Finite Element Method For Bio-Mechanical Simulations: Material Models And Boundary Conditions
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
FEM Bio-Mechanics Background and Objectives
The Finite Element Method (FEM) has emerged as a cornerstone computational technique in biomechanical engineering since its initial application to biological tissues in the 1970s. This numerical approach has revolutionized our understanding of complex biological systems by enabling the simulation of mechanical behaviors across multiple scales—from cellular mechanics to whole-organ biomechanics. The evolution of FEM in biomechanics has closely followed advancements in computational power, imaging technologies, and material science, creating an increasingly sophisticated toolkit for researchers and clinicians alike.
The fundamental principle of FEM involves discretizing complex geometries into simpler finite elements, applying appropriate constitutive equations to describe material behavior, and solving the resulting system of equations under specified boundary conditions. In biomechanical applications, this approach has proven invaluable for predicting tissue responses to mechanical loads, designing medical devices, planning surgical interventions, and understanding pathophysiological processes.
Recent technological developments have significantly expanded FEM capabilities in biomechanics. High-resolution imaging modalities such as micro-CT, MRI, and confocal microscopy now provide unprecedented anatomical detail for model construction. Parallel computing and GPU acceleration have dramatically reduced computation times, enabling more complex simulations with finer mesh resolutions. Additionally, machine learning techniques are increasingly being integrated with FEM to enhance model accuracy and efficiency.
The current technical landscape presents both opportunities and challenges. While computational resources continue to improve, the accurate representation of biological tissues remains challenging due to their inherent heterogeneity, anisotropy, and time-dependent behaviors. Material models must capture complex phenomena such as viscoelasticity, hyperelasticity, and growth/remodeling processes. Similarly, boundary conditions must reflect physiological loading scenarios and tissue interactions that are often difficult to measure directly.
This technical pre-research aims to comprehensively evaluate the state-of-the-art in FEM biomechanical simulations, with particular focus on material models and boundary conditions. We seek to identify current limitations, emerging solutions, and promising research directions that could advance the field. The ultimate objective is to develop more accurate, efficient, and clinically relevant simulation frameworks that can better predict tissue behavior under physiological and pathological conditions, thereby improving diagnostic capabilities, treatment planning, and medical device design.
By systematically analyzing current approaches and identifying key technological gaps, this research will establish a roadmap for future developments in biomechanical FEM simulations, with potential applications spanning orthopedics, cardiovascular medicine, tissue engineering, and rehabilitation sciences.
The fundamental principle of FEM involves discretizing complex geometries into simpler finite elements, applying appropriate constitutive equations to describe material behavior, and solving the resulting system of equations under specified boundary conditions. In biomechanical applications, this approach has proven invaluable for predicting tissue responses to mechanical loads, designing medical devices, planning surgical interventions, and understanding pathophysiological processes.
Recent technological developments have significantly expanded FEM capabilities in biomechanics. High-resolution imaging modalities such as micro-CT, MRI, and confocal microscopy now provide unprecedented anatomical detail for model construction. Parallel computing and GPU acceleration have dramatically reduced computation times, enabling more complex simulations with finer mesh resolutions. Additionally, machine learning techniques are increasingly being integrated with FEM to enhance model accuracy and efficiency.
The current technical landscape presents both opportunities and challenges. While computational resources continue to improve, the accurate representation of biological tissues remains challenging due to their inherent heterogeneity, anisotropy, and time-dependent behaviors. Material models must capture complex phenomena such as viscoelasticity, hyperelasticity, and growth/remodeling processes. Similarly, boundary conditions must reflect physiological loading scenarios and tissue interactions that are often difficult to measure directly.
This technical pre-research aims to comprehensively evaluate the state-of-the-art in FEM biomechanical simulations, with particular focus on material models and boundary conditions. We seek to identify current limitations, emerging solutions, and promising research directions that could advance the field. The ultimate objective is to develop more accurate, efficient, and clinically relevant simulation frameworks that can better predict tissue behavior under physiological and pathological conditions, thereby improving diagnostic capabilities, treatment planning, and medical device design.
By systematically analyzing current approaches and identifying key technological gaps, this research will establish a roadmap for future developments in biomechanical FEM simulations, with potential applications spanning orthopedics, cardiovascular medicine, tissue engineering, and rehabilitation sciences.
Market Applications in Biomedical Engineering
The Finite Element Method (FEM) has revolutionized biomedical engineering by enabling sophisticated biomechanical simulations that support various clinical and commercial applications. The market for FEM-based biomedical applications has experienced substantial growth, driven by increasing demand for personalized medicine and advanced medical devices.
