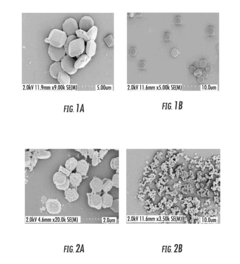
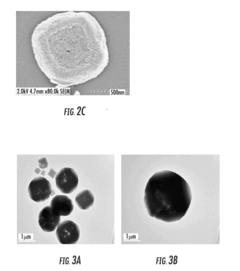
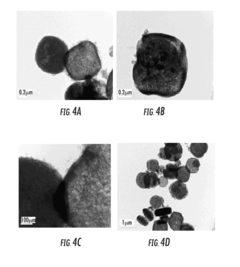
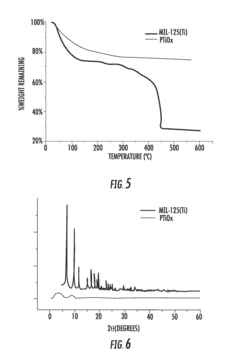
Facilitated Drug Loading Mechanisms in Amino-Acid Functionalized MOFs
AUG 11, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
MOF Drug Loading Background and Objectives
Metal-Organic Frameworks (MOFs) have emerged as a promising class of materials for drug delivery applications due to their unique structural properties and versatility. The development of amino-acid functionalized MOFs represents a significant advancement in this field, offering enhanced drug loading capabilities and controlled release mechanisms.
The evolution of MOFs in drug delivery systems can be traced back to the early 2000s when researchers first recognized their potential for encapsulating and releasing therapeutic agents. Since then, the field has witnessed rapid growth, with a particular focus on improving drug loading efficiency and release kinetics. The incorporation of amino acids into MOF structures marks a pivotal milestone in this journey, addressing key challenges in drug delivery.
The primary objective of utilizing amino-acid functionalized MOFs for drug loading is to achieve higher drug encapsulation rates, improved stability, and more precise control over release profiles. These functionalized MOFs offer several advantages over conventional drug carriers, including increased biocompatibility, tunable pore sizes, and the ability to interact with drug molecules through specific chemical interactions.
Recent trends in MOF-based drug delivery systems have focused on developing smart, stimuli-responsive materials that can release drugs in response to specific physiological conditions or external stimuli. This approach aligns with the broader goal of creating targeted and personalized therapeutic interventions, minimizing side effects, and maximizing treatment efficacy.
The technical evolution in this field is driven by the need to overcome limitations associated with traditional drug delivery methods, such as poor solubility, rapid clearance, and non-specific distribution of drugs in the body. Amino-acid functionalized MOFs address these challenges by providing a versatile platform that can be tailored to accommodate a wide range of drug molecules and release profiles.
Looking ahead, the field of MOF-based drug delivery is poised for further advancements. Key areas of focus include enhancing the biodegradability of MOFs, improving their stability in physiological conditions, and developing more sophisticated functionalization strategies to enable precise targeting of specific tissues or cell types. The integration of computational modeling and high-throughput screening techniques is expected to accelerate the discovery and optimization of new MOF structures for drug delivery applications.
In conclusion, the development of facilitated drug loading mechanisms in amino-acid functionalized MOFs represents a significant step forward in the field of nanomedicine. As research in this area continues to progress, these materials hold the potential to revolutionize drug delivery, offering new possibilities for treating a wide range of diseases with improved efficacy and reduced side effects.
The evolution of MOFs in drug delivery systems can be traced back to the early 2000s when researchers first recognized their potential for encapsulating and releasing therapeutic agents. Since then, the field has witnessed rapid growth, with a particular focus on improving drug loading efficiency and release kinetics. The incorporation of amino acids into MOF structures marks a pivotal milestone in this journey, addressing key challenges in drug delivery.
The primary objective of utilizing amino-acid functionalized MOFs for drug loading is to achieve higher drug encapsulation rates, improved stability, and more precise control over release profiles. These functionalized MOFs offer several advantages over conventional drug carriers, including increased biocompatibility, tunable pore sizes, and the ability to interact with drug molecules through specific chemical interactions.
