Understanding Thermite's Thermodynamics for Better Application
JUN 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Thermite Fundamentals
Thermite, a pyrotechnic composition of metal fuel and metal oxide, has been a subject of fascination and practical application for over a century. This mixture, typically composed of aluminum powder and iron oxide, undergoes an exothermic reduction-oxidation reaction when ignited, producing intense heat and molten iron as a byproduct. The fundamental principles governing thermite reactions are rooted in thermodynamics and chemical kinetics.
The thermodynamic basis of thermite reactions lies in the transfer of oxygen from the metal oxide to the metal fuel. This process is driven by the difference in the heats of formation of the reactants and products. In the case of the classic aluminum-iron oxide thermite, the reaction is highly exothermic due to the strong affinity of aluminum for oxygen compared to iron. The standard enthalpy of reaction for this system is approximately -850 kJ/mol, indicating a substantial release of energy.
The reaction kinetics of thermite are equally crucial to understanding its behavior. Despite the large negative enthalpy of reaction, thermite mixtures are generally stable at room temperature due to kinetic barriers. The initiation of the reaction requires significant activation energy, typically provided by an external heat source or a high-temperature primer. Once initiated, the reaction is self-sustaining due to the heat generated, which propagates through the mixture at velocities ranging from centimeters to meters per second, depending on composition and particle size.
Particle size and distribution play a critical role in thermite reactions. Finer particles increase the surface area available for reaction, enhancing reaction rates and completeness. However, extremely fine particles can lead to safety concerns due to increased sensitivity to ignition. The optimal particle size distribution balances reactivity with handling safety and is a key consideration in thermite formulation.
The stoichiometry of the thermite mixture also significantly influences its performance. While the ideal ratio is determined by the balanced chemical equation, slight deviations can be employed to tailor the reaction characteristics. Fuel-rich mixtures may result in higher temperatures but incomplete oxidation, while oxygen-rich mixtures ensure complete fuel consumption but may reduce overall energy output.
Understanding the thermodynamics of thermite reactions extends beyond the primary aluminum-iron oxide system. Various metal fuels (e.g., magnesium, titanium) and metal oxides (e.g., copper oxide, chromium oxide) can be employed to create thermites with diverse properties. Each combination presents unique thermodynamic profiles, affecting reaction temperatures, energy densities, and product compositions.
The application of thermodynamic principles to thermite systems enables the prediction and optimization of reaction outcomes. Thermochemical calculations, such as those based on Hess's Law and the Born-Haber cycle, allow for the estimation of reaction enthalpies and adiabatic flame temperatures. These theoretical predictions serve as valuable guides for formulation development and performance expectations in practical applications.
The thermodynamic basis of thermite reactions lies in the transfer of oxygen from the metal oxide to the metal fuel. This process is driven by the difference in the heats of formation of the reactants and products. In the case of the classic aluminum-iron oxide thermite, the reaction is highly exothermic due to the strong affinity of aluminum for oxygen compared to iron. The standard enthalpy of reaction for this system is approximately -850 kJ/mol, indicating a substantial release of energy.
The reaction kinetics of thermite are equally crucial to understanding its behavior. Despite the large negative enthalpy of reaction, thermite mixtures are generally stable at room temperature due to kinetic barriers. The initiation of the reaction requires significant activation energy, typically provided by an external heat source or a high-temperature primer. Once initiated, the reaction is self-sustaining due to the heat generated, which propagates through the mixture at velocities ranging from centimeters to meters per second, depending on composition and particle size.
Particle size and distribution play a critical role in thermite reactions. Finer particles increase the surface area available for reaction, enhancing reaction rates and completeness. However, extremely fine particles can lead to safety concerns due to increased sensitivity to ignition. The optimal particle size distribution balances reactivity with handling safety and is a key consideration in thermite formulation.
