How Does Petroleum Ether Residual Impact ICH Q3C Compliance In Drug Substance Workups?
SEP 12, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Petroleum Ether Residual Background and Objectives
Petroleum ether, a mixture of volatile hydrocarbons derived from petroleum distillation, has been widely utilized in pharmaceutical manufacturing processes for decades. Its excellent solvent properties make it particularly valuable in drug substance workups, where it serves as an extraction medium, crystallization agent, and purification tool. The evolution of this solvent's application in pharmaceutical processing can be traced back to the early 20th century, with significant refinements occurring alongside advancements in analytical chemistry and regulatory frameworks.
The technical landscape surrounding petroleum ether usage has undergone substantial transformation following the implementation of the International Council for Harmonisation (ICH) guidelines, particularly ICH Q3C, which establishes permissible daily exposure limits for residual solvents in pharmaceutical products. Initially introduced in 1997 and subsequently revised, these guidelines have progressively shaped industry practices regarding solvent selection, monitoring, and control strategies.
Current trends indicate a growing emphasis on understanding the behavior of petroleum ether residuals throughout the manufacturing process, including their potential for retention in various drug substance matrices and the factors influencing their removal efficiency. This technical evolution has been driven by increasingly sensitive analytical methodologies capable of detecting trace solvent levels, alongside heightened regulatory scrutiny of pharmaceutical impurity profiles.
The primary objective of this technical investigation is to comprehensively evaluate how petroleum ether residuals impact compliance with ICH Q3C guidelines in drug substance workups. This encompasses determining typical residual levels under various processing conditions, identifying critical process parameters affecting residual concentrations, and assessing analytical challenges associated with accurate quantification of these complex hydrocarbon mixtures.
Additionally, this research aims to establish correlations between petroleum ether composition variability and its residual behavior, as commercial petroleum ether products often contain varying proportions of pentanes, hexanes, and other hydrocarbons depending on the supplier and grade. Understanding these relationships is essential for developing robust control strategies that ensure consistent ICH Q3C compliance across manufacturing batches.
Furthermore, this investigation seeks to explore emerging technologies and methodologies that may enhance the removal of petroleum ether residuals or provide alternative processing approaches with improved residual solvent profiles. The ultimate goal is to establish a scientific foundation for risk-based decision-making regarding petroleum ether usage in pharmaceutical manufacturing, balancing process efficiency considerations with patient safety requirements as defined by global regulatory standards.
The technical landscape surrounding petroleum ether usage has undergone substantial transformation following the implementation of the International Council for Harmonisation (ICH) guidelines, particularly ICH Q3C, which establishes permissible daily exposure limits for residual solvents in pharmaceutical products. Initially introduced in 1997 and subsequently revised, these guidelines have progressively shaped industry practices regarding solvent selection, monitoring, and control strategies.
Current trends indicate a growing emphasis on understanding the behavior of petroleum ether residuals throughout the manufacturing process, including their potential for retention in various drug substance matrices and the factors influencing their removal efficiency. This technical evolution has been driven by increasingly sensitive analytical methodologies capable of detecting trace solvent levels, alongside heightened regulatory scrutiny of pharmaceutical impurity profiles.
The primary objective of this technical investigation is to comprehensively evaluate how petroleum ether residuals impact compliance with ICH Q3C guidelines in drug substance workups. This encompasses determining typical residual levels under various processing conditions, identifying critical process parameters affecting residual concentrations, and assessing analytical challenges associated with accurate quantification of these complex hydrocarbon mixtures.
Additionally, this research aims to establish correlations between petroleum ether composition variability and its residual behavior, as commercial petroleum ether products often contain varying proportions of pentanes, hexanes, and other hydrocarbons depending on the supplier and grade. Understanding these relationships is essential for developing robust control strategies that ensure consistent ICH Q3C compliance across manufacturing batches.
Furthermore, this investigation seeks to explore emerging technologies and methodologies that may enhance the removal of petroleum ether residuals or provide alternative processing approaches with improved residual solvent profiles. The ultimate goal is to establish a scientific foundation for risk-based decision-making regarding petroleum ether usage in pharmaceutical manufacturing, balancing process efficiency considerations with patient safety requirements as defined by global regulatory standards.