Recent trends in MOF-based drug delivery systems have focused on developing smart, stimuli-responsive materials that can release drugs in response to specific physiological conditions or external stimuli. This approach aligns with the broader goal of creating targeted and personalized therapeutic interventions, minimizing side effects, and maximizing treatment efficacy.
The technical evolution in this field is driven by the need to overcome limitations associated with traditional drug delivery methods, such as poor solubility, rapid clearance, and non-specific distribution of drugs in the body. Amino-acid functionalized MOFs address these challenges by providing a versatile platform that can be tailored to accommodate a wide range of drug molecules and release profiles.
Looking ahead, the field of MOF-based drug delivery is poised for further advancements. Key areas of focus include enhancing the biodegradability of MOFs, improving their stability in physiological conditions, and developing more sophisticated functionalization strategies to enable precise targeting of specific tissues or cell types. The integration of computational modeling and high-throughput screening techniques is expected to accelerate the discovery and optimization of new MOF structures for drug delivery applications.
In conclusion, the development of facilitated drug loading mechanisms in amino-acid functionalized MOFs represents a significant step forward in the field of nanomedicine. As research in this area continues to progress, these materials hold the potential to revolutionize drug delivery, offering new possibilities for treating a wide range of diseases with improved efficacy and reduced side effects.
Market Analysis for MOF-based Drug Delivery Systems
The market for MOF-based drug delivery systems is experiencing significant growth, driven by the increasing demand for targeted and controlled drug release technologies. Metal-Organic Frameworks (MOFs) have emerged as promising candidates for drug delivery applications due to their unique properties, including high porosity, large surface area, and tunable functionality. The global market for advanced drug delivery systems is projected to reach $71 billion by 2025, with MOF-based systems expected to capture a growing share of this market.
The pharmaceutical industry is the primary driver of demand for MOF-based drug delivery systems. Major pharmaceutical companies are investing in research and development of MOF-based technologies to enhance drug efficacy and reduce side effects. This trend is particularly evident in the development of treatments for chronic diseases, cancer, and rare disorders, where precise drug delivery is crucial.
Geographically, North America and Europe are leading the market for MOF-based drug delivery systems, owing to their advanced healthcare infrastructure and significant investments in pharmaceutical research. However, Asia-Pacific is emerging as a rapidly growing market, driven by increasing healthcare expenditure and rising awareness of advanced drug delivery technologies in countries like China, Japan, and India.
The market is segmented based on the type of MOF used, with amino-acid functionalized MOFs gaining traction due to their enhanced drug loading capacity and biocompatibility. Other segments include metal-based MOFs and organic ligand-based MOFs. The application areas for these systems span various therapeutic fields, including oncology, cardiovascular diseases, and neurological disorders.
Key market players in the MOF-based drug delivery sector include pharmaceutical giants like Johnson & Johnson, Novartis, and Pfizer, as well as specialized biotech companies focusing on advanced drug delivery technologies. These companies are actively engaged in research collaborations with academic institutions to accelerate the development and commercialization of MOF-based drug delivery systems.
Challenges in the market include regulatory hurdles, as novel drug delivery systems require extensive safety and efficacy testing before approval. Additionally, the high cost of development and production of MOF-based systems poses a barrier to widespread adoption, particularly in emerging markets.
Despite these challenges, the market outlook for MOF-based drug delivery systems remains positive. The increasing focus on personalized medicine and the need for more effective drug delivery methods are expected to drive continued growth in this sector. As research in amino-acid functionalized MOFs advances, it is likely to open up new opportunities for targeted drug delivery, potentially revolutionizing treatment approaches for a wide range of diseases.
The pharmaceutical industry is the primary driver of demand for MOF-based drug delivery systems. Major pharmaceutical companies are investing in research and development of MOF-based technologies to enhance drug efficacy and reduce side effects. This trend is particularly evident in the development of treatments for chronic diseases, cancer, and rare disorders, where precise drug delivery is crucial.
