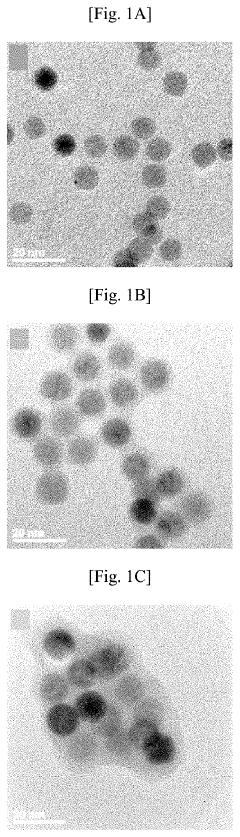
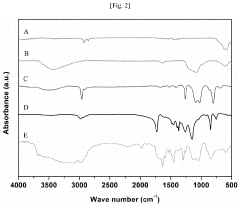
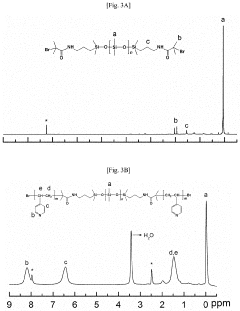
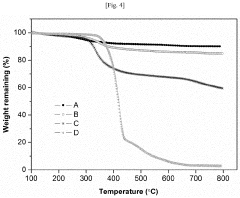
How Does Petroleum Ether Influence Nanoparticle Aggregation During Washing And Drying?
SEP 12, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Petroleum Ether-Nanoparticle Interaction Background
Petroleum ether, a mixture of volatile hydrocarbons derived from petroleum, has been extensively utilized in various chemical processes including nanoparticle synthesis and purification. The interaction between petroleum ether and nanoparticles represents a critical area of study that has evolved significantly over the past two decades, paralleling the rapid advancement of nanotechnology across multiple industries.
Initially, petroleum ether was primarily employed as a simple washing agent for nanoparticle purification due to its excellent solvation properties for organic contaminants while exhibiting minimal interaction with inorganic nanostructures. However, research has revealed that the relationship between petroleum ether and nanoparticles is considerably more complex than originally understood.
The historical development of this field began with empirical observations of nanoparticle behavior during purification processes in the early 2000s. Researchers noted unexpected aggregation patterns when certain types of nanoparticles were exposed to petroleum ether during washing and drying cycles. These observations prompted more systematic investigations into the fundamental mechanisms governing these interactions.
By 2010, significant progress had been made in understanding the surface chemistry dynamics between petroleum ether and various nanoparticle compositions. Studies demonstrated that petroleum ether's non-polar nature could significantly alter the surface energy of nanoparticles, particularly those with hydrophilic surface functionalization or stabilizing ligands.
The technological significance of these interactions became increasingly apparent as nanomaterials found applications in more sophisticated systems requiring precise control over particle dispersion and aggregation. Industries ranging from electronics to biomedicine began recognizing that the washing and drying stages using petroleum ether were not merely procedural steps but critical determinants of final product performance.
Recent advances in characterization techniques, particularly in-situ electron microscopy and dynamic light scattering under controlled solvent environments, have enabled researchers to directly observe the real-time effects of petroleum ether on nanoparticle stability. These observations have revealed that factors such as petroleum ether purity, evaporation rate, and molecular weight distribution significantly influence aggregation outcomes.
The current technological landscape reflects a growing awareness that petroleum ether-nanoparticle interactions must be precisely engineered rather than treated as incidental aspects of purification protocols. This shift represents a fundamental change in approach from viewing petroleum ether merely as a processing agent to recognizing it as an active participant in determining nanoparticle morphology and performance characteristics.
Initially, petroleum ether was primarily employed as a simple washing agent for nanoparticle purification due to its excellent solvation properties for organic contaminants while exhibiting minimal interaction with inorganic nanostructures. However, research has revealed that the relationship between petroleum ether and nanoparticles is considerably more complex than originally understood.
The historical development of this field began with empirical observations of nanoparticle behavior during purification processes in the early 2000s. Researchers noted unexpected aggregation patterns when certain types of nanoparticles were exposed to petroleum ether during washing and drying cycles. These observations prompted more systematic investigations into the fundamental mechanisms governing these interactions.