Pharmaceutical Industry Demand for Solvent Control
The pharmaceutical industry faces increasing regulatory pressure to control residual solvents in drug substances and products. ICH Q3C guidelines, established by the International Council for Harmonisation, categorize solvents based on their toxicity levels and set permissible daily exposure (PDE) limits. These guidelines have been adopted by major regulatory bodies worldwide, including the FDA, EMA, and NMPA, making compliance a global requirement for pharmaceutical manufacturers.
Market research indicates that pharmaceutical companies are investing significantly in analytical technologies and process improvements to ensure solvent control compliance. The global pharmaceutical analytical testing outsourcing market, which includes residual solvent testing, was valued at $6.1 billion in 2021 and is projected to grow at a CAGR of 8.3% through 2028, reflecting the industry's commitment to meeting regulatory standards.
Petroleum ether, a mixture of volatile hydrocarbons commonly used in drug substance workups, presents particular challenges for ICH Q3C compliance. As a Class 2 or Class 3 solvent (depending on specific composition), its residual presence must be strictly controlled. The industry demand for effective petroleum ether control stems from both regulatory requirements and quality considerations that directly impact patient safety.
Recent industry surveys reveal that approximately 65% of pharmaceutical manufacturers report challenges with residual solvent control during scale-up and technology transfer phases. These challenges often lead to production delays, increased costs, and potential regulatory issues during submission reviews.
The economic impact of non-compliance with ICH Q3C guidelines can be substantial. Regulatory rejections due to solvent residual issues can delay market entry by 6-18 months, potentially costing companies millions in lost revenue. Additionally, post-approval changes to manufacturing processes to address solvent issues can cost between $500,000 to $2 million per product, depending on the complexity of the required changes.
Industry stakeholders are increasingly demanding integrated solutions that address solvent control throughout the drug development lifecycle. This includes improved analytical methods with lower detection limits, green chemistry approaches that reduce or eliminate problematic solvents, and robust risk assessment frameworks for solvent selection during process development.
Contract development and manufacturing organizations (CDMOs) report a 40% increase in client requests specifically addressing ICH Q3C compliance strategies in the past five years, highlighting the growing importance of this issue in outsourced pharmaceutical manufacturing relationships.
Market research indicates that pharmaceutical companies are investing significantly in analytical technologies and process improvements to ensure solvent control compliance. The global pharmaceutical analytical testing outsourcing market, which includes residual solvent testing, was valued at $6.1 billion in 2021 and is projected to grow at a CAGR of 8.3% through 2028, reflecting the industry's commitment to meeting regulatory standards.
Petroleum ether, a mixture of volatile hydrocarbons commonly used in drug substance workups, presents particular challenges for ICH Q3C compliance. As a Class 2 or Class 3 solvent (depending on specific composition), its residual presence must be strictly controlled. The industry demand for effective petroleum ether control stems from both regulatory requirements and quality considerations that directly impact patient safety.
Recent industry surveys reveal that approximately 65% of pharmaceutical manufacturers report challenges with residual solvent control during scale-up and technology transfer phases. These challenges often lead to production delays, increased costs, and potential regulatory issues during submission reviews.
The economic impact of non-compliance with ICH Q3C guidelines can be substantial. Regulatory rejections due to solvent residual issues can delay market entry by 6-18 months, potentially costing companies millions in lost revenue. Additionally, post-approval changes to manufacturing processes to address solvent issues can cost between $500,000 to $2 million per product, depending on the complexity of the required changes.
Industry stakeholders are increasingly demanding integrated solutions that address solvent control throughout the drug development lifecycle. This includes improved analytical methods with lower detection limits, green chemistry approaches that reduce or eliminate problematic solvents, and robust risk assessment frameworks for solvent selection during process development.
Contract development and manufacturing organizations (CDMOs) report a 40% increase in client requests specifically addressing ICH Q3C compliance strategies in the past five years, highlighting the growing importance of this issue in outsourced pharmaceutical manufacturing relationships.
ICH Q3C Compliance Challenges for Petroleum Ether
The International Council for Harmonisation (ICH) Q3C guideline establishes residual solvent limits in pharmaceutical products, categorizing solvents based on their toxicity levels. Petroleum ether, a complex mixture of hydrocarbons primarily consisting of pentanes and hexanes, presents significant challenges for pharmaceutical manufacturers striving to meet these regulatory requirements. The variable composition of petroleum ether across different suppliers and batches creates inconsistency in residual solvent profiles, making standardized testing protocols difficult to establish.