Geographically, North America and Europe are leading the market for MOF-based drug delivery systems, owing to their advanced healthcare infrastructure and significant investments in pharmaceutical research. However, Asia-Pacific is emerging as a rapidly growing market, driven by increasing healthcare expenditure and rising awareness of advanced drug delivery technologies in countries like China, Japan, and India.
The market is segmented based on the type of MOF used, with amino-acid functionalized MOFs gaining traction due to their enhanced drug loading capacity and biocompatibility. Other segments include metal-based MOFs and organic ligand-based MOFs. The application areas for these systems span various therapeutic fields, including oncology, cardiovascular diseases, and neurological disorders.
Key market players in the MOF-based drug delivery sector include pharmaceutical giants like Johnson & Johnson, Novartis, and Pfizer, as well as specialized biotech companies focusing on advanced drug delivery technologies. These companies are actively engaged in research collaborations with academic institutions to accelerate the development and commercialization of MOF-based drug delivery systems.
Challenges in the market include regulatory hurdles, as novel drug delivery systems require extensive safety and efficacy testing before approval. Additionally, the high cost of development and production of MOF-based systems poses a barrier to widespread adoption, particularly in emerging markets.
Despite these challenges, the market outlook for MOF-based drug delivery systems remains positive. The increasing focus on personalized medicine and the need for more effective drug delivery methods are expected to drive continued growth in this sector. As research in amino-acid functionalized MOFs advances, it is likely to open up new opportunities for targeted drug delivery, potentially revolutionizing treatment approaches for a wide range of diseases.
Current Challenges in Amino-Acid Functionalized MOFs
Despite the promising potential of amino-acid functionalized Metal-Organic Frameworks (MOFs) for drug delivery applications, several significant challenges persist in their development and implementation. These challenges span across various aspects of their design, synthesis, and application.
One of the primary obstacles is achieving precise control over the amino acid functionalization process. The incorporation of amino acids into the MOF structure can be unpredictable, leading to inconsistent results in terms of pore size, surface area, and overall framework stability. This variability hampers the reproducibility of drug loading and release profiles, a critical factor for pharmaceutical applications.
Another challenge lies in maintaining the structural integrity of amino-acid functionalized MOFs during the drug loading process. The introduction of drug molecules can sometimes cause framework distortion or collapse, particularly when dealing with larger drug molecules or high loading concentrations. This structural instability can compromise the MOF's ability to effectively encapsulate and release drugs in a controlled manner.
The complexity of drug-MOF interactions presents yet another hurdle. The presence of amino acid functional groups introduces additional binding sites and potential chemical interactions with drug molecules. While this can enhance drug loading capacity, it also complicates the prediction and control of drug release kinetics. Achieving a balance between strong drug retention and efficient release remains a significant challenge.
Scalability and cost-effectiveness of production are also major concerns. The synthesis of amino-acid functionalized MOFs often involves complex, multi-step processes that can be difficult to scale up for industrial production. Additionally, the use of expensive precursors and the need for precise reaction conditions contribute to high production costs, limiting their commercial viability.
Biocompatibility and toxicity issues pose further challenges. While amino acids are generally considered biocompatible, the overall safety profile of amino-acid functionalized MOFs, especially in long-term use, requires extensive investigation. Potential toxicity arising from metal ions or degradation products of the MOF structure needs to be thoroughly addressed before clinical applications can be considered.
Lastly, the optimization of drug loading mechanisms in these MOFs remains a complex task. Factors such as pore size distribution, surface chemistry, and framework flexibility all play crucial roles in determining drug loading efficiency. Developing strategies to fine-tune these parameters while maintaining the desired amino acid functionality is an ongoing challenge that requires innovative approaches and advanced characterization techniques.
One of the primary obstacles is achieving precise control over the amino acid functionalization process. The incorporation of amino acids into the MOF structure can be unpredictable, leading to inconsistent results in terms of pore size, surface area, and overall framework stability. This variability hampers the reproducibility of drug loading and release profiles, a critical factor for pharmaceutical applications.