Orthopedic implant design represents one of the largest market segments utilizing biomechanical FEM simulations. Companies like Zimmer Biomet, Stryker, and Johnson & Johnson employ these simulations to optimize implant geometries, material selection, and fixation techniques. This approach has significantly reduced development cycles and improved implant longevity, addressing a market valued at over $45 billion globally.
Cardiovascular device development constitutes another critical application area. FEM simulations enable the design and testing of stents, heart valves, and vascular grafts under physiologically realistic conditions. These simulations incorporate complex material models that capture the hyperelastic and anisotropic behavior of blood vessels, allowing manufacturers to predict device performance and reduce animal testing requirements.
The dental industry has widely adopted FEM for designing dental implants and prosthetics. Companies utilize biomechanical simulations to analyze stress distributions in dental structures, optimizing implant designs for different bone densities and loading conditions. This market segment continues to expand as digital dentistry becomes increasingly prevalent.
Surgical planning software represents an emerging high-growth market application. Patient-specific FEM models derived from medical imaging data allow surgeons to simulate procedures before entering the operating room. This technology is particularly valuable in complex orthopedic, craniofacial, and cardiovascular surgeries, where it can reduce operating times and improve outcomes.
Tissue engineering and regenerative medicine applications are gaining significant traction. FEM simulations help design scaffolds with appropriate mechanical properties to support cell growth and tissue development. These applications require sophisticated material models that capture the time-dependent behavior of biological tissues and biodegradable materials.
Regulatory bodies increasingly recognize FEM simulation data as supporting evidence for medical device approval. The FDA's Medical Device Development Tools (MDDT) program now includes computational modeling as a recognized methodology, creating market opportunities for simulation software providers and consultancies specializing in regulatory submissions.
The global market for biomechanical simulation software and services continues to expand, with specialized players like Simulia (Dassault Systèmes), Ansys, and Materialise competing alongside industry-specific solution providers. As computational capabilities advance and material models become more sophisticated, the market applications for FEM in biomedical engineering will continue to diversify and grow.
Orthopedic implant design represents one of the largest market segments utilizing biomechanical FEM simulations. Companies like Zimmer Biomet, Stryker, and Johnson & Johnson employ these simulations to optimize implant geometries, material selection, and fixation techniques. This approach has significantly reduced development cycles and improved implant longevity, addressing a market valued at over $45 billion globally.
Cardiovascular device development constitutes another critical application area. FEM simulations enable the design and testing of stents, heart valves, and vascular grafts under physiologically realistic conditions. These simulations incorporate complex material models that capture the hyperelastic and anisotropic behavior of blood vessels, allowing manufacturers to predict device performance and reduce animal testing requirements.
The dental industry has widely adopted FEM for designing dental implants and prosthetics. Companies utilize biomechanical simulations to analyze stress distributions in dental structures, optimizing implant designs for different bone densities and loading conditions. This market segment continues to expand as digital dentistry becomes increasingly prevalent.
Surgical planning software represents an emerging high-growth market application. Patient-specific FEM models derived from medical imaging data allow surgeons to simulate procedures before entering the operating room. This technology is particularly valuable in complex orthopedic, craniofacial, and cardiovascular surgeries, where it can reduce operating times and improve outcomes.
Tissue engineering and regenerative medicine applications are gaining significant traction. FEM simulations help design scaffolds with appropriate mechanical properties to support cell growth and tissue development. These applications require sophisticated material models that capture the time-dependent behavior of biological tissues and biodegradable materials.
Regulatory bodies increasingly recognize FEM simulation data as supporting evidence for medical device approval. The FDA's Medical Device Development Tools (MDDT) program now includes computational modeling as a recognized methodology, creating market opportunities for simulation software providers and consultancies specializing in regulatory submissions.
The global market for biomechanical simulation software and services continues to expand, with specialized players like Simulia (Dassault Systèmes), Ansys, and Materialise competing alongside industry-specific solution providers. As computational capabilities advance and material models become more sophisticated, the market applications for FEM in biomedical engineering will continue to diversify and grow.
Current Challenges in Bio-Mechanical FEM
Despite significant advancements in bio-mechanical finite element modeling, several critical challenges persist that limit the accuracy and applicability of these simulations. One fundamental issue is the accurate characterization of biological tissue properties, which exhibit complex behaviors including anisotropy, viscoelasticity, and heterogeneity. Unlike traditional engineering materials, biological tissues demonstrate strain-dependent responses and time-dependent behaviors that are difficult to capture in conventional constitutive models.
