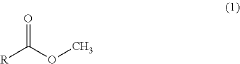How Does Petroleum Ether Vapor Manage To Reach LEL In Typical Lab Setups And How Is Risk Reduced?
SEP 12, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Petroleum Ether Vapor Hazards and Safety Objectives
Petroleum ether is a highly volatile and flammable hydrocarbon mixture commonly used in laboratory settings as a solvent for extractions, chromatography, and cleaning procedures. Its low boiling point range (typically 30-60°C) contributes to its rapid evaporation at room temperature, creating significant fire and explosion hazards when proper safety protocols are not followed.
The primary concern with petroleum ether is its ability to form explosive vapor-air mixtures. The Lower Explosive Limit (LEL) for petroleum ether ranges from 1.1% to 1.4% by volume in air, which can be reached surprisingly quickly in confined laboratory spaces. This presents a substantial risk as the vapor is heavier than air and tends to accumulate at floor level, potentially traveling considerable distances to ignition sources.
Laboratory incidents involving petroleum ether have resulted in serious injuries, fatalities, and facility damage. Notable cases include the 2016 University of Hawaii explosion that severely injured a researcher working with a similar volatile solvent, and multiple incidents in pharmaceutical research facilities where improper handling led to flash fires and explosions.
The technical objectives for petroleum ether safety management focus on preventing vapor concentrations from reaching LEL through engineered controls and procedural safeguards. Primary goals include maintaining vapor concentrations below 10% of LEL (the widely accepted safety threshold), eliminating potential ignition sources, and implementing robust ventilation systems capable of maintaining negative pressure in work areas.
Safety objectives extend to developing comprehensive risk assessment methodologies specific to petroleum ether applications. This includes quantitative modeling of vapor dispersion patterns in laboratory settings, establishing standardized protocols for safe handling volumes, and implementing real-time monitoring systems capable of detecting vapor concentrations well below LEL.
Additional technical goals involve the development of inherently safer alternatives with comparable solvent properties but reduced volatility and flammability. Research into green chemistry alternatives such as supercritical CO2, aqueous systems, and bio-based solvents represents an important direction for reducing petroleum ether dependence in laboratory applications.
Regulatory compliance objectives include alignment with international standards such as NFPA 45 (Fire Protection for Laboratories), OSHA laboratory standards, and European ATEX directives. These frameworks provide the foundation for technical specifications regarding ventilation requirements, electrical equipment classifications, and storage limitations for petroleum ether in laboratory environments.
The primary concern with petroleum ether is its ability to form explosive vapor-air mixtures. The Lower Explosive Limit (LEL) for petroleum ether ranges from 1.1% to 1.4% by volume in air, which can be reached surprisingly quickly in confined laboratory spaces. This presents a substantial risk as the vapor is heavier than air and tends to accumulate at floor level, potentially traveling considerable distances to ignition sources.
Laboratory incidents involving petroleum ether have resulted in serious injuries, fatalities, and facility damage. Notable cases include the 2016 University of Hawaii explosion that severely injured a researcher working with a similar volatile solvent, and multiple incidents in pharmaceutical research facilities where improper handling led to flash fires and explosions.
The technical objectives for petroleum ether safety management focus on preventing vapor concentrations from reaching LEL through engineered controls and procedural safeguards. Primary goals include maintaining vapor concentrations below 10% of LEL (the widely accepted safety threshold), eliminating potential ignition sources, and implementing robust ventilation systems capable of maintaining negative pressure in work areas.
Safety objectives extend to developing comprehensive risk assessment methodologies specific to petroleum ether applications. This includes quantitative modeling of vapor dispersion patterns in laboratory settings, establishing standardized protocols for safe handling volumes, and implementing real-time monitoring systems capable of detecting vapor concentrations well below LEL.
Additional technical goals involve the development of inherently safer alternatives with comparable solvent properties but reduced volatility and flammability. Research into green chemistry alternatives such as supercritical CO2, aqueous systems, and bio-based solvents represents an important direction for reducing petroleum ether dependence in laboratory applications.
Regulatory compliance objectives include alignment with international standards such as NFPA 45 (Fire Protection for Laboratories), OSHA laboratory standards, and European ATEX directives. These frameworks provide the foundation for technical specifications regarding ventilation requirements, electrical equipment classifications, and storage limitations for petroleum ether in laboratory environments.