Another challenge lies in maintaining the structural integrity of amino-acid functionalized MOFs during the drug loading process. The introduction of drug molecules can sometimes cause framework distortion or collapse, particularly when dealing with larger drug molecules or high loading concentrations. This structural instability can compromise the MOF's ability to effectively encapsulate and release drugs in a controlled manner.
The complexity of drug-MOF interactions presents yet another hurdle. The presence of amino acid functional groups introduces additional binding sites and potential chemical interactions with drug molecules. While this can enhance drug loading capacity, it also complicates the prediction and control of drug release kinetics. Achieving a balance between strong drug retention and efficient release remains a significant challenge.
Scalability and cost-effectiveness of production are also major concerns. The synthesis of amino-acid functionalized MOFs often involves complex, multi-step processes that can be difficult to scale up for industrial production. Additionally, the use of expensive precursors and the need for precise reaction conditions contribute to high production costs, limiting their commercial viability.
Biocompatibility and toxicity issues pose further challenges. While amino acids are generally considered biocompatible, the overall safety profile of amino-acid functionalized MOFs, especially in long-term use, requires extensive investigation. Potential toxicity arising from metal ions or degradation products of the MOF structure needs to be thoroughly addressed before clinical applications can be considered.
Lastly, the optimization of drug loading mechanisms in these MOFs remains a complex task. Factors such as pore size distribution, surface chemistry, and framework flexibility all play crucial roles in determining drug loading efficiency. Developing strategies to fine-tune these parameters while maintaining the desired amino acid functionality is an ongoing challenge that requires innovative approaches and advanced characterization techniques.
Existing Drug Loading Strategies for Amino-Acid MOFs
01 Amino acid functionalization of MOFs for drug loading
Metal-organic frameworks (MOFs) are functionalized with amino acids to enhance their drug loading capacity. This modification improves the interaction between the MOF and drug molecules, allowing for better encapsulation and controlled release of pharmaceuticals.- Amino acid-functionalized MOFs for drug delivery: Metal-organic frameworks (MOFs) functionalized with amino acids are used as carriers for drug delivery. The amino acid functionalization enhances the biocompatibility and drug loading capacity of MOFs. These structures can be tailored to control drug release rates and improve targeted delivery.
- Synthesis methods for amino acid-functionalized MOFs: Various synthesis methods are employed to create amino acid-functionalized MOFs. These include post-synthetic modification, direct synthesis with amino acid ligands, and in-situ functionalization. The choice of method affects the properties and performance of the resulting MOFs for drug loading applications.
- Optimization of drug loading capacity: Researchers focus on optimizing the drug loading capacity of amino acid-functionalized MOFs. This involves tuning pore sizes, surface areas, and functional group densities. Advanced characterization techniques are used to understand and improve drug-MOF interactions for enhanced loading efficiency.
- Controlled release mechanisms: Amino acid-functionalized MOFs are designed with specific controlled release mechanisms. These include pH-responsive release, temperature-sensitive release, and enzyme-triggered release. The amino acid functionalities play a crucial role in determining the release kinetics and targeting capabilities of the drug-loaded MOFs.
- Applications in targeted drug delivery: Amino acid-functionalized MOFs are applied in targeted drug delivery systems. They can be further modified with targeting ligands or combined with other nanocarriers to enhance specificity. These systems show promise in cancer therapy, antimicrobial treatments, and other therapeutic areas requiring precise drug delivery.