The multi-scale nature of biological systems presents another significant challenge. Phenomena occurring at molecular, cellular, tissue, and organ levels all contribute to overall mechanical behavior, yet developing models that effectively bridge these scales remains problematic. Current computational limitations often force researchers to make simplifications that may compromise biological fidelity.
Patient-specific modeling represents a frontier challenge, as biological variability between individuals necessitates customized material parameters. The acquisition of in vivo material properties without invasive procedures remains technically difficult, limiting the personalization of models. Additionally, the validation of these models against experimental data is complicated by ethical constraints and measurement difficulties in living tissues.
Boundary conditions present particular difficulties in bio-mechanical FEM. The complex interactions between different tissue types at interfaces, such as bone-cartilage or muscle-tendon junctions, are not easily represented by standard contact algorithms. Furthermore, accurately capturing physiological loading conditions, especially for dynamic processes like gait or mastication, requires sophisticated approaches that can represent time-varying forces and constraints.
Computational efficiency remains a persistent challenge, particularly for simulations involving large deformations, contact problems, or fluid-structure interactions common in biological systems. Real-time applications for surgical planning or medical device testing are limited by these computational demands.
The integration of growth, remodeling, and adaptation processes into FEM frameworks represents an emerging challenge. Biological tissues continuously respond to mechanical stimuli through processes like bone remodeling or soft tissue growth, yet incorporating these time-dependent adaptive responses into simulations requires complex coupling of mechanical models with biological rule sets.
Finally, there is a significant gap between the development of sophisticated bio-mechanical models in research settings and their practical implementation in clinical environments. User-friendly interfaces, robust validation protocols, and clinical interpretation guidelines are needed to translate these advanced modeling capabilities into practical tools for medical applications.
The multi-scale nature of biological systems presents another significant challenge. Phenomena occurring at molecular, cellular, tissue, and organ levels all contribute to overall mechanical behavior, yet developing models that effectively bridge these scales remains problematic. Current computational limitations often force researchers to make simplifications that may compromise biological fidelity.
Patient-specific modeling represents a frontier challenge, as biological variability between individuals necessitates customized material parameters. The acquisition of in vivo material properties without invasive procedures remains technically difficult, limiting the personalization of models. Additionally, the validation of these models against experimental data is complicated by ethical constraints and measurement difficulties in living tissues.
Boundary conditions present particular difficulties in bio-mechanical FEM. The complex interactions between different tissue types at interfaces, such as bone-cartilage or muscle-tendon junctions, are not easily represented by standard contact algorithms. Furthermore, accurately capturing physiological loading conditions, especially for dynamic processes like gait or mastication, requires sophisticated approaches that can represent time-varying forces and constraints.
Computational efficiency remains a persistent challenge, particularly for simulations involving large deformations, contact problems, or fluid-structure interactions common in biological systems. Real-time applications for surgical planning or medical device testing are limited by these computational demands.
The integration of growth, remodeling, and adaptation processes into FEM frameworks represents an emerging challenge. Biological tissues continuously respond to mechanical stimuli through processes like bone remodeling or soft tissue growth, yet incorporating these time-dependent adaptive responses into simulations requires complex coupling of mechanical models with biological rule sets.
Finally, there is a significant gap between the development of sophisticated bio-mechanical models in research settings and their practical implementation in clinical environments. User-friendly interfaces, robust validation protocols, and clinical interpretation guidelines are needed to translate these advanced modeling capabilities into practical tools for medical applications.
State-of-Art Material Models for Biological Tissues
01 Material models for finite element analysis
Various material models can be implemented in finite element method (FEM) simulations to accurately represent the physical behavior of different materials. These models include linear elastic, non-linear elastic, plastic, viscoelastic, and composite material models. The selection of an appropriate material model is crucial for obtaining accurate simulation results that reflect real-world behavior under various loading conditions. Advanced material models can account for anisotropy, temperature dependence, and strain rate effects.- Material modeling in finite element analysis: Various material models can be implemented in finite element method (FEM) simulations to accurately represent the physical behavior of different materials. These models include linear elastic, non-linear, viscoelastic, and plastic material behaviors. The selection of appropriate material models is crucial for achieving accurate simulation results that reflect real-world material responses under various loading conditions. Advanced material models can account for complex behaviors such as strain hardening, creep, and temperature-dependent properties.
- Boundary condition implementation techniques: Boundary conditions define the constraints and external influences on a finite element model. Proper implementation of boundary conditions is essential for accurate simulation results. Techniques include fixed constraints, displacement constraints, symmetry conditions, and load applications. Advanced boundary condition implementations can handle contact interfaces, thermal boundaries, and time-dependent constraints. The accuracy of simulation results heavily depends on how well the boundary conditions represent the physical reality of the problem being solved.