Regulatory bodies worldwide have adopted ICH Q3C guidelines with varying degrees of stringency. The European Medicines Agency (EMA) and the U.S. Food and Drug Administration (FDA) have fully implemented these guidelines, while some emerging markets maintain different thresholds or enforcement mechanisms. This regulatory landscape complexity creates additional compliance burdens for pharmaceutical companies operating globally.
The primary challenge stems from petroleum ether's classification under ICH Q3C. Components like n-hexane fall under Class 2 solvents with a permitted daily exposure (PDE) of 290 ppm, while other constituents may be classified differently. This mixed classification necessitates comprehensive analytical approaches to ensure all components are appropriately monitored and controlled.
Analytical detection presents another significant hurdle. Standard gas chromatography methods may not adequately separate all petroleum ether components, potentially leading to incomplete characterization. The low detection limits required for certain components (particularly n-hexane) demand highly sensitive analytical techniques that may not be readily available in all manufacturing environments.
Manufacturing processes utilizing petroleum ether face substantial validation challenges. Process parameters must be optimized to ensure consistent and effective removal of residual solvents. This often requires extensive development work to establish robust drying conditions, appropriate hold times, and effective purification steps that reliably reduce petroleum ether residuals below acceptable limits.
Risk assessment frameworks for petroleum ether usage in pharmaceutical manufacturing remain underdeveloped compared to single-component solvents. Companies must develop customized approaches to evaluate the potential impact of petroleum ether residuals on patient safety, product stability, and regulatory compliance, often without clear industry precedents to follow.
The economic implications of petroleum ether compliance challenges are substantial. Manufacturers must weigh the cost advantages of petroleum ether against the increased analytical burden, potential regulatory scrutiny, and possible manufacturing delays associated with its use. Many companies are exploring alternative solvents with more straightforward compliance pathways despite potentially higher direct costs.
Regulatory bodies worldwide have adopted ICH Q3C guidelines with varying degrees of stringency. The European Medicines Agency (EMA) and the U.S. Food and Drug Administration (FDA) have fully implemented these guidelines, while some emerging markets maintain different thresholds or enforcement mechanisms. This regulatory landscape complexity creates additional compliance burdens for pharmaceutical companies operating globally.
The primary challenge stems from petroleum ether's classification under ICH Q3C. Components like n-hexane fall under Class 2 solvents with a permitted daily exposure (PDE) of 290 ppm, while other constituents may be classified differently. This mixed classification necessitates comprehensive analytical approaches to ensure all components are appropriately monitored and controlled.
Analytical detection presents another significant hurdle. Standard gas chromatography methods may not adequately separate all petroleum ether components, potentially leading to incomplete characterization. The low detection limits required for certain components (particularly n-hexane) demand highly sensitive analytical techniques that may not be readily available in all manufacturing environments.
Manufacturing processes utilizing petroleum ether face substantial validation challenges. Process parameters must be optimized to ensure consistent and effective removal of residual solvents. This often requires extensive development work to establish robust drying conditions, appropriate hold times, and effective purification steps that reliably reduce petroleum ether residuals below acceptable limits.
Risk assessment frameworks for petroleum ether usage in pharmaceutical manufacturing remain underdeveloped compared to single-component solvents. Companies must develop customized approaches to evaluate the potential impact of petroleum ether residuals on patient safety, product stability, and regulatory compliance, often without clear industry precedents to follow.
The economic implications of petroleum ether compliance challenges are substantial. Manufacturers must weigh the cost advantages of petroleum ether against the increased analytical burden, potential regulatory scrutiny, and possible manufacturing delays associated with its use. Many companies are exploring alternative solvents with more straightforward compliance pathways despite potentially higher direct costs.