The stoichiometry of the thermite mixture also significantly influences its performance. While the ideal ratio is determined by the balanced chemical equation, slight deviations can be employed to tailor the reaction characteristics. Fuel-rich mixtures may result in higher temperatures but incomplete oxidation, while oxygen-rich mixtures ensure complete fuel consumption but may reduce overall energy output.
Understanding the thermodynamics of thermite reactions extends beyond the primary aluminum-iron oxide system. Various metal fuels (e.g., magnesium, titanium) and metal oxides (e.g., copper oxide, chromium oxide) can be employed to create thermites with diverse properties. Each combination presents unique thermodynamic profiles, affecting reaction temperatures, energy densities, and product compositions.
The application of thermodynamic principles to thermite systems enables the prediction and optimization of reaction outcomes. Thermochemical calculations, such as those based on Hess's Law and the Born-Haber cycle, allow for the estimation of reaction enthalpies and adiabatic flame temperatures. These theoretical predictions serve as valuable guides for formulation development and performance expectations in practical applications.
Market Applications
Thermite's unique thermodynamic properties have led to its widespread adoption across various industries, with applications ranging from welding and metal purification to pyrotechnics and military uses. In the manufacturing sector, thermite reactions are extensively utilized for welding railway tracks, creating seamless joints that enhance the durability and safety of rail networks. The high-temperature exothermic reaction allows for on-site welding of large metal components, reducing transportation costs and improving efficiency in infrastructure projects.
The metal production industry benefits significantly from thermite's ability to produce high-purity metals through aluminothermic reduction. This process is particularly valuable in the production of chromium, manganese, and other specialty metals, where traditional smelting methods may be less efficient or cost-effective. The thermite reaction's capacity to generate temperatures exceeding 2500°C enables the separation of desired metals from their ores with minimal impurities, meeting the stringent quality requirements of aerospace and advanced materials industries.
In the field of pyrotechnics, thermite compositions are essential for creating visual effects in fireworks displays and special effects for film and television productions. The intense light and heat generated by thermite reactions provide unique visual spectacles that cannot be easily replicated by other means. This application has created a niche market within the entertainment industry, driving innovation in safe and controlled thermite formulations.
The military and defense sector has long recognized the potential of thermite in incendiary devices and for the destruction of sensitive equipment. Its ability to generate intense heat rapidly makes it an effective tool for disabling vehicles, weapons systems, and electronic devices in combat situations. Additionally, thermite is used in emergency destruction protocols for classified materials, ensuring that sensitive information does not fall into unauthorized hands.
Emerging applications in the field of materials science are exploring the use of thermite reactions for the synthesis of advanced ceramics and composite materials. The extreme temperatures and rapid cooling rates achievable with thermite reactions allow for the creation of unique microstructures and properties in materials, opening new avenues for research and development in high-performance materials for aerospace, energy, and electronics industries.
The growing focus on renewable energy and environmental sustainability has also led to investigations into thermite's potential role in energy storage systems. Researchers are exploring the use of controlled thermite reactions as a means of storing and releasing thermal energy, which could have applications in solar thermal power plants and industrial heat management systems.
The metal production industry benefits significantly from thermite's ability to produce high-purity metals through aluminothermic reduction. This process is particularly valuable in the production of chromium, manganese, and other specialty metals, where traditional smelting methods may be less efficient or cost-effective. The thermite reaction's capacity to generate temperatures exceeding 2500°C enables the separation of desired metals from their ores with minimal impurities, meeting the stringent quality requirements of aerospace and advanced materials industries.
In the field of pyrotechnics, thermite compositions are essential for creating visual effects in fireworks displays and special effects for film and television productions. The intense light and heat generated by thermite reactions provide unique visual spectacles that cannot be easily replicated by other means. This application has created a niche market within the entertainment industry, driving innovation in safe and controlled thermite formulations.
