinking water sources.
The use of thermite in industrial applications, such as welding or metal purification, also raises concerns about the proper disposal of reaction byproducts. Improper handling or disposal of these materials can lead to long-term environmental contamination and pose risks to human health. This necessitates the development and implementation of strict protocols for the management of thermite-related waste.
In addition to direct environmental impacts, the production and transportation of thermite components contribute to the overall environmental footprint of its use. The mining and processing of raw materials, particularly aluminum, can have significant environmental implications, including habitat destruction, energy consumption, and greenhouse gas emissions.
To mitigate these environmental impacts, ongoing research is focused on developing more environmentally friendly thermite compositions and reaction processes. This includes exploring alternative materials that produce fewer harmful byproducts and investigating methods to contain and control the reaction more effectively. Additionally, efforts are being made to improve the recycling and safe disposal of thermite reaction residues, aiming to minimize long-term environmental contamination.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!