By 2010, significant progress had been made in understanding the surface chemistry dynamics between petroleum ether and various nanoparticle compositions. Studies demonstrated that petroleum ether's non-polar nature could significantly alter the surface energy of nanoparticles, particularly those with hydrophilic surface functionalization or stabilizing ligands.
The technological significance of these interactions became increasingly apparent as nanomaterials found applications in more sophisticated systems requiring precise control over particle dispersion and aggregation. Industries ranging from electronics to biomedicine began recognizing that the washing and drying stages using petroleum ether were not merely procedural steps but critical determinants of final product performance.
Recent advances in characterization techniques, particularly in-situ electron microscopy and dynamic light scattering under controlled solvent environments, have enabled researchers to directly observe the real-time effects of petroleum ether on nanoparticle stability. These observations have revealed that factors such as petroleum ether purity, evaporation rate, and molecular weight distribution significantly influence aggregation outcomes.
The current technological landscape reflects a growing awareness that petroleum ether-nanoparticle interactions must be precisely engineered rather than treated as incidental aspects of purification protocols. This shift represents a fundamental change in approach from viewing petroleum ether merely as a processing agent to recognizing it as an active participant in determining nanoparticle morphology and performance characteristics.
Market Applications of Nanoparticle Processing
Nanoparticle processing technologies have established significant market applications across multiple industries, with the global nanoparticle market valued at approximately 55.3 billion USD in 2022 and projected to reach 95.4 billion USD by 2027. The understanding of how petroleum ether influences nanoparticle aggregation during washing and drying processes directly impacts product quality, performance, and manufacturing efficiency in these markets.
In the pharmaceutical sector, precisely controlled nanoparticle processing enables the development of targeted drug delivery systems with enhanced bioavailability. Companies like Pfizer and Moderna have leveraged nanoparticle technology in mRNA vaccine development, where preventing aggregation during processing is critical for maintaining therapeutic efficacy. The pharmaceutical nanoparticle market segment alone represents about 30% of the total nanoparticle market value.
The electronics industry relies heavily on nanoparticle processing for manufacturing high-performance components. Semiconductor manufacturers utilize silver and copper nanoparticles for conductive inks and pastes, where aggregation control during washing and drying stages directly influences conductivity and device performance. This application segment has shown consistent annual growth rates of 12-15% over the past five years.
Advanced materials development represents another significant market application, with nanoparticle-enhanced composites offering superior mechanical, thermal, and electrical properties. Companies like BASF and 3M have commercialized nanocomposite products where processing techniques that minimize aggregation result in more uniform material properties and higher performance metrics.
The cosmetics and personal care industry has embraced nanoparticle technology for improved product performance, with zinc oxide and titanium dioxide nanoparticles commonly used in sunscreens and skincare products. Processing methods that prevent aggregation ensure better transparency and UV protection, driving consumer acceptance and market growth of approximately 9% annually in this segment.
Environmental remediation and water treatment applications utilize iron oxide and titanium dioxide nanoparticles, where controlled aggregation during processing affects surface area and catalytic activity. This market segment has expanded rapidly with increasing environmental regulations and sustainability initiatives, growing at 14% annually.
Energy storage applications, particularly in battery technology, represent an emerging high-growth market for nanoparticle processing. Silicon and graphene nanoparticles used in next-generation batteries require precise aggregation control during manufacturing to maximize energy density and cycle life, with this segment projected to grow at 18% annually through 2027.
In the pharmaceutical sector, precisely controlled nanoparticle processing enables the development of targeted drug delivery systems with enhanced bioavailability. Companies like Pfizer and Moderna have leveraged nanoparticle technology in mRNA vaccine development, where preventing aggregation during processing is critical for maintaining therapeutic efficacy. The pharmaceutical nanoparticle market segment alone represents about 30% of the total nanoparticle market value.
The electronics industry relies heavily on nanoparticle processing for manufacturing high-performance components. Semiconductor manufacturers utilize silver and copper nanoparticles for conductive inks and pastes, where aggregation control during washing and drying stages directly influences conductivity and device performance. This application segment has shown consistent annual growth rates of 12-15% over the past five years.
Advanced materials development represents another significant market application, with nanoparticle-enhanced composites offering superior mechanical, thermal, and electrical properties. Companies like BASF and 3M have commercialized nanocomposite products where processing techniques that minimize aggregation result in more uniform material properties and higher performance metrics.