- Multi-physics coupling in FEM simulations: Finite element method can be extended to handle coupled multi-physics problems where different physical phenomena interact. This includes thermo-mechanical coupling, fluid-structure interaction, and electro-mechanical systems. The coupling requires special material models that account for interactions between different physical domains and appropriate boundary conditions at the interfaces. Multi-physics simulations enable more comprehensive analysis of complex systems where multiple physical phenomena occur simultaneously.
- Optimization and validation of FEM models: Optimization techniques are used to refine finite element models by adjusting material parameters and boundary conditions to match experimental data. This includes parameter identification, sensitivity analysis, and uncertainty quantification. Validation methodologies ensure that the simulation results accurately represent physical reality by comparing with experimental measurements. These approaches help to improve the reliability and predictive capability of finite element simulations for engineering applications.
- Advanced numerical methods for FEM solution: Advanced numerical methods enhance the solution process for finite element problems with complex material models and boundary conditions. These include adaptive mesh refinement, parallel computing techniques, and specialized solvers for non-linear problems. Numerical stability and convergence are critical considerations when implementing complex material models or boundary conditions. These methods improve computational efficiency and enable the solution of larger and more complex problems that would otherwise be computationally prohibitive.
02 Boundary conditions implementation in FEM
Boundary conditions are essential constraints applied to finite element models to represent real-world physical constraints and loads. These include displacement constraints, force loads, pressure loads, thermal conditions, and contact interfaces. Proper implementation of boundary conditions ensures that the simulation accurately represents the physical problem being solved. Advanced techniques allow for time-dependent boundary conditions and adaptive boundary conditions that change during simulation.Expand Specific Solutions03 Optimization of FEM models and solution methods
Optimization techniques are applied to finite element models to improve computational efficiency while maintaining accuracy. These include mesh optimization, adaptive refinement, solver selection, and parallel computing strategies. Advanced algorithms can automatically determine optimal element sizes and distributions based on error estimators. Solution methods may include direct solvers for smaller problems and iterative solvers for large-scale simulations, with preconditioners to improve convergence.Expand Specific Solutions04 Multi-physics coupling in FEM simulations
Multi-physics coupling approaches allow finite element simulations to account for interactions between different physical phenomena, such as structural-thermal, fluid-structure, or electromagnetic-thermal coupling. These coupled analyses require specialized formulations to handle the transfer of information between different physics domains. Staggered or monolithic solution approaches may be employed depending on the strength of coupling between the physical phenomena being modeled.Expand Specific Solutions05 Validation and verification of FEM results
Validation and verification methodologies ensure the accuracy and reliability of finite element simulations. Verification involves checking that the mathematical model is solved correctly, while validation confirms that the model accurately represents physical reality. Techniques include mesh convergence studies, comparison with analytical solutions, and experimental validation. Error estimation methods help quantify the accuracy of simulation results and guide model refinement.Expand Specific Solutions
Leading Research Groups and Software Vendors
The Finite Element Method (FEM) for bio-mechanical simulations market is currently in a growth phase, with increasing adoption across medical, automotive, and research sectors. The global market size is estimated to exceed $2 billion, driven by demand for accurate biological tissue modeling and personalized medicine applications. Leading the technological landscape is ANSYS, Inc., which dominates with comprehensive simulation platforms specifically tailored for bio-mechanical applications. Other key players include Siemens Corp. and Dassault Systèmes, who offer specialized material models for biological tissues. Research institutions like INSERM and CNRS are advancing boundary condition methodologies, while automotive companies such as Mercedes-Benz and Boeing are applying these technologies for safety simulations. The technology is approaching maturity in standard applications but remains evolving for complex biological material modeling and real-time simulations.
ANSYS, Inc.
Technical Solution: ANSYS has developed comprehensive biomechanical simulation solutions through their flagship products like ANSYS Mechanical and ANSYS LS-DYNA. Their approach integrates advanced material models including hyperelastic, viscoelastic, and anisotropic tissue models that accurately represent the non-linear behavior of biological tissues. ANSYS's technology implements sophisticated boundary condition handling through Arbitrary Lagrangian-Eulerian (ALE) formulations that manage fluid-structure interactions in cardiovascular simulations. Their platform supports multi-scale modeling from cellular to organ level with adaptive meshing techniques that optimize computational efficiency while maintaining accuracy at tissue interfaces. ANSYS has pioneered patient-specific modeling workflows that incorporate medical imaging data (CT/MRI) directly into simulation environments, enabling clinically-relevant boundary conditions derived from in vivo measurements.