Current Analytical Methods for Petroleum Ether Detection
01 ICH Q3C compliance methods for petroleum ether residuals
Methods for ensuring compliance with ICH Q3C guidelines for petroleum ether residuals in pharmaceutical products. These methods involve specific analytical techniques to detect and quantify petroleum ether residuals to ensure they remain within acceptable limits. The approaches include chromatographic methods, spectroscopic analysis, and validation protocols specifically designed to meet regulatory requirements for residual solvent testing.- ICH Q3C compliance methods for petroleum ether residuals: Methods for ensuring compliance with ICH Q3C guidelines for petroleum ether residuals in pharmaceutical products. These methods involve analytical techniques to detect, quantify, and control residual petroleum ether solvents to meet regulatory requirements. The approaches include specialized testing protocols, validation procedures, and quality control measures specifically designed to address the Class 2 and Class 3 solvents present in petroleum ether fractions according to ICH Q3C guidelines.
- Extraction and purification techniques to reduce petroleum ether residuals: Various extraction and purification techniques are employed to reduce petroleum ether residuals in pharmaceutical and chemical products. These techniques include solvent extraction, distillation, chromatographic separation, and filtration methods that effectively remove or minimize petroleum ether residues. The processes are designed to ensure that final products meet the stringent residual solvent limits established by ICH Q3C guidelines while maintaining product quality and efficacy.
- Analytical methods for detection and quantification of petroleum ether residuals: Specialized analytical methods for the detection and quantification of petroleum ether residuals in pharmaceutical and chemical products. These methods primarily utilize gas chromatography, mass spectrometry, headspace analysis, and other sensitive analytical techniques to accurately measure trace amounts of petroleum ether components. The analytical procedures are validated according to ICH guidelines to ensure reliable and reproducible results that can demonstrate compliance with established safety limits.
- Risk assessment and control strategies for petroleum ether residuals: Comprehensive risk assessment and control strategies for managing petroleum ether residuals in pharmaceutical manufacturing processes. These strategies include risk evaluation frameworks, implementation of process analytical technology (PAT), establishment of acceptance criteria, and development of control measures throughout the manufacturing process. The approaches focus on identifying critical control points and implementing appropriate measures to ensure that petroleum ether residuals remain below ICH Q3C specified limits.
- Alternative solvents and green chemistry approaches: Development and implementation of alternative solvents and green chemistry approaches to replace petroleum ether in various applications. These approaches focus on using environmentally friendly solvents with better safety profiles that comply with ICH Q3C guidelines without requiring extensive residual testing. The alternative methods include the use of bio-based solvents, supercritical fluids, ionic liquids, and water-based systems that can achieve similar technical outcomes while eliminating concerns about petroleum ether residuals.
02 Extraction and purification techniques to reduce petroleum ether residuals
Various extraction and purification techniques are employed to reduce petroleum ether residuals in pharmaceutical products. These include liquid-liquid extraction, solid-phase extraction, and distillation processes designed to remove or minimize petroleum ether content. These techniques are crucial for ensuring that final products meet ICH Q3C guidelines for residual solvent limits.Expand Specific Solutions03 Analytical equipment for petroleum ether residual detection
Specialized analytical equipment and systems designed for the detection and quantification of petroleum ether residuals in pharmaceutical products. These systems typically incorporate gas chromatography, mass spectrometry, or other sensitive detection methods capable of measuring trace amounts of petroleum ether components to ensure compliance with ICH Q3C guidelines.Expand Specific Solutions04 Alternative solvents compliant with ICH Q3C guidelines
Development and use of alternative solvents that can replace petroleum ether in pharmaceutical processes while maintaining compliance with ICH Q3C guidelines. These alternative solvents are selected based on their safety profiles, effectiveness in the intended applications, and reduced toxicity compared to petroleum ether, helping manufacturers meet stringent regulatory requirements for residual solvents.Expand Specific Solutions05 Quality control processes for petroleum ether residual monitoring
Comprehensive quality control processes specifically designed for monitoring petroleum ether residuals throughout the manufacturing process. These include sampling protocols, testing frequencies, documentation requirements, and corrective action procedures to ensure consistent compliance with ICH Q3C guidelines. Implementation of these quality control processes helps manufacturers maintain regulatory compliance and product safety.Expand Specific Solutions
Key Pharmaceutical Companies and Regulatory Bodies
Petroleum ether residual management in drug substance workups represents a critical challenge for ICH Q3C compliance, currently in a mature regulatory phase but with evolving technical solutions. The global pharmaceutical solvent market, valued at approximately $1.5 billion, shows steady growth as regulatory scrutiny intensifies. Leading pharmaceutical companies including Teva Pharmaceutical, Novartis AG, and Astellas Pharma have developed sophisticated analytical methods and purification techniques to address residual solvent concerns. Research institutions like The Scripps Research Institute and University of Southern California contribute significant advancements in analytical methodology. The competitive landscape features both established pharmaceutical manufacturers implementing robust quality control systems and specialized service providers offering compliance solutions, creating a dynamic ecosystem focused on patient safety and regulatory adherence.