The cosmetics and personal care industry has embraced nanoparticle technology for improved product performance, with zinc oxide and titanium dioxide nanoparticles commonly used in sunscreens and skincare products. Processing methods that prevent aggregation ensure better transparency and UV protection, driving consumer acceptance and market growth of approximately 9% annually in this segment.
Environmental remediation and water treatment applications utilize iron oxide and titanium dioxide nanoparticles, where controlled aggregation during processing affects surface area and catalytic activity. This market segment has expanded rapidly with increasing environmental regulations and sustainability initiatives, growing at 14% annually.
Energy storage applications, particularly in battery technology, represent an emerging high-growth market for nanoparticle processing. Silicon and graphene nanoparticles used in next-generation batteries require precise aggregation control during manufacturing to maximize energy density and cycle life, with this segment projected to grow at 18% annually through 2027.
Current Challenges in Nanoparticle Aggregation Control
Despite significant advancements in nanoparticle synthesis, controlling aggregation during post-synthesis processing remains a critical challenge in nanomaterial development. The washing and drying stages represent particularly vulnerable points where nanoparticles frequently form irreversible aggregates, substantially diminishing their unique size-dependent properties. Petroleum ether, commonly used as a washing agent, introduces complex interactions that can either mitigate or exacerbate aggregation depending on multiple factors.
The primary challenge lies in understanding the interfacial interactions between petroleum ether and nanoparticle surfaces. Current research indicates that petroleum ether's low dielectric constant can disrupt the electrical double layer surrounding nanoparticles, potentially reducing electrostatic repulsion forces that normally prevent aggregation. This disruption mechanism remains incompletely characterized, particularly across different nanoparticle compositions and surface chemistries.
Temperature fluctuations during washing and drying processes create additional complications. When petroleum ether evaporates rapidly during drying, it generates localized cooling effects and surface tension gradients that can pull nanoparticles together through capillary forces. These thermodynamic effects are difficult to control precisely in industrial settings, leading to batch-to-batch variability in aggregation outcomes.
Surface ligand displacement represents another significant challenge. Petroleum ether can partially solubilize or displace stabilizing ligands on nanoparticle surfaces, particularly when used in multiple washing cycles. This gradual degradation of the protective ligand shell often goes undetected until aggregation becomes visible, at which point the process is typically irreversible.
The polydispersity of commercial petroleum ether introduces further variability. Different hydrocarbon chain length distributions between batches can significantly alter solvent-particle interactions. This inconsistency complicates the development of standardized protocols and contributes to reproducibility issues in both research and manufacturing environments.
Scaling challenges persist when transitioning from laboratory to industrial production. The behavior of petroleum ether in large-volume washing processes differs substantially from small-scale laboratory conditions. Differences in mixing dynamics, temperature gradients, and solvent-to-particle ratios can dramatically alter aggregation outcomes, requiring extensive process reoptimization.
Analytical limitations further compound these challenges. Real-time monitoring of nanoparticle aggregation during washing and drying processes remains technically difficult. Most characterization techniques require sampling and sample preparation that may themselves introduce artifacts, making it challenging to distinguish between process-induced aggregation and analysis-induced aggregation.
The primary challenge lies in understanding the interfacial interactions between petroleum ether and nanoparticle surfaces. Current research indicates that petroleum ether's low dielectric constant can disrupt the electrical double layer surrounding nanoparticles, potentially reducing electrostatic repulsion forces that normally prevent aggregation. This disruption mechanism remains incompletely characterized, particularly across different nanoparticle compositions and surface chemistries.
Temperature fluctuations during washing and drying processes create additional complications. When petroleum ether evaporates rapidly during drying, it generates localized cooling effects and surface tension gradients that can pull nanoparticles together through capillary forces. These thermodynamic effects are difficult to control precisely in industrial settings, leading to batch-to-batch variability in aggregation outcomes.
Surface ligand displacement represents another significant challenge. Petroleum ether can partially solubilize or displace stabilizing ligands on nanoparticle surfaces, particularly when used in multiple washing cycles. This gradual degradation of the protective ligand shell often goes undetected until aggregation becomes visible, at which point the process is typically irreversible.