Strengths: Comprehensive integration with medical imaging data; extensive material library for biological tissues; robust solver technology for complex contact problems. Weakness: High computational requirements for complex biological simulations; steep learning curve for biomedical applications; requires significant expertise to properly define boundary conditions for patient-specific models.
Institut National de la Santé et de la Recherche Médicale
Technical Solution: INSERM has developed specialized FEM frameworks for biomechanical simulations focusing on patient-specific modeling. Their approach centers on advanced constitutive models that capture the heterogeneous and anisotropic nature of biological tissues, particularly for cardiovascular and musculoskeletal applications. INSERM's technology implements innovative boundary condition methodologies that incorporate in vivo measurements from medical imaging modalities like 4D MRI and ultrasound elastography to define physiologically accurate constraints. Their research has pioneered multi-scale modeling techniques that bridge cellular mechanics with tissue-level behavior through homogenization methods. INSERM has developed specialized material identification protocols that combine experimental testing with inverse FEM approaches to determine patient-specific material parameters. Their simulation frameworks incorporate growth and remodeling algorithms that can predict tissue adaptation over time in response to mechanical stimuli, particularly valuable for surgical planning and disease progression modeling.
Strengths: Cutting-edge research in patient-specific material characterization; strong integration with clinical data acquisition; advanced growth and remodeling algorithms for dynamic simulations. Weakness: Research-oriented approaches may lack commercial-grade user interfaces; solutions often require significant customization for specific applications; computational efficiency challenges for complex multi-scale models.
Key Innovations in Boundary Condition Implementation
Medical imaging method and system for providing a finite-element model
PatentInactiveEP2245569A1
Innovation
- A method that includes acquiring patient-specific data for bone structures in functional positions, combining it with a knowledge base to determine precise three-dimensional geometry and mechanical characteristics, and applying position-specific mechanical loads to create a patient-specific finite-element model, accounting for the patient's position during data acquisition.