Teva Pharmaceutical Industries Ltd.
Technical Solution: Teva has developed a comprehensive solvent management system specifically addressing petroleum ether residuals in active pharmaceutical ingredients (APIs). Their approach involves a multi-stage purification process that combines vacuum distillation with supercritical fluid extraction to reduce petroleum ether residuals below ICH Q3C limits. The company employs real-time monitoring systems with gas chromatography-mass spectrometry (GC-MS) detection limits as low as 1 ppm to ensure compliance throughout manufacturing. Teva has also implemented a risk-based assessment framework that categorizes different drug substances based on their solvent retention profiles, allowing for customized purification protocols. Their method includes validation procedures that account for different formulation matrices and potential solvent-drug interactions that could affect residual levels.
Strengths: Industry-leading analytical capabilities with high sensitivity detection systems; integrated quality management system that tracks solvent usage from raw materials to final product. Weaknesses: Their approach requires specialized equipment and expertise, potentially increasing production costs; the multi-stage purification process may extend manufacturing timelines.
Novartis AG
Technical Solution: Novartis has pioneered an innovative approach to petroleum ether residual management through their "Green Chemistry Initiative" for API production. Their technology utilizes a combination of membrane-based separation techniques and modified crystallization processes specifically designed to minimize Class 2 solvent residuals. The company has developed proprietary computational models that predict solvent-API interactions and retention behaviors, enabling process optimization before manufacturing begins. Novartis implements a hierarchical control strategy that first focuses on solvent substitution where possible, followed by process parameter optimization to minimize usage, and finally advanced purification techniques when necessary. Their analytical platform incorporates headspace GC with flame ionization detection coupled with mass spectrometry confirmation, achieving detection limits well below ICH Q3C requirements. The company has also established a global database of solvent residual profiles across different manufacturing sites to ensure consistency in compliance approaches.
Strengths: Strong emphasis on green chemistry principles that address solvent issues at source; sophisticated predictive modeling capabilities that reduce experimental iterations. Weaknesses: Their approach may be difficult to implement in contract manufacturing organizations without similar technological infrastructure; higher initial investment costs compared to traditional methods.
Critical Patents in Residual Solvent Analysis
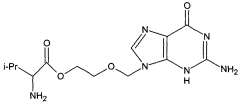
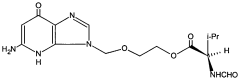
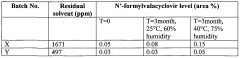
Method for reducing residual alcohols in crystalline valacyclovir hydrochloride
PatentWO2006011874A1
Innovation
- The method involves statically or dynamically contacting valacyclovir hydrochloride with a humid gas, particularly humid air of at least 50% relative humidity, in a fluidized bed apparatus to reduce residual process alcohols to below 5000 ppm, thereby stabilizing the compound against impurity formation.
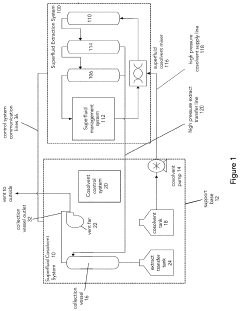
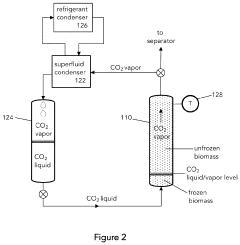
Liquid carbon dioxide and cosolvent biomass extraction method and system
PatentActiveUS20230092529A1
Innovation
- A method involving cryogenic cooling of biomass with liquid carbon dioxide to 0° C. or less, followed by monosolvent and cosolvent extraction steps using subcritical or supercritical liquid carbon dioxide, with controlled cosolvent addition and removal, and subsequent evaporation to obtain extractant oils, along with a cosolvent system that includes a solvent pump, high-pressure fluid lines, and a control system for managing pressure and solvent transfer.