Laboratory Safety Market Analysis and Compliance Needs
The laboratory safety market has experienced significant growth in recent years, driven by increasing regulatory requirements and heightened awareness of workplace safety. Currently valued at approximately $2.5 billion globally, this market is projected to grow at a CAGR of 7.2% through 2028, with chemical safety equipment and monitoring systems representing the largest segment.
Petroleum ether handling represents a critical area within laboratory safety protocols due to its high volatility and flammability characteristics. Market analysis indicates that approximately 65% of academic and industrial laboratories regularly work with petroleum ether or similar volatile organic compounds, creating substantial demand for specialized safety equipment and monitoring solutions.
Regulatory compliance remains a primary market driver, with OSHA standards in the United States, REACH regulations in Europe, and similar frameworks worldwide mandating specific safety measures for handling flammable solvents. Non-compliance penalties have increased by nearly 30% over the past five years, compelling organizations to invest in comprehensive safety solutions.
The market for LEL (Lower Explosive Limit) monitoring systems specifically designed for laboratory environments has seen 12% annual growth, outpacing the broader safety equipment market. This growth reflects increasing recognition of vapor accumulation risks in standard laboratory setups, particularly in facilities with older ventilation infrastructure.
Demand segmentation shows distinct patterns across different laboratory types. Research institutions and academic laboratories account for 42% of safety compliance spending, while industrial R&D facilities represent 35%, and commercial testing laboratories comprise 23%. Each segment demonstrates different purchasing behaviors and compliance priorities.
Regional analysis reveals that North America leads in laboratory safety spending at 38% of the global market, followed by Europe (31%), Asia-Pacific (22%), and rest of world (9%). However, the Asia-Pacific region shows the fastest growth rate at 9.8% annually, driven by rapid expansion of research facilities in China and India.
The competitive landscape features both specialized safety equipment manufacturers and diversified scientific supply companies. Major players include Thermo Fisher Scientific, 3M Safety Products, Honeywell Analytics, and Drägerwerk, collectively controlling approximately 47% of the market share for laboratory safety solutions specific to volatile compound handling.
Customer needs assessment indicates growing demand for integrated safety systems that combine real-time monitoring with automated emergency response capabilities. Survey data shows that 78% of laboratory managers prioritize solutions that offer continuous vapor concentration monitoring with alert systems, while 63% seek systems with direct integration to building management systems.
Petroleum ether handling represents a critical area within laboratory safety protocols due to its high volatility and flammability characteristics. Market analysis indicates that approximately 65% of academic and industrial laboratories regularly work with petroleum ether or similar volatile organic compounds, creating substantial demand for specialized safety equipment and monitoring solutions.
Regulatory compliance remains a primary market driver, with OSHA standards in the United States, REACH regulations in Europe, and similar frameworks worldwide mandating specific safety measures for handling flammable solvents. Non-compliance penalties have increased by nearly 30% over the past five years, compelling organizations to invest in comprehensive safety solutions.
The market for LEL (Lower Explosive Limit) monitoring systems specifically designed for laboratory environments has seen 12% annual growth, outpacing the broader safety equipment market. This growth reflects increasing recognition of vapor accumulation risks in standard laboratory setups, particularly in facilities with older ventilation infrastructure.
Demand segmentation shows distinct patterns across different laboratory types. Research institutions and academic laboratories account for 42% of safety compliance spending, while industrial R&D facilities represent 35%, and commercial testing laboratories comprise 23%. Each segment demonstrates different purchasing behaviors and compliance priorities.
Regional analysis reveals that North America leads in laboratory safety spending at 38% of the global market, followed by Europe (31%), Asia-Pacific (22%), and rest of world (9%). However, the Asia-Pacific region shows the fastest growth rate at 9.8% annually, driven by rapid expansion of research facilities in China and India.
The competitive landscape features both specialized safety equipment manufacturers and diversified scientific supply companies. Major players include Thermo Fisher Scientific, 3M Safety Products, Honeywell Analytics, and Drägerwerk, collectively controlling approximately 47% of the market share for laboratory safety solutions specific to volatile compound handling.
Customer needs assessment indicates growing demand for integrated safety systems that combine real-time monitoring with automated emergency response capabilities. Survey data shows that 78% of laboratory managers prioritize solutions that offer continuous vapor concentration monitoring with alert systems, while 63% seek systems with direct integration to building management systems.