The military and defense sector has long recognized the potential of thermite in incendiary devices and for the destruction of sensitive equipment. Its ability to generate intense heat rapidly makes it an effective tool for disabling vehicles, weapons systems, and electronic devices in combat situations. Additionally, thermite is used in emergency destruction protocols for classified materials, ensuring that sensitive information does not fall into unauthorized hands.
Emerging applications in the field of materials science are exploring the use of thermite reactions for the synthesis of advanced ceramics and composite materials. The extreme temperatures and rapid cooling rates achievable with thermite reactions allow for the creation of unique microstructures and properties in materials, opening new avenues for research and development in high-performance materials for aerospace, energy, and electronics industries.
The growing focus on renewable energy and environmental sustainability has also led to investigations into thermite's potential role in energy storage systems. Researchers are exploring the use of controlled thermite reactions as a means of storing and releasing thermal energy, which could have applications in solar thermal power plants and industrial heat management systems.
Current Challenges
The current challenges in understanding thermite's thermodynamics for better application are multifaceted and complex. One of the primary obstacles is the difficulty in accurately modeling and predicting the behavior of thermite reactions under various conditions. The extreme temperatures and rapid reaction rates involved make it challenging to obtain precise measurements and data during the reaction process.
Another significant challenge lies in controlling the reaction rate and energy release of thermite mixtures. While the high energy density of thermite is advantageous for many applications, it also poses safety risks and can lead to uncontrolled reactions. Developing methods to fine-tune the reaction kinetics and achieve controlled, sustained energy release remains a key area of research.
The heterogeneous nature of thermite mixtures presents additional complications. The interface between the metal fuel and metal oxide oxidizer plays a crucial role in the reaction dynamics, but understanding and optimizing this interface at the microscopic level is still an ongoing challenge. Factors such as particle size, shape, and distribution significantly affect the reaction characteristics, making it difficult to achieve consistent and reproducible results across different batches or scales.
Environmental factors also pose challenges in thermite applications. The sensitivity of thermite mixtures to moisture, temperature, and other external conditions can affect their stability and performance. Developing robust formulations that maintain their properties under varying environmental conditions is essential for expanding the range of practical applications.
Furthermore, the high temperatures generated during thermite reactions can lead to material degradation and unwanted side reactions. This is particularly problematic in applications where the integrity of surrounding materials must be maintained. Finding ways to manage and dissipate heat effectively without compromising the desired energy output is a significant challenge.
The scaling of thermite reactions from laboratory to industrial applications presents another set of challenges. Phenomena that are well-controlled at small scales may behave unpredictably when scaled up, necessitating careful consideration of heat transfer, reaction propagation, and safety measures in larger systems.
Lastly, the development of more environmentally friendly and sustainable thermite formulations is an emerging challenge. Traditional thermite mixtures often contain materials that can have negative environmental impacts. Research into alternative, greener compositions that maintain the desired thermodynamic properties while reducing environmental footprint is becoming increasingly important in the field.
Another significant challenge lies in controlling the reaction rate and energy release of thermite mixtures. While the high energy density of thermite is advantageous for many applications, it also poses safety risks and can lead to uncontrolled reactions. Developing methods to fine-tune the reaction kinetics and achieve controlled, sustained energy release remains a key area of research.
The heterogeneous nature of thermite mixtures presents additional complications. The interface between the metal fuel and metal oxide oxidizer plays a crucial role in the reaction dynamics, but understanding and optimizing this interface at the microscopic level is still an ongoing challenge. Factors such as particle size, shape, and distribution significantly affect the reaction characteristics, making it difficult to achieve consistent and reproducible results across different batches or scales.
Environmental factors also pose challenges in thermite applications. The sensitivity of thermite mixtures to moisture, temperature, and other external conditions can affect their stability and performance. Developing robust formulations that maintain their properties under varying environmental conditions is essential for expanding the range of practical applications.
Furthermore, the high temperatures generated during thermite reactions can lead to material degradation and unwanted side reactions. This is particularly problematic in applications where the integrity of surrounding materials must be maintained. Finding ways to manage and dissipate heat effectively without compromising the desired energy output is a significant challenge.