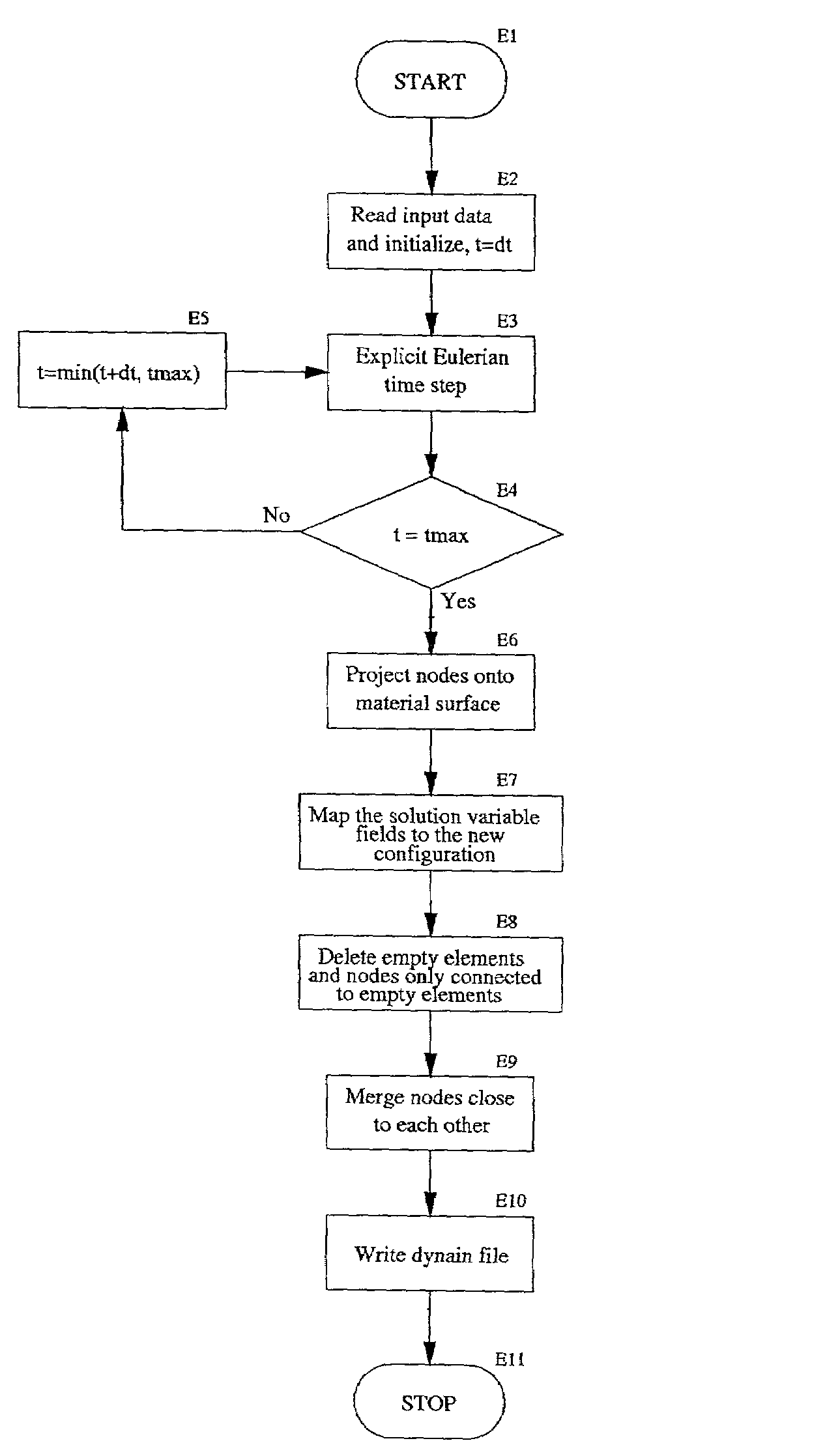
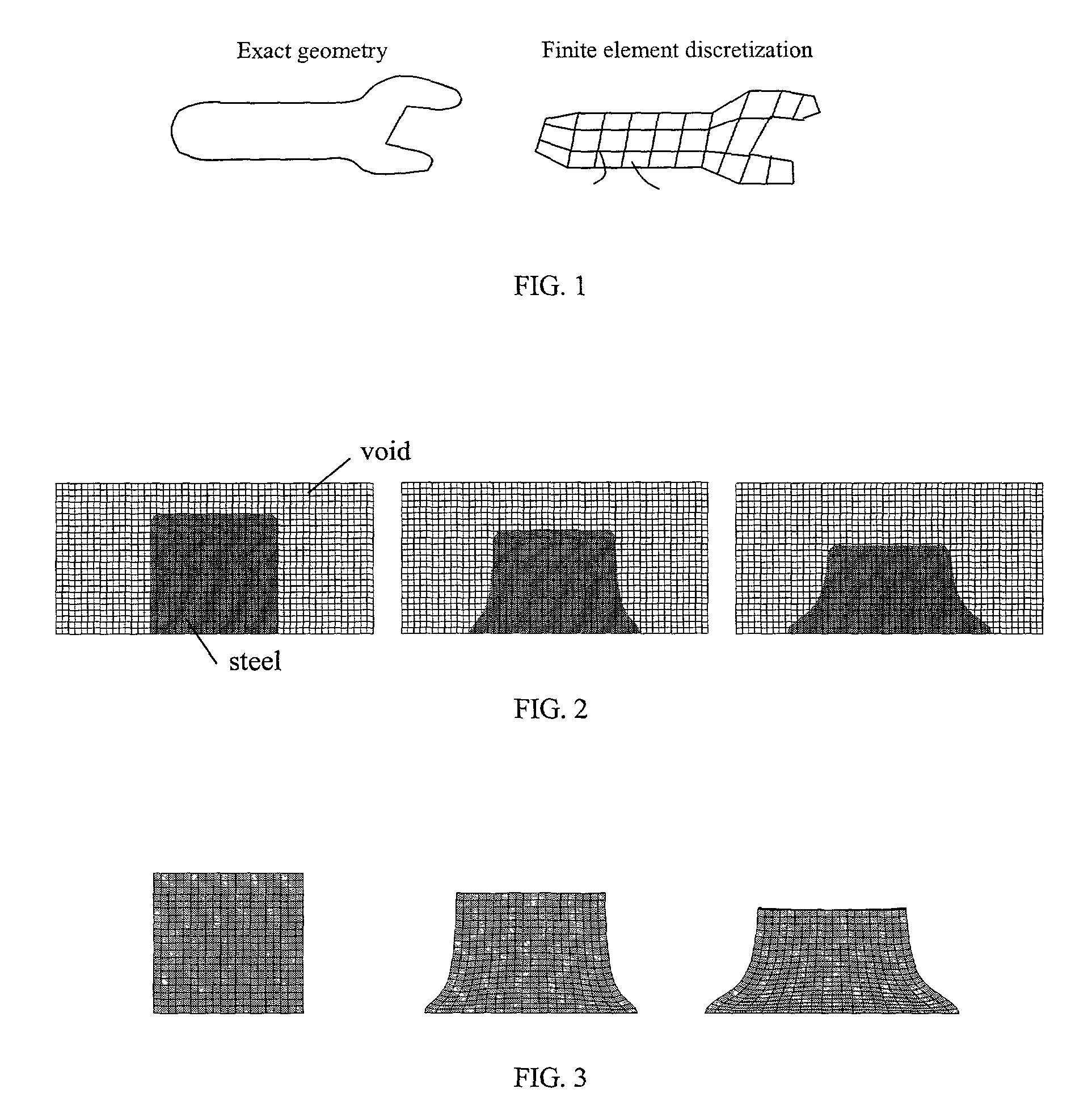
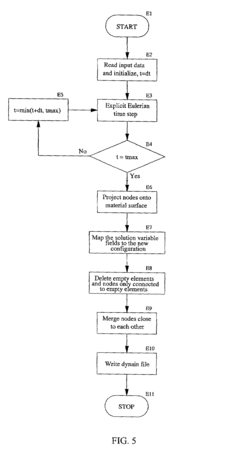
Eulerian-Lagrangian mapping for finite element analysis
PatentInactiveUS7167816B1
Innovation
- A method that switches from an Eulerian to a Lagrangian formulation during a finite element simulation, allowing for a combination of both methods to effectively handle large deformations and subsequent small deformation processes, such as forging and springback, by mapping the Eulerian mesh onto a new Lagrangian mesh for quasi-static analysis.
Validation Methods for Bio-Mechanical Simulations
Validation of bio-mechanical simulations using the Finite Element Method (FEM) requires rigorous methodologies to ensure computational models accurately represent real-world biological systems. The validation process typically follows a multi-tiered approach, beginning with verification against analytical solutions for simplified cases where closed-form solutions exist. This establishes the mathematical accuracy of the numerical implementation before proceeding to more complex biological scenarios.
Experimental validation forms the cornerstone of bio-mechanical simulation assessment, comparing computational predictions with in vitro and in vivo measurements. For soft tissue simulations, this often involves mechanical testing protocols such as uniaxial tension, biaxial tension, and shear tests to validate material model parameters. Advanced imaging techniques including Digital Image Correlation (DIC), Magnetic Resonance Imaging (MRI), and micro-CT provide displacement field data that can be directly compared with FEM predictions at multiple spatial scales.
Statistical methods play a crucial role in validation, with uncertainty quantification techniques addressing the inherent variability in biological tissues. Sensitivity analyses identify which material parameters and boundary conditions most significantly impact simulation outcomes, guiding refinement efforts. Metrics such as Root Mean Square Error (RMSE), correlation coefficients, and Bland-Altman plots quantify the agreement between computational and experimental results.