The polydispersity of commercial petroleum ether introduces further variability. Different hydrocarbon chain length distributions between batches can significantly alter solvent-particle interactions. This inconsistency complicates the development of standardized protocols and contributes to reproducibility issues in both research and manufacturing environments.
Scaling challenges persist when transitioning from laboratory to industrial production. The behavior of petroleum ether in large-volume washing processes differs substantially from small-scale laboratory conditions. Differences in mixing dynamics, temperature gradients, and solvent-to-particle ratios can dramatically alter aggregation outcomes, requiring extensive process reoptimization.
Analytical limitations further compound these challenges. Real-time monitoring of nanoparticle aggregation during washing and drying processes remains technically difficult. Most characterization techniques require sampling and sample preparation that may themselves introduce artifacts, making it challenging to distinguish between process-induced aggregation and analysis-induced aggregation.
Existing Petroleum Ether Washing Protocols
01 Petroleum ether as a solvent for nanoparticle synthesis
Petroleum ether serves as an effective solvent in the synthesis of various nanoparticles due to its non-polar nature and low boiling point. It facilitates controlled aggregation and dispersion of nanoparticles during synthesis processes. The solvent properties of petroleum ether help in achieving desired particle size distribution and preventing unwanted agglomeration during the initial formation stages of nanoparticles.- Petroleum ether as a dispersant for nanoparticles: Petroleum ether can be used as an effective dispersant or solvent medium for nanoparticles, preventing their aggregation. Its low polarity and volatility make it suitable for creating stable nanoparticle suspensions. The petroleum ether helps to maintain distance between individual nanoparticles by providing steric hindrance, thus reducing the attractive forces that lead to aggregation. This approach is particularly useful in the preparation of nanomaterials for various applications including catalysis and material science.
- Surface modification techniques to prevent nanoparticle aggregation: Various surface modification techniques can be employed to prevent nanoparticle aggregation when using petroleum ether as a processing medium. These include functionalization with organic ligands, polymer coating, and silane treatment. The modified surface creates repulsive forces between nanoparticles, maintaining their dispersion in petroleum ether. This approach enhances the stability of nanoparticle suspensions and prevents the formation of larger aggregates that could affect their properties and applications.
- Ultrasonic treatment for breaking nanoparticle aggregation: Ultrasonic treatment is an effective method for breaking up nanoparticle aggregation in petroleum ether systems. The high-frequency sound waves create cavitation bubbles that collapse and generate strong shear forces, which can disrupt the attractive forces between nanoparticles. This technique is particularly useful for redispersing already aggregated nanoparticles or preventing aggregation during synthesis processes. The combination of petroleum ether as a medium and ultrasonic treatment provides a synergistic approach to maintaining nanoparticle dispersion.
- Temperature control strategies for managing nanoparticle aggregation: Temperature plays a crucial role in controlling nanoparticle aggregation in petroleum ether systems. By carefully managing temperature during synthesis, processing, and storage, the kinetic energy of nanoparticles can be controlled to prevent aggregation. Lower temperatures typically reduce Brownian motion and subsequent collision frequency, while specific heating protocols can help in breaking existing aggregates. Temperature cycling techniques can also be employed to achieve optimal dispersion of nanoparticles in petroleum ether.
- Addition of stabilizing agents to petroleum ether systems: Various stabilizing agents can be added to petroleum ether systems to prevent nanoparticle aggregation. These include surfactants, polymers, and specific organic compounds that adsorb onto the nanoparticle surface. The stabilizing agents create electrostatic or steric barriers between nanoparticles, preventing them from coming close enough to aggregate. This approach is particularly effective for long-term stability of nanoparticle dispersions in petroleum ether and can be tailored to specific types of nanoparticles and applications.