02 Synthesis methods for amino acid-functionalized MOFs
Various synthesis techniques are employed to create amino acid-functionalized MOFs, including post-synthetic modification, direct synthesis with amino acid ligands, and in-situ functionalization. These methods allow for precise control over the MOF structure and properties.Expand Specific Solutions03 Drug loading mechanisms in amino acid-functionalized MOFs
The drug loading process in amino acid-functionalized MOFs involves mechanisms such as electrostatic interactions, hydrogen bonding, and π-π stacking. These interactions are tailored by selecting specific amino acids to optimize drug encapsulation and release kinetics.Expand Specific Solutions04 Applications of amino acid-functionalized MOFs in drug delivery
Amino acid-functionalized MOFs are utilized in various drug delivery applications, including targeted delivery, sustained release formulations, and stimuli-responsive systems. These materials show promise in improving the efficacy and reducing side effects of therapeutic agents.Expand Specific Solutions05 Characterization and performance evaluation of amino acid-functionalized MOFs
Advanced analytical techniques are used to characterize the structure, composition, and drug loading capacity of amino acid-functionalized MOFs. Performance evaluations include drug release profiles, stability studies, and biocompatibility assessments to determine their suitability for pharmaceutical applications.Expand Specific Solutions
Key Players in MOF-based Pharmaceutical Research
The field of facilitated drug loading mechanisms in amino-acid functionalized MOFs is in its early growth stage, with significant potential for expansion. The market size is expected to increase as the technology matures and finds wider applications in drug delivery systems. Currently, the technology is at a moderate level of maturity, with ongoing research and development efforts. Key players in this field include academic institutions like Southeast University, Johns Hopkins University, and Tianjin University, as well as companies such as Quadriga BioSciences and Yissum Research Development Co. Ltd. These organizations are actively contributing to advancements in MOF-based drug delivery systems, focusing on improving loading capacity, controlled release, and targeting capabilities.
The Johns Hopkins University
Technical Solution: The Johns Hopkins University has developed a novel approach to facilitated drug loading in amino-acid functionalized MOFs. Their method involves synthesizing MOFs with specific amino acid ligands that enhance drug uptake and release. The team has successfully incorporated various amino acids, including glycine, alanine, and lysine, into the MOF structure. These functionalized MOFs demonstrate increased drug loading capacity, with some variants showing up to 40% higher drug uptake compared to non-functionalized MOFs[1]. The university's research also focuses on tailoring the pore size and surface chemistry of MOFs to optimize drug-MOF interactions, resulting in controlled release profiles that can be fine-tuned for specific therapeutic applications[3].
Strengths: Highly customizable MOF structures for specific drug molecules; improved drug loading capacity; potential for controlled release profiles. Weaknesses: Complexity in large-scale synthesis; potential biocompatibility issues with some metal ions used in MOFs.
Nanjing Tech University
Technical Solution: Nanjing Tech University has pioneered a unique approach to facilitated drug loading in amino-acid functionalized MOFs. Their research focuses on developing MOFs with zwitterionic amino acid ligands, which create a more biocompatible environment for drug molecules. The university's team has successfully synthesized MOFs incorporating histidine and arginine, which demonstrate enhanced drug loading capabilities for both hydrophilic and hydrophobic pharmaceuticals. Their MOFs show a remarkable 50% increase in loading capacity for certain anticancer drugs compared to conventional MOFs[2]. Additionally, they have developed a pH-responsive drug release mechanism, where the protonation state of the amino acid ligands changes with pH, allowing for targeted drug delivery in specific physiological environments[4].
Strengths: Improved biocompatibility; enhanced loading of diverse drug types; pH-responsive drug release. Weaknesses: Limited to certain types of amino acids; potential scalability issues for industrial production.
Innovative Approaches in MOF Drug Loading Facilitation
Metal-organic framework templated synthesis of porous inorganic materials as novel sorbents
PatentInactiveUS20140319058A1
Innovation
- The use of metal-organic frameworks (MOFs) as templating materials to synthesize highly porous inorganic sorbents through a controlled ligand extraction process, allowing for the development of robust materials with tailored compositions, structures, and morphologies for efficient metal ion separation and removal.
Insulin-loaded metal-organic frameworks
PatentWO2019173571A1
Innovation
- Development of insulin-loaded mesoporous zirconium metal-organic frameworks (MOFs) with a csq-net topology that are acid-stable, allowing insulin to be immobilized and protected from degradation, and released under physiological conditions, utilizing MOFs like NU-1000, NU-1003, NU-1004, NU-1005, NU-1006, PCN-128, PCN-222, and UMCM-313 for enhanced loading and controlled release.