Current LEL Detection Technologies and Challenges
Lower Explosive Limit (LEL) detection technologies in laboratory environments have evolved significantly over the past decades, yet several challenges persist in effectively monitoring petroleum ether vapor concentrations. Current detection systems primarily utilize four main technologies: catalytic bead sensors, infrared (IR) sensors, photoionization detectors (PIDs), and electrochemical sensors.
Catalytic bead sensors remain the most widely deployed technology in laboratory settings due to their cost-effectiveness and reliability. These sensors operate by catalytically oxidizing combustible gases on a heated element, with the resulting temperature change indicating gas concentration. However, they suffer from sensitivity to poisoning agents commonly found in laboratories, such as silicones and sulfur compounds, which can significantly reduce their detection capabilities over time.
Infrared sensors offer improved selectivity by measuring the absorption of infrared radiation at wavelengths specific to hydrocarbon molecules. While these sensors provide excellent resistance to poisoning and require less frequent calibration, their higher cost and bulkier form factor limit widespread adoption in space-constrained laboratory environments. Additionally, their cross-sensitivity to water vapor can produce false readings in humid laboratory conditions.
Photoionization detectors (PIDs) excel at detecting low concentrations of volatile organic compounds including petroleum ether components. Their exceptional sensitivity allows for early warning before LEL concentrations are reached. However, PIDs require regular lamp cleaning and calibration, and their readings can be affected by dust and humidity, common variables in laboratory settings.
Electrochemical sensors, while highly specific for certain gases, have limited application for petroleum ether vapor detection due to cross-sensitivity issues and relatively slow response times compared to other technologies. Their primary use in laboratories tends to be as secondary verification systems rather than primary LEL monitors.
A significant challenge across all detection technologies is the proper placement of sensors in laboratory environments. Petroleum ether vapor, being heavier than air, tends to accumulate near floor level, yet many fixed detection systems are installed at breathing height or higher, creating potential blind spots in detection coverage.
Calibration frequency represents another persistent challenge, with many laboratory facilities failing to maintain proper calibration schedules. Studies indicate that up to 60% of fixed gas detectors in laboratory environments may be operating with outdated calibration, potentially providing false security.
Integration with laboratory ventilation systems presents additional complications, as many detection systems lack seamless communication with automated ventilation controls. This disconnect can delay appropriate ventilation responses when petroleum ether concentrations begin approaching dangerous levels.
Emerging challenges include the need for wireless, portable detection solutions that can provide real-time monitoring data to laboratory personnel through mobile devices, enabling more responsive risk management practices while maintaining compliance with increasingly stringent safety regulations.
Catalytic bead sensors remain the most widely deployed technology in laboratory settings due to their cost-effectiveness and reliability. These sensors operate by catalytically oxidizing combustible gases on a heated element, with the resulting temperature change indicating gas concentration. However, they suffer from sensitivity to poisoning agents commonly found in laboratories, such as silicones and sulfur compounds, which can significantly reduce their detection capabilities over time.
Infrared sensors offer improved selectivity by measuring the absorption of infrared radiation at wavelengths specific to hydrocarbon molecules. While these sensors provide excellent resistance to poisoning and require less frequent calibration, their higher cost and bulkier form factor limit widespread adoption in space-constrained laboratory environments. Additionally, their cross-sensitivity to water vapor can produce false readings in humid laboratory conditions.
Photoionization detectors (PIDs) excel at detecting low concentrations of volatile organic compounds including petroleum ether components. Their exceptional sensitivity allows for early warning before LEL concentrations are reached. However, PIDs require regular lamp cleaning and calibration, and their readings can be affected by dust and humidity, common variables in laboratory settings.
Electrochemical sensors, while highly specific for certain gases, have limited application for petroleum ether vapor detection due to cross-sensitivity issues and relatively slow response times compared to other technologies. Their primary use in laboratories tends to be as secondary verification systems rather than primary LEL monitors.
A significant challenge across all detection technologies is the proper placement of sensors in laboratory environments. Petroleum ether vapor, being heavier than air, tends to accumulate near floor level, yet many fixed detection systems are installed at breathing height or higher, creating potential blind spots in detection coverage.
Calibration frequency represents another persistent challenge, with many laboratory facilities failing to maintain proper calibration schedules. Studies indicate that up to 60% of fixed gas detectors in laboratory environments may be operating with outdated calibration, potentially providing false security.