The scaling of thermite reactions from laboratory to industrial applications presents another set of challenges. Phenomena that are well-controlled at small scales may behave unpredictably when scaled up, necessitating careful consideration of heat transfer, reaction propagation, and safety measures in larger systems.
Lastly, the development of more environmentally friendly and sustainable thermite formulations is an emerging challenge. Traditional thermite mixtures often contain materials that can have negative environmental impacts. Research into alternative, greener compositions that maintain the desired thermodynamic properties while reducing environmental footprint is becoming increasingly important in the field.
Existing Solutions
01 Thermite reaction principles and applications
Thermite reactions involve the exothermic reduction of metal oxides by aluminum, producing intense heat and molten metal. This principle is applied in various fields, including welding, incendiary devices, and metallurgy. The high temperatures generated can be harnessed for specific industrial processes or controlled demolition.- Thermite reaction principles and applications: Thermite reactions involve the exothermic reduction of metal oxides by aluminum, producing intense heat and molten metal. This principle is applied in various fields, including welding, incendiary devices, and metallurgy. The high temperatures generated can be harnessed for specific industrial processes or military applications.
- Composition and formulation of thermite mixtures: The composition of thermite mixtures can be tailored for specific applications by adjusting the ratio of metal oxide to aluminum, as well as incorporating additives. These formulations can influence reaction rate, temperature, and byproducts. Advanced compositions may include nanomaterials or other reactive metals to enhance performance or achieve desired properties.
- Ignition and control mechanisms for thermite reactions: Various methods are employed to initiate and control thermite reactions, including electrical ignition, chemical primers, and mechanical activation. Precise control over the ignition process and reaction propagation is crucial for safety and effectiveness in applications such as welding and metal purification.
- Heat transfer and energy utilization in thermite systems: The intense heat generated by thermite reactions can be harnessed for various purposes, including thermal energy storage, heat engines, and materials processing. Efficient heat transfer mechanisms and thermal management strategies are essential for maximizing energy utilization and controlling the reaction environment.
- Safety considerations and containment of thermite reactions: Due to the high temperatures and potential for rapid energy release, safety is a critical aspect of thermite applications. Containment systems, protective equipment, and handling procedures are developed to mitigate risks associated with thermite reactions. This includes strategies for controlling byproducts, managing slag, and preventing unintended ignition.
02 Composition and formulation of thermite mixtures
The composition of thermite mixtures can be tailored for specific applications by adjusting the ratio of metal oxide to aluminum, or by incorporating additives. These formulations can influence reaction rate, temperature, and byproducts. Advanced compositions may include nanomaterials or catalysts to enhance performance or control the reaction.Expand Specific Solutions03 Ignition and control mechanisms for thermite reactions
Various methods are employed to initiate and control thermite reactions, including electrical ignition, chemical initiators, and mechanical means. Precise control over the ignition process and subsequent reaction propagation is crucial for safety and effectiveness in applications ranging from welding to pyrotechnics.Expand Specific Solutions04 Thermodynamic modeling and analysis of thermite reactions
Advanced computational methods and thermodynamic models are used to predict and analyze the behavior of thermite reactions. These tools help in optimizing mixture compositions, understanding heat transfer mechanisms, and assessing the efficiency of energy release. Such analyses are crucial for developing new applications and improving existing ones.Expand Specific Solutions05 Safety considerations and containment of thermite reactions
Given the high temperatures and potential for rapid energy release, safety is a paramount concern in thermite applications. Specialized containment systems, protective equipment, and handling procedures are developed to mitigate risks associated with thermite reactions. This includes methods for controlling slag formation and managing byproducts in industrial settings.Expand Specific Solutions
Industry Leaders
The thermite reaction market is in a growth phase, driven by increasing applications in various industries. The global market size for thermite-based products and technologies is expanding, with projections indicating continued growth. Technologically, thermite reactions are well-established, but research for improved efficiency and novel applications is ongoing. Companies like Naval Research Laboratory, Lockheed Martin Corp., and Battelle Memorial Institute are at the forefront of advancing thermite technology. Academic institutions such as Nanjing University of Science & Technology and Beijing Institute of Technology are contributing to fundamental research. The industry sees a mix of established players and innovative startups, with companies like BiSN Oil Tools Ltd. and Spectre Primer Technologies, Inc. developing specialized applications, indicating a maturing but still evolving technological landscape.