Toxicological Assessment of Petroleum Ether Components
Petroleum ether, a mixture of various hydrocarbons primarily consisting of pentanes and hexanes, presents significant toxicological concerns when residual amounts remain in pharmaceutical drug substances. The toxicity profiles of these components vary considerably, with n-hexane being particularly concerning due to its established neurotoxicity through its metabolite 2,5-hexanedione, which can cause peripheral neuropathy with chronic exposure.
The primary components of petroleum ether include n-pentane, isopentane, n-hexane, and various branched hexanes. Each component carries distinct toxicological implications. N-hexane demonstrates the highest toxicity potential, with established Permitted Daily Exposure (PDE) values of 2.9 mg/day according to ICH Q3C guidelines. Other components like n-pentane and isopentane generally exhibit lower toxicity profiles but still require careful monitoring.
Acute exposure to petroleum ether components primarily affects the central nervous system, causing symptoms ranging from headaches and dizziness to unconsciousness at high concentrations. Chronic exposure presents more serious concerns, particularly with n-hexane, which can lead to permanent neurological damage through axonal degeneration in peripheral nerves.
The toxicokinetics of petroleum ether components involves rapid absorption through inhalation and dermal routes, with distribution primarily to lipid-rich tissues due to their lipophilic nature. Metabolism occurs primarily in the liver through cytochrome P450 enzymes, with n-hexane's metabolism to 2,5-hexanedione being particularly significant from a toxicological perspective.
Regulatory bodies worldwide have established exposure limits for petroleum ether components. The American Conference of Governmental Industrial Hygienists (ACGIH) has set Threshold Limit Values (TLVs) for n-hexane at 50 ppm for an 8-hour workday. The European Chemicals Agency (ECHA) has similar restrictions, emphasizing the importance of minimizing exposure in pharmaceutical manufacturing.
Risk assessment methodologies for petroleum ether residuals in pharmaceuticals must consider both the composition of the specific petroleum ether used and the potential for differential evaporation during processing, which can concentrate more toxic components. Analytical techniques must be capable of identifying and quantifying individual components rather than merely measuring total hydrocarbon content to accurately assess toxicological risk.
Recent toxicological studies have revealed potential endocrine disrupting effects and reproductive toxicity concerns with certain petroleum ether components, particularly at chronic low-dose exposures. These findings emphasize the importance of stringent residual solvent controls in pharmaceutical manufacturing processes to ensure patient safety and regulatory compliance.
The primary components of petroleum ether include n-pentane, isopentane, n-hexane, and various branched hexanes. Each component carries distinct toxicological implications. N-hexane demonstrates the highest toxicity potential, with established Permitted Daily Exposure (PDE) values of 2.9 mg/day according to ICH Q3C guidelines. Other components like n-pentane and isopentane generally exhibit lower toxicity profiles but still require careful monitoring.
Acute exposure to petroleum ether components primarily affects the central nervous system, causing symptoms ranging from headaches and dizziness to unconsciousness at high concentrations. Chronic exposure presents more serious concerns, particularly with n-hexane, which can lead to permanent neurological damage through axonal degeneration in peripheral nerves.
The toxicokinetics of petroleum ether components involves rapid absorption through inhalation and dermal routes, with distribution primarily to lipid-rich tissues due to their lipophilic nature. Metabolism occurs primarily in the liver through cytochrome P450 enzymes, with n-hexane's metabolism to 2,5-hexanedione being particularly significant from a toxicological perspective.
Regulatory bodies worldwide have established exposure limits for petroleum ether components. The American Conference of Governmental Industrial Hygienists (ACGIH) has set Threshold Limit Values (TLVs) for n-hexane at 50 ppm for an 8-hour workday. The European Chemicals Agency (ECHA) has similar restrictions, emphasizing the importance of minimizing exposure in pharmaceutical manufacturing.
Risk assessment methodologies for petroleum ether residuals in pharmaceuticals must consider both the composition of the specific petroleum ether used and the potential for differential evaporation during processing, which can concentrate more toxic components. Analytical techniques must be capable of identifying and quantifying individual components rather than merely measuring total hydrocarbon content to accurately assess toxicological risk.