Integration with laboratory ventilation systems presents additional complications, as many detection systems lack seamless communication with automated ventilation controls. This disconnect can delay appropriate ventilation responses when petroleum ether concentrations begin approaching dangerous levels.
Emerging challenges include the need for wireless, portable detection solutions that can provide real-time monitoring data to laboratory personnel through mobile devices, enabling more responsive risk management practices while maintaining compliance with increasingly stringent safety regulations.
Existing Vapor Control Solutions in Laboratory Settings
01 Lower Explosive Limit (LEL) detection systems for petroleum ether vapor
Detection systems designed to monitor and alert when petroleum ether vapor concentrations approach the lower explosive limit. These systems typically include sensors that can detect hydrocarbon vapors at concentrations well below the LEL, providing early warning to prevent potential explosions. The systems may incorporate alarms, automatic shutdown mechanisms, and continuous monitoring capabilities to ensure safety in environments where petroleum ether is present.- Lower Explosive Limit (LEL) detection systems for petroleum ether vapor: Detection systems designed to monitor and alert when petroleum ether vapor concentrations approach the lower explosive limit. These systems typically include sensors that can detect hydrocarbon vapors and trigger alarms when concentrations reach dangerous levels. Such systems are crucial for preventing explosions in environments where petroleum ether is used or stored.
- Safety measures for handling petroleum ether below LEL: Various safety protocols and equipment designed to maintain petroleum ether vapor concentrations below the lower explosive limit. These include ventilation systems, vapor recovery units, and operational procedures that minimize vapor release. Implementing these safety measures is essential in industrial settings to prevent the formation of explosive atmospheres.
- Monitoring and control systems for petroleum ether environments: Automated systems that continuously monitor petroleum ether vapor concentrations and implement control measures to maintain levels below the lower explosive limit. These systems often integrate sensors, controllers, and actuators to provide real-time monitoring and automatic response to dangerous conditions, helping to prevent potential explosion hazards.
- Explosion prevention in petroleum ether processing equipment: Specialized equipment and design features that prevent the formation of explosive petroleum ether vapor concentrations. These include explosion-proof electrical components, flame arrestors, and pressure relief systems. Such equipment is critical in processing facilities where petroleum ether is handled in large quantities.
- LEL measurement techniques for petroleum ether vapor: Methods and instruments used to accurately measure the concentration of petroleum ether vapor relative to its lower explosive limit. These techniques include catalytic sensors, infrared analyzers, and photoionization detectors that provide precise measurements of vapor concentrations. Accurate measurement is essential for maintaining safe operating conditions in environments where petroleum ether is present.
02 Safety measures for handling petroleum ether below LEL
Various safety protocols and equipment designed to maintain petroleum ether vapor concentrations below the lower explosive limit. These include ventilation systems, vapor recovery units, and specialized handling procedures. By implementing these safety measures, facilities can prevent the accumulation of petroleum ether vapors to dangerous levels, reducing the risk of fire or explosion in industrial settings where petroleum ether is used or stored.Expand Specific Solutions03 Petroleum ether vapor control in extraction and processing
Methods and systems for controlling petroleum ether vapor during extraction and processing operations. These include closed-loop systems that capture and recycle petroleum ether vapors, condensation techniques to recover volatile components, and process modifications to minimize vapor generation. Such control measures are essential in pharmaceutical, chemical, and oil processing industries where petroleum ether is commonly used as a solvent or extraction medium.Expand Specific Solutions04 LEL monitoring technology for petroleum ether environments
Advanced technologies for continuous monitoring of petroleum ether vapor concentrations relative to the lower explosive limit. These technologies include wireless sensor networks, infrared spectroscopy, catalytic bead sensors, and integrated monitoring systems. Modern LEL monitors provide real-time data, remote monitoring capabilities, and integration with facility management systems to ensure comprehensive safety coverage in areas where petroleum ether is present.Expand Specific Solutions05 Risk assessment and prevention strategies related to petroleum ether LEL