Naval Research Laboratory
Technical Solution: The Naval Research Laboratory has developed advanced thermite compositions with enhanced energy release and controlled reaction rates. Their research focuses on optimizing particle size distribution and incorporating additives to improve ignition sensitivity and combustion efficiency. They have also explored novel manufacturing techniques, such as 3D printing, to create precisely structured thermite materials for specific applications.
Strengths: Access to advanced research facilities and expertise in material science. Weaknesses: Limited commercial focus and potential restrictions on technology transfer.
Xi'an Modern Chemistry Research Institute
Technical Solution: The Xi'an Modern Chemistry Research Institute has conducted extensive research on thermite reactions, focusing on the development of novel compositions for specialized applications. Their work includes the study of rare earth metal-based thermites, which offer unique properties such as enhanced energy density and tailored reaction temperatures. They have also investigated the use of composite structures to improve the stability and shelf life of thermite materials.
Strengths: Expertise in rare earth materials and access to specialized equipment. Weaknesses: Potential challenges in scaling up production for commercial applications.
Key Innovations
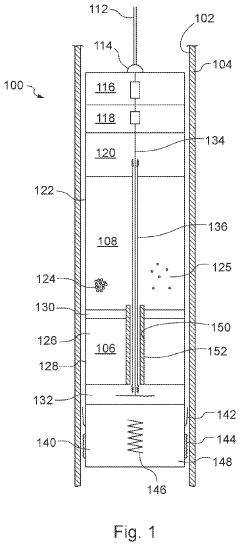
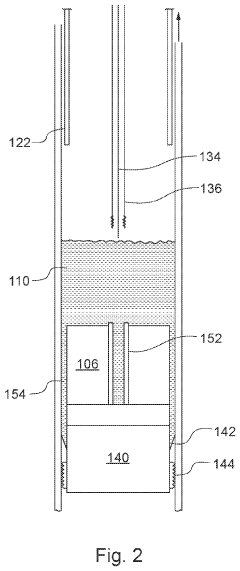
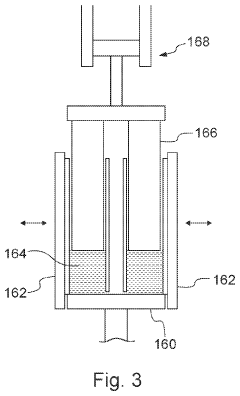
Downhole apparatus and method
PatentActiveUS20240011367A1
Innovation
- A downhole apparatus and method utilizing compressed thermite within a container, which reduces porosity and increases density, facilitating efficient heat transfer and reaction, and is combined with a low-melt-point alloy to form a solid plug, allowing for effective sealing without the need for binding agents and maintaining structural strength under pressure.
Thermite with tailored properties
PatentUndeterminedRO129221A2
Innovation
- Modifying the proportions of iron oxides (FeO, Fe2O3, Fe3O4) in the termite mixture by heating them in controlled atmospheres within specific temperature ranges to adjust the molding temperature of the steel, allowing for tailored thermite recipes with optimized properties for various applications.
Safety Regulations
The safe handling and application of thermite require strict adherence to comprehensive safety regulations due to its highly exothermic nature and potential hazards. These regulations encompass various aspects of thermite use, from storage and transportation to application and disposal.