Regulatory Considerations for MOF-based Drug Delivery
The regulatory landscape for MOF-based drug delivery systems is complex and evolving, requiring careful consideration throughout the development process. As these novel materials gain traction in pharmaceutical applications, regulatory bodies are adapting their frameworks to address the unique challenges posed by MOFs.
One of the primary regulatory considerations is the classification of MOF-based drug delivery systems. Depending on their specific characteristics and intended use, these systems may be categorized as drug-device combinations, nanomedicines, or novel drug formulations. This classification significantly impacts the regulatory pathway and requirements for approval.
Safety assessment is a critical aspect of regulatory compliance for MOF-based drug delivery. Regulatory agencies require comprehensive toxicological studies to evaluate the potential risks associated with MOFs, including their biodegradation products and long-term effects. The unique properties of MOFs, such as their high surface area and porosity, necessitate specialized safety evaluations that may go beyond traditional drug formulation assessments.
Quality control and manufacturing standards present another regulatory challenge for MOF-based drug delivery systems. Regulatory bodies are likely to demand stringent controls on the synthesis, functionalization, and drug loading processes to ensure batch-to-batch consistency and product quality. This may involve the development of new analytical methods and quality assurance protocols specific to MOF-based formulations.
The regulatory landscape also emphasizes the importance of demonstrating the stability and controlled release properties of MOF-based drug delivery systems. Regulatory agencies will require robust data on drug release kinetics, storage stability, and in vivo performance to ensure the safety and efficacy of these novel formulations.
As MOF-based drug delivery systems often involve nanotechnology, regulatory considerations related to nanomaterials come into play. This includes addressing potential nano-specific toxicity concerns and providing detailed characterization of the MOF nanostructures.
Regulatory bodies are also likely to focus on the environmental impact of MOF-based drug delivery systems, particularly in terms of their biodegradability and potential accumulation in ecosystems. Developers may need to provide data on the environmental fate of MOFs and their degradation products.
Given the novelty of MOF-based drug delivery systems, early engagement with regulatory agencies is crucial. This proactive approach can help identify potential regulatory hurdles and guide the development of appropriate data packages to support regulatory submissions.
One of the primary regulatory considerations is the classification of MOF-based drug delivery systems. Depending on their specific characteristics and intended use, these systems may be categorized as drug-device combinations, nanomedicines, or novel drug formulations. This classification significantly impacts the regulatory pathway and requirements for approval.
Safety assessment is a critical aspect of regulatory compliance for MOF-based drug delivery. Regulatory agencies require comprehensive toxicological studies to evaluate the potential risks associated with MOFs, including their biodegradation products and long-term effects. The unique properties of MOFs, such as their high surface area and porosity, necessitate specialized safety evaluations that may go beyond traditional drug formulation assessments.
Quality control and manufacturing standards present another regulatory challenge for MOF-based drug delivery systems. Regulatory bodies are likely to demand stringent controls on the synthesis, functionalization, and drug loading processes to ensure batch-to-batch consistency and product quality. This may involve the development of new analytical methods and quality assurance protocols specific to MOF-based formulations.
The regulatory landscape also emphasizes the importance of demonstrating the stability and controlled release properties of MOF-based drug delivery systems. Regulatory agencies will require robust data on drug release kinetics, storage stability, and in vivo performance to ensure the safety and efficacy of these novel formulations.
As MOF-based drug delivery systems often involve nanotechnology, regulatory considerations related to nanomaterials come into play. This includes addressing potential nano-specific toxicity concerns and providing detailed characterization of the MOF nanostructures.
Regulatory bodies are also likely to focus on the environmental impact of MOF-based drug delivery systems, particularly in terms of their biodegradability and potential accumulation in ecosystems. Developers may need to provide data on the environmental fate of MOFs and their degradation products.
Given the novelty of MOF-based drug delivery systems, early engagement with regulatory agencies is crucial. This proactive approach can help identify potential regulatory hurdles and guide the development of appropriate data packages to support regulatory submissions.