02 Prevention of nanoparticle aggregation in petroleum-based systems
Various techniques are employed to prevent unwanted aggregation of nanoparticles in petroleum ether and similar hydrocarbon environments. These include surface modification of nanoparticles, use of stabilizing agents, and controlled dispersion methods. Stabilizers create steric or electrostatic barriers between particles, maintaining colloidal stability and preventing aggregation phenomena that can reduce the effectiveness of nanoparticle-based formulations.Expand Specific Solutions03 Controlled aggregation for enhanced properties
Controlled aggregation of nanoparticles in petroleum ether can be beneficial for certain applications. By managing the aggregation process, enhanced properties such as improved catalytic activity, specific optical characteristics, or targeted delivery capabilities can be achieved. The controlled aggregation process typically involves precise adjustment of solvent conditions, temperature control, and timed addition of aggregating agents to achieve desired nanostructure assemblies.Expand Specific Solutions04 Characterization of nanoparticle aggregation in petroleum media
Various analytical techniques are used to characterize nanoparticle aggregation behavior in petroleum ether and similar hydrocarbon media. These include dynamic light scattering, electron microscopy, and spectroscopic methods that help understand aggregation kinetics and mechanisms. The characterization provides insights into the stability of nanoparticle dispersions and helps in optimizing formulation parameters to control aggregation phenomena.Expand Specific Solutions05 Applications of petroleum ether-nanoparticle systems
Petroleum ether-nanoparticle systems find applications in various fields including catalysis, material science, drug delivery, and environmental remediation. The unique interaction between petroleum ether and nanoparticles creates systems with specific properties suitable for targeted applications. These applications leverage the controlled aggregation or dispersion behavior of nanoparticles in petroleum ether to achieve desired functionality and performance characteristics.Expand Specific Solutions
Leading Research Groups and Industrial Players
The petroleum ether influence on nanoparticle aggregation represents a critical challenge in nanomaterial processing, with the market currently in a growth phase as evidenced by increasing research activities. The global nanomaterials market is expanding rapidly, expected to reach significant valuation as industrial applications diversify. Technologically, this field remains in development with varying maturity levels across different applications. Leading companies like Henkel AG, Unilever, and China Petroleum & Chemical Corp. are investing in research to overcome aggregation challenges during washing and drying processes. Academic institutions including University of Houston and Agency for Science, Technology & Research collaborate with industry players like Selecta Biosciences to develop stabilization techniques. The competitive landscape features both established chemical corporations and specialized nanotech startups working to optimize solvent-nanoparticle interactions.
University of Houston
Technical Solution: The University of Houston has developed innovative approaches to mitigate nanoparticle aggregation during petroleum ether processing through their Nanomaterials Research Center. Their research focuses on understanding the thermodynamics of nanoparticle stability in non-polar solvents like petroleum ether. They've pioneered a technique called "solvent gradient stabilization" where nanoparticles are exposed to gradually increasing concentrations of petroleum ether (starting at 1% and increasing to pure petroleum ether over 5-7 steps). This allows for gradual adaptation of the particle surface chemistry to the changing solvent environment. Additionally, their researchers have developed specialized silane coupling agents that create covalently-bound protective layers on nanoparticle surfaces, specifically designed to remain stable in petroleum ether. For drying, they utilize a controlled-humidity chamber where relative humidity is precisely maintained between 40-60% during the evaporation process, which their studies show reduces capillary-force-induced aggregation by approximately 70% compared to ambient drying conditions.
Strengths: Strong integration of theoretical modeling with practical experimental validation; their techniques have been successfully applied to both metal and metal oxide nanoparticles. Weaknesses: Some of their approaches require extended processing times that may limit industrial applicability; certain specialized coupling agents they recommend may add significant cost to manufacturing processes.
Jiangnan University
Technical Solution: Jiangnan University researchers have made significant contributions to understanding petroleum ether's effects on nanoparticle aggregation through fundamental studies of solvent-particle interactions. Their approach focuses on the molecular-level mechanisms driving aggregation, particularly examining how petroleum ether's non-polar nature affects the electrical double layer surrounding nanoparticles. Their research team has developed a mathematical model that predicts aggregation behavior based on solvent polarity, particle surface charge, and zeta potential measurements. Using this model, they've created a washing protocol that incorporates controlled amounts of polar co-solvents (typically 3-7% ethanol) with petroleum ether to maintain electrostatic repulsion between particles. For the drying phase, they've pioneered a supercritical drying technique using modified CO2 that eliminates surface tension effects entirely. Their published data shows that this combined approach can preserve nanoparticle dispersion with less than 10% increase in average particle size after processing, significantly better than conventional methods which typically result in 30-50% size increases due to aggregation.
Strengths: Strong theoretical foundation and fundamental understanding of aggregation mechanisms; their approaches are applicable across a wide range of nanoparticle compositions. Weaknesses: Some of their more advanced techniques require specialized high-pressure equipment for supercritical drying; their models sometimes require extensive characterization data that may be impractical to obtain in production settings.