Storage regulations for thermite mandate secure, dry environments with controlled temperature and humidity. Facilities must be equipped with appropriate fire suppression systems and be located away from populated areas. Access to storage areas should be restricted to authorized personnel only, with proper documentation and inventory control measures in place.
Transportation of thermite is subject to stringent guidelines set by regulatory bodies such as the Department of Transportation (DOT) in the United States. These guidelines specify packaging requirements, labeling standards, and transportation routes to minimize risks during transit. Vehicles used for thermite transportation must meet specific safety standards and be operated by trained personnel.
Personal protective equipment (PPE) regulations for thermite handling are extensive. Workers must wear fire-resistant clothing, face shields, and respiratory protection. Specialized gloves and footwear are also required to protect against thermal and chemical hazards. Regular training and certification programs ensure that personnel are up-to-date with safety protocols and emergency procedures.
Application procedures for thermite are governed by strict safety protocols. These include site preparation, establishment of safety perimeters, and implementation of fire prevention measures. Ignition methods must be carefully controlled, with redundant safety systems in place. Weather conditions, particularly wind speed and direction, must be monitored and factored into the application process.
Disposal of thermite residues and byproducts is regulated to prevent environmental contamination and ensure worker safety. Proper cooling and containment procedures must be followed, and disposal must occur at approved facilities capable of handling hazardous materials.
Emergency response plans are a critical component of thermite safety regulations. These plans outline procedures for fire suppression, chemical spills, and personnel evacuation. Regular drills and simulations are required to maintain readiness for potential incidents.
Regulatory compliance and documentation are essential aspects of thermite safety. Companies must maintain detailed records of thermite use, storage, and disposal. Regular audits and inspections by regulatory agencies ensure ongoing compliance with safety standards.
International regulations for thermite vary, necessitating careful consideration when operating across borders. Companies must navigate different regulatory frameworks and ensure compliance with local, national, and international standards.
As research into thermite's thermodynamics advances, safety regulations continue to evolve. Ongoing studies focus on improving ignition control, developing safer formulations, and enhancing containment strategies. These efforts aim to balance the practical applications of thermite with the paramount concern for safety in its use and handling.
Storage regulations for thermite mandate secure, dry environments with controlled temperature and humidity. Facilities must be equipped with appropriate fire suppression systems and be located away from populated areas. Access to storage areas should be restricted to authorized personnel only, with proper documentation and inventory control measures in place.
Transportation of thermite is subject to stringent guidelines set by regulatory bodies such as the Department of Transportation (DOT) in the United States. These guidelines specify packaging requirements, labeling standards, and transportation routes to minimize risks during transit. Vehicles used for thermite transportation must meet specific safety standards and be operated by trained personnel.
Personal protective equipment (PPE) regulations for thermite handling are extensive. Workers must wear fire-resistant clothing, face shields, and respiratory protection. Specialized gloves and footwear are also required to protect against thermal and chemical hazards. Regular training and certification programs ensure that personnel are up-to-date with safety protocols and emergency procedures.
Application procedures for thermite are governed by strict safety protocols. These include site preparation, establishment of safety perimeters, and implementation of fire prevention measures. Ignition methods must be carefully controlled, with redundant safety systems in place. Weather conditions, particularly wind speed and direction, must be monitored and factored into the application process.
Disposal of thermite residues and byproducts is regulated to prevent environmental contamination and ensure worker safety. Proper cooling and containment procedures must be followed, and disposal must occur at approved facilities capable of handling hazardous materials.
Emergency response plans are a critical component of thermite safety regulations. These plans outline procedures for fire suppression, chemical spills, and personnel evacuation. Regular drills and simulations are required to maintain readiness for potential incidents.
Regulatory compliance and documentation are essential aspects of thermite safety. Companies must maintain detailed records of thermite use, storage, and disposal. Regular audits and inspections by regulatory agencies ensure ongoing compliance with safety standards.