Comprehensive approaches to evaluate and mitigate risks associated with petroleum ether vapor reaching its lower explosive limit. These include hazard identification methodologies, risk assessment frameworks, emergency response planning, and preventive maintenance protocols. By implementing these strategies, facilities can systematically identify potential hazards, evaluate their likelihood and consequences, and establish appropriate controls to prevent petroleum ether vapor concentrations from reaching dangerous levels.Expand Specific Solutions
Key Laboratory Safety Equipment Manufacturers
The petroleum ether vapor risk management landscape is currently in a mature development phase, with established safety protocols evolving alongside technological advancements. The market for laboratory safety solutions addressing LEL (Lower Explosive Limit) concerns is growing steadily, driven by stringent regulatory requirements and increased awareness of chemical hazards. Leading players like China Petroleum & Chemical Corp., PetroChina, and Saudi Arabian Oil Co. have developed comprehensive risk mitigation technologies, while specialized safety firms such as Vitalong Fire Safety Group offer targeted solutions. Academic institutions including China Petroleum University Beijing and Shandong University of Science & Technology contribute significant research to improve vapor containment systems. Equipment manufacturers like Agilent Technologies and Praxair Technology have integrated advanced ventilation and monitoring capabilities into laboratory setups, effectively reducing explosion risks through engineering controls.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed comprehensive petroleum ether vapor management systems for laboratory environments that focus on multi-layered safety protocols. Their approach combines advanced ventilation systems with real-time vapor concentration monitoring technology. The system employs specialized fume hoods designed specifically for volatile petroleum solvents that maintain negative pressure gradients to prevent vapor escape. Sinopec's solution incorporates proprietary vapor detection sensors calibrated specifically for petroleum ether's properties, capable of detecting concentrations as low as 0.1% of LEL (Lower Explosive Limit). Their integrated safety system includes automatic shutdown protocols that activate when vapor concentrations reach 10% of LEL, well before reaching dangerous levels. Additionally, they've developed specialized storage cabinets with built-in ventilation and fire suppression systems specifically designed for petroleum ether storage in laboratory settings.
Strengths: Comprehensive integration of detection and prevention systems; highly sensitive vapor monitoring technology; automated safety response mechanisms. Weaknesses: System complexity requires significant initial investment and specialized maintenance; may be overengineered for smaller laboratory settings; requires consistent power supply and backup systems to maintain safety protocols.
Celanese International Corp.
Technical Solution: Celanese International Corp. has developed a sophisticated petroleum ether vapor management system specifically designed for research and quality control laboratories. Their approach centers on their ChemSecure™ platform that integrates multiple safety technologies into a cohesive system. The solution incorporates specialized ventilation systems with optimized air exchange rates calibrated specifically for petroleum ether's vapor density and dispersion characteristics. Celanese's system features their proprietary VaporGuard™ technology that uses selective catalytic oxidation to convert petroleum ether vapors into less hazardous compounds before exhaust. Their approach includes continuous monitoring systems with redundant sensor technologies that provide fail-safe detection capabilities. The system incorporates smart laboratory furniture with built-in local exhaust ventilation at common petroleum ether handling points. Celanese has also developed specialized procedural controls and workflow optimization techniques that minimize the quantity of petroleum ether in open containers at any given time, significantly reducing vapor generation potential.
Strengths: Innovative vapor conversion technology reduces hazardous emissions; integrated approach combining engineering and procedural controls; redundant safety systems provide fail-safe operation. Weaknesses: Higher implementation and operational costs; requires specialized maintenance for catalytic systems; limited adaptability to laboratories with highly variable workflows.
Critical Innovations in LEL Prevention Systems
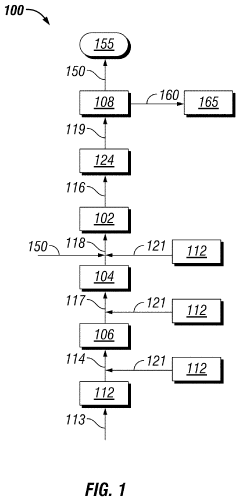
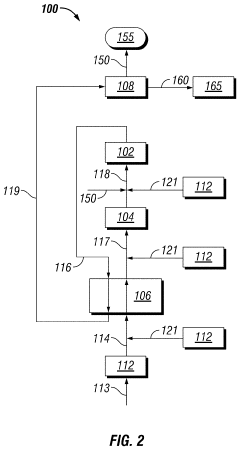
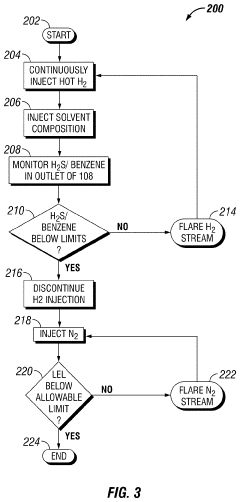
Solvent System for Cleaning Low-Temperature Fixed-Bed Reactor Catalyst in Situ
PatentActiveUS20210023548A1
Innovation
- A method involving a hot hydrogen stream with a fatty acid methyl ester and an oxygenated solvent is circulated through equipment to dissolve and disaggregate deposits, followed by a nitrogen stream to reduce vapor concentrations, allowing for safe and efficient removal of contaminants.