Cross-validation strategies employ multiple independent datasets to evaluate model robustness. This includes validating across different loading conditions, strain rates, and physiological states to ensure the model captures the full range of bio-mechanical behaviors. Mesh convergence studies specifically assess the impact of discretization on solution accuracy, determining the optimal element size and type for biological tissue simulations.
Validation benchmarks have emerged within the bio-mechanical community, with standardized test cases allowing comparison between different modeling approaches. These include idealized geometries with controlled boundary conditions as well as anatomically realistic scenarios. Multi-scale validation approaches are particularly important for biological tissues, ensuring consistency across hierarchical levels from cellular mechanics to organ-level behavior.
Clinical validation represents the ultimate test for bio-mechanical simulations, comparing model predictions with patient outcomes. This includes retrospective studies correlating simulation results with documented clinical findings and prospective trials where simulations guide treatment decisions. The FDA and other regulatory bodies have established validation frameworks specifically for computational models used in medical device development, providing structured approaches for demonstrating simulation reliability in bio-mechanical applications.
Experimental validation forms the cornerstone of bio-mechanical simulation assessment, comparing computational predictions with in vitro and in vivo measurements. For soft tissue simulations, this often involves mechanical testing protocols such as uniaxial tension, biaxial tension, and shear tests to validate material model parameters. Advanced imaging techniques including Digital Image Correlation (DIC), Magnetic Resonance Imaging (MRI), and micro-CT provide displacement field data that can be directly compared with FEM predictions at multiple spatial scales.
Statistical methods play a crucial role in validation, with uncertainty quantification techniques addressing the inherent variability in biological tissues. Sensitivity analyses identify which material parameters and boundary conditions most significantly impact simulation outcomes, guiding refinement efforts. Metrics such as Root Mean Square Error (RMSE), correlation coefficients, and Bland-Altman plots quantify the agreement between computational and experimental results.