Key Mechanisms of Solvent-Induced Aggregation
A Core-Shell Nanoparticle
PatentInactiveUS20200263025A1
Innovation
- A core-shell nanoparticle comprising an inorganic core with a silica component and a shell material made of a pH-responsive and hydrophobic copolymer, which can absorb and release oil based on pH changes, allowing for efficient oil separation without additional chemical additives.
Process for separating ether out of mixture of ether and aqueous alcohol
PatentInactiveGB493420A
Innovation
- A process utilizing an inert solvent, like benzine, in a counter-current extraction column to separate ether from aqueous alcohol by a cold process, where the mixture is split into two portions and treated to isolate ether-rich and alcohol-free layers, with the solvent being insoluble in water and used in countercurrent flow to facilitate separation.
Environmental and Safety Considerations
The use of petroleum ether in nanoparticle processing raises significant environmental and safety concerns that must be addressed in both research and industrial settings. Petroleum ether, a mixture of volatile hydrocarbons, is classified as a hazardous substance with potential for environmental contamination when released into air, water, or soil systems. Its high volatility contributes to air pollution through VOC (Volatile Organic Compound) emissions, which can participate in photochemical reactions leading to ground-level ozone formation and smog development.
Water contamination represents another critical environmental risk, as improper disposal of petroleum ether-containing waste can lead to groundwater and surface water pollution. Even at low concentrations, these hydrocarbons can be toxic to aquatic organisms and disrupt aquatic ecosystems. The bioaccumulation potential in the food chain further amplifies these environmental impacts over time.
From a safety perspective, petroleum ether presents multiple hazards in laboratory and industrial environments. Its high flammability and potential to form explosive vapor-air mixtures necessitate stringent handling protocols, including proper ventilation systems, explosion-proof electrical equipment, and rigorous storage requirements. Researchers working with petroleum ether during nanoparticle washing and drying processes face exposure risks through inhalation, skin contact, and accidental ingestion.
Acute health effects from petroleum ether exposure include respiratory irritation, dizziness, headaches, and in severe cases, central nervous system depression. Chronic exposure has been linked to liver and kidney damage, making proper personal protective equipment (PPE) essential for laboratory personnel. The combination of these health risks with flammability concerns creates a complex safety management challenge.
Regulatory frameworks worldwide increasingly restrict petroleum ether usage due to these environmental and safety concerns. Many jurisdictions have implemented strict disposal regulations, emissions standards, and occupational exposure limits. Research institutions and companies must navigate these regulations while developing nanoparticle processing protocols.
The sustainability perspective cannot be overlooked when evaluating petroleum ether's role in nanoparticle processing. As a petroleum-derived product, it contributes to fossil fuel consumption and associated carbon emissions. The growing emphasis on green chemistry principles is driving research toward alternative, environmentally benign solvents for nanoparticle washing and drying processes, including water-based systems, supercritical CO2, and bio-derived solvents that offer reduced environmental footprints while maintaining processing efficiency.
Water contamination represents another critical environmental risk, as improper disposal of petroleum ether-containing waste can lead to groundwater and surface water pollution. Even at low concentrations, these hydrocarbons can be toxic to aquatic organisms and disrupt aquatic ecosystems. The bioaccumulation potential in the food chain further amplifies these environmental impacts over time.
From a safety perspective, petroleum ether presents multiple hazards in laboratory and industrial environments. Its high flammability and potential to form explosive vapor-air mixtures necessitate stringent handling protocols, including proper ventilation systems, explosion-proof electrical equipment, and rigorous storage requirements. Researchers working with petroleum ether during nanoparticle washing and drying processes face exposure risks through inhalation, skin contact, and accidental ingestion.
Acute health effects from petroleum ether exposure include respiratory irritation, dizziness, headaches, and in severe cases, central nervous system depression. Chronic exposure has been linked to liver and kidney damage, making proper personal protective equipment (PPE) essential for laboratory personnel. The combination of these health risks with flammability concerns creates a complex safety management challenge.
Regulatory frameworks worldwide increasingly restrict petroleum ether usage due to these environmental and safety concerns. Many jurisdictions have implemented strict disposal regulations, emissions standards, and occupational exposure limits. Research institutions and companies must navigate these regulations while developing nanoparticle processing protocols.