System and method for vapor space monitoring and control
PatentWO2025155820A1
Innovation
- A system and method that utilizes both Lower Explosive Limit (LEL) gas monitors and oxygen sensors to detect potentially explosive gas mixtures, triggering a control system to halt mechanical equipment operations when unsafe concentrations are detected, thereby preventing ignition.
Regulatory Framework for Laboratory Chemical Safety
The regulatory landscape governing laboratory chemical safety is extensive and multi-layered, encompassing international standards, national legislation, and institutional policies. For petroleum ether specifically, which presents significant flammability hazards, these regulations are particularly stringent.
At the international level, the Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides standardized hazard communication elements for petroleum ether, classifying it as a highly flammable liquid with specific labeling requirements. This system has been widely adopted across jurisdictions to ensure consistent hazard identification.
In the United States, OSHA's Laboratory Standard (29 CFR 1910.1450) mandates the development of Chemical Hygiene Plans that must specifically address flammable materials like petroleum ether. This regulation requires risk assessments, proper ventilation systems, and emergency protocols for handling potential vapor accumulation scenarios.
The National Fire Protection Association (NFPA) provides additional regulatory guidance through NFPA 45 (Standard on Fire Protection for Laboratories Using Chemicals), which establishes maximum allowable quantities of flammable liquids per control area and specific storage requirements to prevent vapor accumulation reaching LEL.
European laboratories operate under the ATEX Directive (2014/34/EU) which regulates equipment and protective systems intended for use in potentially explosive atmospheres. This framework requires zoning of laboratory areas where petroleum ether vapors might accumulate, with corresponding equipment specifications.
Institutional implementation of these regulations typically manifests through Standard Operating Procedures (SOPs) that detail specific handling protocols for petroleum ether. These include requirements for fume hood use, specifications for explosion-proof refrigeration, and guidelines for waste disposal that prevent vapor accumulation.
Regulatory compliance mechanisms include mandatory laboratory inspections, certification of ventilation systems, and documentation of staff training on flammable material handling. Many jurisdictions require periodic testing of ventilation systems to ensure they maintain sufficient air changes per hour to prevent petroleum ether vapor from approaching LEL concentrations.
Recent regulatory trends show increasing emphasis on quantitative risk assessment approaches, with some jurisdictions now requiring continuous monitoring systems for volatile organic compounds in laboratories where significant quantities of petroleum ether are used. This represents a shift from prescriptive to performance-based regulatory frameworks.
At the international level, the Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides standardized hazard communication elements for petroleum ether, classifying it as a highly flammable liquid with specific labeling requirements. This system has been widely adopted across jurisdictions to ensure consistent hazard identification.
In the United States, OSHA's Laboratory Standard (29 CFR 1910.1450) mandates the development of Chemical Hygiene Plans that must specifically address flammable materials like petroleum ether. This regulation requires risk assessments, proper ventilation systems, and emergency protocols for handling potential vapor accumulation scenarios.
The National Fire Protection Association (NFPA) provides additional regulatory guidance through NFPA 45 (Standard on Fire Protection for Laboratories Using Chemicals), which establishes maximum allowable quantities of flammable liquids per control area and specific storage requirements to prevent vapor accumulation reaching LEL.
European laboratories operate under the ATEX Directive (2014/34/EU) which regulates equipment and protective systems intended for use in potentially explosive atmospheres. This framework requires zoning of laboratory areas where petroleum ether vapors might accumulate, with corresponding equipment specifications.
Institutional implementation of these regulations typically manifests through Standard Operating Procedures (SOPs) that detail specific handling protocols for petroleum ether. These include requirements for fume hood use, specifications for explosion-proof refrigeration, and guidelines for waste disposal that prevent vapor accumulation.
Regulatory compliance mechanisms include mandatory laboratory inspections, certification of ventilation systems, and documentation of staff training on flammable material handling. Many jurisdictions require periodic testing of ventilation systems to ensure they maintain sufficient air changes per hour to prevent petroleum ether vapor from approaching LEL concentrations.