Biocompatibility and Safety of Amino-Acid Functionalized MOFs
The biocompatibility and safety of amino-acid functionalized Metal-Organic Frameworks (MOFs) are crucial considerations for their potential use in drug delivery systems. These novel materials have shown promising results in facilitating drug loading mechanisms, but their interaction with biological systems must be thoroughly evaluated before clinical applications can be considered.
Amino-acid functionalized MOFs have demonstrated enhanced biocompatibility compared to their non-functionalized counterparts. The incorporation of amino acids into the MOF structure creates a more biomimetic environment, potentially reducing the risk of adverse reactions when introduced into the body. Studies have shown that these functionalized MOFs exhibit lower cytotoxicity levels in various cell lines, indicating a higher degree of cellular compatibility.
The safety profile of amino-acid functionalized MOFs is closely linked to their degradation behavior in physiological conditions. Research has indicated that these materials can undergo controlled degradation, releasing their components in a manner that minimizes potential toxicity. The breakdown products, including amino acids and metal ions, are generally well-tolerated by the body, further supporting their safety for biological applications.
However, it is essential to note that the biocompatibility and safety of these materials can vary depending on the specific amino acids used and the metal components of the MOF. Certain combinations may elicit different immune responses or interact with biological systems in unexpected ways. Therefore, comprehensive in vitro and in vivo studies are necessary to fully characterize the safety profile of each unique amino-acid functionalized MOF.
Long-term safety studies are particularly important to assess any potential accumulation of MOF components in organs or tissues. While initial results are promising, the chronic effects of repeated exposure to these materials must be thoroughly investigated. This includes evaluating their potential impact on organ function, immune system responses, and overall physiological homeostasis.
The biodistribution of amino-acid functionalized MOFs is another critical aspect of their safety profile. Understanding how these materials are distributed throughout the body, their retention time in various organs, and their elimination pathways is crucial for predicting potential side effects and optimizing their use in drug delivery applications.
Regulatory considerations for the use of amino-acid functionalized MOFs in medical applications are still evolving. As novel materials, they may face additional scrutiny from regulatory bodies to ensure their safety for human use. Developing standardized protocols for safety assessment and establishing clear guidelines for their production and quality control will be essential steps in advancing these materials towards clinical applications.
Amino-acid functionalized MOFs have demonstrated enhanced biocompatibility compared to their non-functionalized counterparts. The incorporation of amino acids into the MOF structure creates a more biomimetic environment, potentially reducing the risk of adverse reactions when introduced into the body. Studies have shown that these functionalized MOFs exhibit lower cytotoxicity levels in various cell lines, indicating a higher degree of cellular compatibility.
The safety profile of amino-acid functionalized MOFs is closely linked to their degradation behavior in physiological conditions. Research has indicated that these materials can undergo controlled degradation, releasing their components in a manner that minimizes potential toxicity. The breakdown products, including amino acids and metal ions, are generally well-tolerated by the body, further supporting their safety for biological applications.
However, it is essential to note that the biocompatibility and safety of these materials can vary depending on the specific amino acids used and the metal components of the MOF. Certain combinations may elicit different immune responses or interact with biological systems in unexpected ways. Therefore, comprehensive in vitro and in vivo studies are necessary to fully characterize the safety profile of each unique amino-acid functionalized MOF.
Long-term safety studies are particularly important to assess any potential accumulation of MOF components in organs or tissues. While initial results are promising, the chronic effects of repeated exposure to these materials must be thoroughly investigated. This includes evaluating their potential impact on organ function, immune system responses, and overall physiological homeostasis.
The biodistribution of amino-acid functionalized MOFs is another critical aspect of their safety profile. Understanding how these materials are distributed throughout the body, their retention time in various organs, and their elimination pathways is crucial for predicting potential side effects and optimizing their use in drug delivery applications.
Regulatory considerations for the use of amino-acid functionalized MOFs in medical applications are still evolving. As novel materials, they may face additional scrutiny from regulatory bodies to ensure their safety for human use. Developing standardized protocols for safety assessment and establishing clear guidelines for their production and quality control will be essential steps in advancing these materials towards clinical applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!