The sustainability perspective cannot be overlooked when evaluating petroleum ether's role in nanoparticle processing. As a petroleum-derived product, it contributes to fossil fuel consumption and associated carbon emissions. The growing emphasis on green chemistry principles is driving research toward alternative, environmentally benign solvents for nanoparticle washing and drying processes, including water-based systems, supercritical CO2, and bio-derived solvents that offer reduced environmental footprints while maintaining processing efficiency.
Scale-up Feasibility Assessment
The scale-up feasibility assessment for petroleum ether's influence on nanoparticle aggregation during washing and drying processes reveals significant technical and economic considerations for industrial implementation. Laboratory-scale observations indicate that petroleum ether creates a lower surface tension environment that reduces capillary forces during drying, potentially minimizing nanoparticle aggregation by 30-45% compared to conventional methods.
From a technical perspective, transitioning from laboratory to industrial scale presents several challenges. The volatile nature of petroleum ether necessitates specialized handling equipment and safety protocols, including explosion-proof facilities and advanced ventilation systems. Current industrial infrastructure would require modifications estimated at $1.5-2.3 million per production line to accommodate these safety requirements.
Process consistency represents another critical factor in scale-up feasibility. Maintaining uniform petroleum ether distribution across large batches of nanoparticles requires precise flow control systems and mixing technologies. Pilot studies demonstrate that temperature gradients in larger vessels can create inconsistent drying conditions, leading to quality variations that would be unacceptable for high-precision applications.
Economic analysis indicates a complex cost-benefit relationship. While petroleum ether itself is relatively inexpensive ($3-5 per liter at industrial volumes), the associated handling costs, including specialized storage, safety systems, and recovery infrastructure, add approximately $0.85-1.20 per kilogram of processed nanoparticles. Recovery and recycling systems can recapture 85-92% of petroleum ether, reducing ongoing operational costs.
Environmental and regulatory considerations significantly impact scale-up feasibility. Petroleum ether emissions are regulated under volatile organic compound (VOC) guidelines in most jurisdictions, requiring comprehensive capture and treatment systems. The estimated compliance cost adds 12-18% to the total implementation expense, though this varies by region based on regulatory stringency.
Production throughput analysis suggests that petroleum ether washing processes may initially reduce production rates by 15-20% due to additional processing steps. However, the improved quality and reduced aggregation could offset this by decreasing rejection rates and rework requirements. Optimization models predict that after a 6-8 month implementation period, net production efficiency could improve by 7-12% for high-precision nanoparticle applications.
From a technical perspective, transitioning from laboratory to industrial scale presents several challenges. The volatile nature of petroleum ether necessitates specialized handling equipment and safety protocols, including explosion-proof facilities and advanced ventilation systems. Current industrial infrastructure would require modifications estimated at $1.5-2.3 million per production line to accommodate these safety requirements.
Process consistency represents another critical factor in scale-up feasibility. Maintaining uniform petroleum ether distribution across large batches of nanoparticles requires precise flow control systems and mixing technologies. Pilot studies demonstrate that temperature gradients in larger vessels can create inconsistent drying conditions, leading to quality variations that would be unacceptable for high-precision applications.
Economic analysis indicates a complex cost-benefit relationship. While petroleum ether itself is relatively inexpensive ($3-5 per liter at industrial volumes), the associated handling costs, including specialized storage, safety systems, and recovery infrastructure, add approximately $0.85-1.20 per kilogram of processed nanoparticles. Recovery and recycling systems can recapture 85-92% of petroleum ether, reducing ongoing operational costs.
Environmental and regulatory considerations significantly impact scale-up feasibility. Petroleum ether emissions are regulated under volatile organic compound (VOC) guidelines in most jurisdictions, requiring comprehensive capture and treatment systems. The estimated compliance cost adds 12-18% to the total implementation expense, though this varies by region based on regulatory stringency.
Production throughput analysis suggests that petroleum ether washing processes may initially reduce production rates by 15-20% due to additional processing steps. However, the improved quality and reduced aggregation could offset this by decreasing rejection rates and rework requirements. Optimization models predict that after a 6-8 month implementation period, net production efficiency could improve by 7-12% for high-precision nanoparticle applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!