Recent regulatory trends show increasing emphasis on quantitative risk assessment approaches, with some jurisdictions now requiring continuous monitoring systems for volatile organic compounds in laboratories where significant quantities of petroleum ether are used. This represents a shift from prescriptive to performance-based regulatory frameworks.
Risk Assessment Methodologies for Volatile Solvents
Risk assessment for volatile solvents like petroleum ether requires systematic methodologies to identify, evaluate, and mitigate potential hazards in laboratory environments. These methodologies typically follow a structured approach beginning with hazard identification, where the specific properties of petroleum ether—including its extremely low flash point (-40°C), wide flammability range, and high vapor density—are documented and analyzed.
Quantitative risk assessment techniques form the cornerstone of volatile solvent safety protocols. These include Failure Mode and Effects Analysis (FMEA), which systematically evaluates potential failure points in laboratory setups where petroleum ether is used, and Layer of Protection Analysis (LOPA), which assesses the effectiveness of multiple safeguards designed to prevent vapor accumulation from reaching Lower Explosive Limit (LEL) concentrations.
The Bow-Tie Analysis method has gained prominence in laboratory safety management, providing visual representation of pathways between hazard causes (petroleum ether vapor release) and consequences (fire or explosion). This methodology identifies preventive barriers on the left side of the "bow-tie" and mitigation measures on the right, offering a comprehensive view of risk control strategies.
Process Hazard Analysis (PHA) techniques, particularly HAZOP (Hazard and Operability Study), examine deviations from intended operations in laboratory processes involving petroleum ether. This structured approach evaluates parameters such as ventilation rates, temperature controls, and containment systems to identify scenarios where LEL might be reached.
Modern risk assessment increasingly incorporates computational fluid dynamics (CFD) modeling to simulate vapor dispersion patterns in laboratory spaces. These models account for room geometry, ventilation systems, and potential release scenarios to predict where petroleum ether vapors might accumulate and reach dangerous concentrations.
Exposure monitoring methodologies complement theoretical assessments by providing real-time data on actual vapor concentrations. Portable gas detectors calibrated specifically for petroleum ether can be strategically placed in laboratories to alert personnel before concentrations approach 10% of the LEL, providing an early warning system.
Risk matrices are commonly employed to prioritize identified hazards by plotting likelihood against severity. For petroleum ether, the severity is typically rated high due to potential for fire or explosion, while likelihood varies based on specific laboratory controls. This prioritization guides resource allocation for risk reduction measures and helps establish acceptable risk thresholds for laboratory operations.
Quantitative risk assessment techniques form the cornerstone of volatile solvent safety protocols. These include Failure Mode and Effects Analysis (FMEA), which systematically evaluates potential failure points in laboratory setups where petroleum ether is used, and Layer of Protection Analysis (LOPA), which assesses the effectiveness of multiple safeguards designed to prevent vapor accumulation from reaching Lower Explosive Limit (LEL) concentrations.
The Bow-Tie Analysis method has gained prominence in laboratory safety management, providing visual representation of pathways between hazard causes (petroleum ether vapor release) and consequences (fire or explosion). This methodology identifies preventive barriers on the left side of the "bow-tie" and mitigation measures on the right, offering a comprehensive view of risk control strategies.
Process Hazard Analysis (PHA) techniques, particularly HAZOP (Hazard and Operability Study), examine deviations from intended operations in laboratory processes involving petroleum ether. This structured approach evaluates parameters such as ventilation rates, temperature controls, and containment systems to identify scenarios where LEL might be reached.
Modern risk assessment increasingly incorporates computational fluid dynamics (CFD) modeling to simulate vapor dispersion patterns in laboratory spaces. These models account for room geometry, ventilation systems, and potential release scenarios to predict where petroleum ether vapors might accumulate and reach dangerous concentrations.
Exposure monitoring methodologies complement theoretical assessments by providing real-time data on actual vapor concentrations. Portable gas detectors calibrated specifically for petroleum ether can be strategically placed in laboratories to alert personnel before concentrations approach 10% of the LEL, providing an early warning system.
Risk matrices are commonly employed to prioritize identified hazards by plotting likelihood against severity. For petroleum ether, the severity is typically rated high due to potential for fire or explosion, while likelihood varies based on specific laboratory controls. This prioritization guides resource allocation for risk reduction measures and helps establish acceptable risk thresholds for laboratory operations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!