Cross-validation strategies employ multiple independent datasets to evaluate model robustness. This includes validating across different loading conditions, strain rates, and physiological states to ensure the model captures the full range of bio-mechanical behaviors. Mesh convergence studies specifically assess the impact of discretization on solution accuracy, determining the optimal element size and type for biological tissue simulations.
Validation benchmarks have emerged within the bio-mechanical community, with standardized test cases allowing comparison between different modeling approaches. These include idealized geometries with controlled boundary conditions as well as anatomically realistic scenarios. Multi-scale validation approaches are particularly important for biological tissues, ensuring consistency across hierarchical levels from cellular mechanics to organ-level behavior.
Clinical validation represents the ultimate test for bio-mechanical simulations, comparing model predictions with patient outcomes. This includes retrospective studies correlating simulation results with documented clinical findings and prospective trials where simulations guide treatment decisions. The FDA and other regulatory bodies have established validation frameworks specifically for computational models used in medical device development, providing structured approaches for demonstrating simulation reliability in bio-mechanical applications.
Clinical Translation and Regulatory Considerations
The translation of finite element biomechanical simulations into clinical practice requires navigating complex regulatory frameworks and addressing specific clinical implementation challenges. Currently, the FDA and EMA have established guidelines for computational modeling in medical device development, requiring validation studies that demonstrate the accuracy of material models and boundary conditions in predicting real-world biological responses. These regulatory bodies increasingly accept simulation data as supplementary evidence in approval processes, though comprehensive physical testing remains mandatory for final validation.
Clinical translation faces significant hurdles in patient-specific modeling, where individual anatomical and physiological variations necessitate customized material parameters and boundary conditions. The development of standardized protocols for acquiring patient-specific data through imaging techniques like MRI and CT scans has improved this process, but challenges remain in real-time data integration and model adjustment during clinical procedures.
Verification and validation requirements present another critical consideration, with regulatory bodies demanding multi-level validation approaches. These typically include bench testing against cadaveric specimens, comparison with clinical outcomes data, and uncertainty quantification analyses that account for biological variability. The FDA's guidance on "Reporting of Computational Modeling Studies in Medical Device Submissions" specifically addresses how FEM simulations should be documented and validated for regulatory review.
Ethical and legal considerations also impact clinical implementation, particularly regarding liability when treatment decisions are informed by computational models. Questions about responsibility distribution between software developers, clinicians, and institutions remain unresolved in many jurisdictions, creating barriers to widespread adoption despite technical readiness.
Recent developments in regulatory science show promising trends toward "in silico clinical trials" where virtual patient cohorts supplement traditional clinical studies. The Virtual Physiological Human initiative in Europe and similar FDA programs in the US are developing frameworks to incorporate biomechanical simulations into regulatory decision-making processes, potentially reducing the cost and time of bringing new medical technologies to market.
For successful clinical translation, interdisciplinary collaboration between engineers, clinicians, and regulatory experts is essential. Training programs for clinicians to interpret simulation results and understand their limitations have emerged as a critical component of implementation strategies, bridging the knowledge gap between technical capabilities and practical clinical application.
Clinical translation faces significant hurdles in patient-specific modeling, where individual anatomical and physiological variations necessitate customized material parameters and boundary conditions. The development of standardized protocols for acquiring patient-specific data through imaging techniques like MRI and CT scans has improved this process, but challenges remain in real-time data integration and model adjustment during clinical procedures.
Verification and validation requirements present another critical consideration, with regulatory bodies demanding multi-level validation approaches. These typically include bench testing against cadaveric specimens, comparison with clinical outcomes data, and uncertainty quantification analyses that account for biological variability. The FDA's guidance on "Reporting of Computational Modeling Studies in Medical Device Submissions" specifically addresses how FEM simulations should be documented and validated for regulatory review.
Ethical and legal considerations also impact clinical implementation, particularly regarding liability when treatment decisions are informed by computational models. Questions about responsibility distribution between software developers, clinicians, and institutions remain unresolved in many jurisdictions, creating barriers to widespread adoption despite technical readiness.
Recent developments in regulatory science show promising trends toward "in silico clinical trials" where virtual patient cohorts supplement traditional clinical studies. The Virtual Physiological Human initiative in Europe and similar FDA programs in the US are developing frameworks to incorporate biomechanical simulations into regulatory decision-making processes, potentially reducing the cost and time of bringing new medical technologies to market.
For successful clinical translation, interdisciplinary collaboration between engineers, clinicians, and regulatory experts is essential. Training programs for clinicians to interpret simulation results and understand their limitations have emerged as a critical component of implementation strategies, bridging the knowledge gap between technical capabilities and practical clinical application.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!