International regulations for thermite vary, necessitating careful consideration when operating across borders. Companies must navigate different regulatory frameworks and ensure compliance with local, national, and international standards.
As research into thermite's thermodynamics advances, safety regulations continue to evolve. Ongoing studies focus on improving ignition control, developing safer formulations, and enhancing containment strategies. These efforts aim to balance the practical applications of thermite with the paramount concern for safety in its use and handling.
Environmental Impact
The environmental impact of thermite reactions is a critical consideration in their application and development. Thermite reactions, characterized by their high-temperature exothermic nature, can have significant effects on the surrounding environment. These impacts range from localized thermal effects to potential long-term ecological consequences.
One of the primary environmental concerns associated with thermite reactions is the release of heat and light. The intense temperatures generated during the reaction can cause localized heating of the surrounding area, potentially affecting nearby flora and fauna. In industrial applications, proper containment and heat management strategies are essential to mitigate these thermal impacts and prevent unintended damage to the ecosystem.
Particulate emissions are another environmental factor to consider. The reaction products of thermite, typically metal oxides and elemental metals, can be released as fine particles into the air. These particulates may contribute to air pollution and pose respiratory risks if not properly controlled. Implementation of effective filtration systems and emission control measures is crucial in industrial settings where thermite reactions are utilized.
The potential for soil and water contamination is also a significant environmental concern. Residual products from thermite reactions, if not properly managed, can leach into soil and water systems. This is particularly relevant in applications such as welding or metal purification, where thermite reactions are conducted in outdoor or less controlled environments. Proper disposal and containment of reaction byproducts are essential to prevent contamination of natural resources.
Long-term ecological effects of thermite reactions, especially in cases of repeated or large-scale use, require careful study. The alteration of soil chemistry, potential impacts on microbial communities, and effects on plant growth in areas exposed to thermite reactions are areas of ongoing research. Understanding these long-term impacts is crucial for developing sustainable practices in industries that rely on thermite technology.
In the context of improving thermite applications, addressing these environmental concerns is paramount. Research into more environmentally friendly thermite compositions, development of advanced containment technologies, and implementation of rigorous safety and disposal protocols are key areas of focus. By minimizing the environmental footprint of thermite reactions, industries can ensure the sustainable use of this powerful technology while preserving ecological balance.
One of the primary environmental concerns associated with thermite reactions is the release of heat and light. The intense temperatures generated during the reaction can cause localized heating of the surrounding area, potentially affecting nearby flora and fauna. In industrial applications, proper containment and heat management strategies are essential to mitigate these thermal impacts and prevent unintended damage to the ecosystem.
Particulate emissions are another environmental factor to consider. The reaction products of thermite, typically metal oxides and elemental metals, can be released as fine particles into the air. These particulates may contribute to air pollution and pose respiratory risks if not properly controlled. Implementation of effective filtration systems and emission control measures is crucial in industrial settings where thermite reactions are utilized.
The potential for soil and water contamination is also a significant environmental concern. Residual products from thermite reactions, if not properly managed, can leach into soil and water systems. This is particularly relevant in applications such as welding or metal purification, where thermite reactions are conducted in outdoor or less controlled environments. Proper disposal and containment of reaction byproducts are essential to prevent contamination of natural resources.
Long-term ecological effects of thermite reactions, especially in cases of repeated or large-scale use, require careful study. The alteration of soil chemistry, potential impacts on microbial communities, and effects on plant growth in areas exposed to thermite reactions are areas of ongoing research. Understanding these long-term impacts is crucial for developing sustainable practices in industries that rely on thermite technology.
In the context of improving thermite applications, addressing these environmental concerns is paramount. Research into more environmentally friendly thermite compositions, development of advanced containment technologies, and implementation of rigorous safety and disposal protocols are key areas of focus. By minimizing the environmental footprint of thermite reactions, industries can ensure the sustainable use of this powerful technology while preserving ecological balance.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!