Recent toxicological studies have revealed potential endocrine disrupting effects and reproductive toxicity concerns with certain petroleum ether components, particularly at chronic low-dose exposures. These findings emphasize the importance of stringent residual solvent controls in pharmaceutical manufacturing processes to ensure patient safety and regulatory compliance.
Global Regulatory Harmonization Strategies
The global pharmaceutical industry faces significant challenges in harmonizing regulatory standards for residual solvents, particularly petroleum ether, across different markets. ICH Q3C guidelines represent a cornerstone effort to establish consistent global standards for residual solvent limits in pharmaceutical products. However, implementation varies considerably across regulatory jurisdictions, creating compliance complexities for manufacturers operating in multiple markets.
Regulatory bodies including the FDA, EMA, and NMPA have adopted ICH Q3C guidelines with varying degrees of modification and implementation timelines. These differences necessitate sophisticated harmonization strategies for pharmaceutical companies developing drug substances with potential petroleum ether residuals. The FDA's approach emphasizes risk-based assessment, while the EMA focuses on comprehensive documentation of control strategies.
A key harmonization challenge involves the classification of petroleum ether components, which may contain multiple hydrocarbons with varying toxicity profiles. Different regulatory authorities may prioritize specific components for monitoring, creating divergent compliance requirements. Companies must develop comprehensive analytical methods capable of satisfying the most stringent requirements across all target markets.
Successful global harmonization strategies typically involve the development of a "regulatory superset" approach—implementing controls that satisfy the most stringent requirements across all relevant jurisdictions. This approach requires sophisticated understanding of regional variations in ICH Q3C interpretation and enforcement practices related to petroleum ether residuals.
Cross-functional regulatory intelligence teams have emerged as a best practice, maintaining current knowledge of evolving global requirements and facilitating early engagement with regulatory authorities. These teams can identify potential compliance issues during drug substance development and implement appropriate control strategies before significant resources are committed to specific manufacturing processes.
Pharmaceutical companies are increasingly participating in international harmonization initiatives through industry associations and regulatory forums. These collaborative efforts aim to reduce unnecessary variations in regulatory interpretation while maintaining appropriate safety standards. The International Pharmaceutical Regulators Programme (IPRP) has established working groups specifically focused on residual solvent harmonization issues.
Digital compliance management systems represent another emerging strategy, enabling companies to maintain current regulatory intelligence and automate compliance verification across multiple jurisdictions. These systems can significantly reduce the risk of non-compliance by providing real-time alerts when petroleum ether residual levels approach regulatory thresholds in specific markets.
Regulatory bodies including the FDA, EMA, and NMPA have adopted ICH Q3C guidelines with varying degrees of modification and implementation timelines. These differences necessitate sophisticated harmonization strategies for pharmaceutical companies developing drug substances with potential petroleum ether residuals. The FDA's approach emphasizes risk-based assessment, while the EMA focuses on comprehensive documentation of control strategies.
A key harmonization challenge involves the classification of petroleum ether components, which may contain multiple hydrocarbons with varying toxicity profiles. Different regulatory authorities may prioritize specific components for monitoring, creating divergent compliance requirements. Companies must develop comprehensive analytical methods capable of satisfying the most stringent requirements across all target markets.
Successful global harmonization strategies typically involve the development of a "regulatory superset" approach—implementing controls that satisfy the most stringent requirements across all relevant jurisdictions. This approach requires sophisticated understanding of regional variations in ICH Q3C interpretation and enforcement practices related to petroleum ether residuals.
Cross-functional regulatory intelligence teams have emerged as a best practice, maintaining current knowledge of evolving global requirements and facilitating early engagement with regulatory authorities. These teams can identify potential compliance issues during drug substance development and implement appropriate control strategies before significant resources are committed to specific manufacturing processes.
Pharmaceutical companies are increasingly participating in international harmonization initiatives through industry associations and regulatory forums. These collaborative efforts aim to reduce unnecessary variations in regulatory interpretation while maintaining appropriate safety standards. The International Pharmaceutical Regulators Programme (IPRP) has established working groups specifically focused on residual solvent harmonization issues.
Digital compliance management systems represent another emerging strategy, enabling companies to maintain current regulatory intelligence and automate compliance verification across multiple jurisdictions. These systems can significantly reduce the risk of non-compliance by providing real-time alerts when petroleum ether residual levels approach regulatory thresholds in specific markets.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!