How Ozonation Achieves Disinfection Targets Without Exceeding Bromate Limits?
SEP 18, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ozonation Disinfection Background and Objectives
Ozonation has evolved significantly since its first application in water treatment in the late 19th century. Initially used primarily for taste and odor control, ozonation has become a cornerstone technology in modern water disinfection systems. The evolution of this technology has been driven by increasing understanding of waterborne pathogens and the need for effective disinfection methods that minimize harmful disinfection by-products (DBPs).
The fundamental principle of ozonation involves the generation of ozone (O₃) through electrical discharge in oxygen-containing gas, which is then dissolved into water. Ozone's powerful oxidizing properties enable it to inactivate a wide range of microorganisms including bacteria, viruses, and protozoan cysts that are resistant to traditional chlorination. This oxidation process disrupts cellular components and metabolic functions of microorganisms, rendering them inactive.
Recent technological advancements have significantly improved ozonation efficiency, including enhanced ozone generation methods, improved gas-liquid contacting systems, and sophisticated monitoring and control systems. These developments have made ozonation more energy-efficient and cost-effective, expanding its application across various water treatment scenarios.
However, a critical challenge in ozonation technology is the formation of bromate (BrO₃⁻) when ozone reacts with naturally occurring bromide (Br⁻) in water. Bromate is classified as a potential human carcinogen, and regulatory agencies worldwide have established strict limits on its concentration in drinking water. The United States Environmental Protection Agency (USEPA) and the World Health Organization (WHO) have set the maximum contaminant level for bromate at 10 μg/L.
The primary objective of this technical research is to investigate and evaluate strategies that enable water treatment facilities to achieve required disinfection targets while maintaining bromate formation below regulatory limits. This involves optimizing ozonation parameters such as ozone dose, contact time, pH, and temperature, as well as exploring innovative approaches like two-stage ozonation, pH depression during ozonation, and ammonia addition.
Additionally, this research aims to assess the integration of ozonation with other treatment processes, including biological filtration, advanced oxidation processes (AOPs), and membrane filtration, to enhance overall treatment efficiency while minimizing bromate formation. The goal is to develop a comprehensive understanding of the factors influencing the balance between effective disinfection and bromate formation, ultimately providing practical guidelines for water treatment facilities to implement ozonation strategies that comply with increasingly stringent water quality regulations.
The fundamental principle of ozonation involves the generation of ozone (O₃) through electrical discharge in oxygen-containing gas, which is then dissolved into water. Ozone's powerful oxidizing properties enable it to inactivate a wide range of microorganisms including bacteria, viruses, and protozoan cysts that are resistant to traditional chlorination. This oxidation process disrupts cellular components and metabolic functions of microorganisms, rendering them inactive.
Recent technological advancements have significantly improved ozonation efficiency, including enhanced ozone generation methods, improved gas-liquid contacting systems, and sophisticated monitoring and control systems. These developments have made ozonation more energy-efficient and cost-effective, expanding its application across various water treatment scenarios.
However, a critical challenge in ozonation technology is the formation of bromate (BrO₃⁻) when ozone reacts with naturally occurring bromide (Br⁻) in water. Bromate is classified as a potential human carcinogen, and regulatory agencies worldwide have established strict limits on its concentration in drinking water. The United States Environmental Protection Agency (USEPA) and the World Health Organization (WHO) have set the maximum contaminant level for bromate at 10 μg/L.
The primary objective of this technical research is to investigate and evaluate strategies that enable water treatment facilities to achieve required disinfection targets while maintaining bromate formation below regulatory limits. This involves optimizing ozonation parameters such as ozone dose, contact time, pH, and temperature, as well as exploring innovative approaches like two-stage ozonation, pH depression during ozonation, and ammonia addition.
Additionally, this research aims to assess the integration of ozonation with other treatment processes, including biological filtration, advanced oxidation processes (AOPs), and membrane filtration, to enhance overall treatment efficiency while minimizing bromate formation. The goal is to develop a comprehensive understanding of the factors influencing the balance between effective disinfection and bromate formation, ultimately providing practical guidelines for water treatment facilities to implement ozonation strategies that comply with increasingly stringent water quality regulations.
Market Analysis of Ozone-Based Water Treatment Systems
The global market for ozone-based water treatment systems has experienced significant growth in recent years, driven by increasing concerns about water quality and the need for effective disinfection solutions that minimize harmful byproducts. Currently valued at approximately $1.2 billion, this market is projected to grow at a compound annual growth rate of 6.8% through 2028, reaching an estimated $1.7 billion.
Municipal water treatment represents the largest application segment, accounting for roughly 42% of the total market share. This dominance stems from stringent regulations regarding drinking water quality and the growing adoption of ozone technology as a primary or supplementary disinfection method in urban water systems. The industrial sector follows closely, comprising about 35% of the market, with particular strength in food and beverage, pharmaceutical, and electronics manufacturing applications.
Geographically, North America and Europe currently lead the market with a combined share of 58%, attributed to their advanced water infrastructure and strict regulatory frameworks. However, the Asia-Pacific region is emerging as the fastest-growing market with a projected growth rate of 8.5% annually, driven by rapid industrialization, urbanization, and increasing government investments in water treatment infrastructure.
A key market trend is the growing demand for integrated water treatment solutions that combine ozonation with other technologies such as UV treatment, advanced oxidation processes, and biological filtration. This integration helps water utilities achieve comprehensive disinfection while maintaining bromate levels below regulatory limits, typically 10 μg/L in most developed countries.
The competitive landscape features both established players and innovative startups. Major companies like Xylem, Suez, Evoqua Water Technologies, and Ozonia (Suez) control approximately 65% of the global market. These industry leaders are increasingly focusing on developing smart ozonation systems with precise dosing controls and real-time monitoring capabilities to address the bromate formation challenge.
Customer segmentation reveals distinct needs across different sectors. Municipal utilities prioritize compliance with regulatory standards and operational efficiency, while industrial users focus on application-specific performance and return on investment. The healthcare sector, representing a smaller but growing segment at 8% of the market, demands ultra-pure water with zero tolerance for microbial contamination.
Price sensitivity varies significantly by region and application. While initial capital costs for ozone systems remain higher than conventional chlorination, the total cost of ownership analysis increasingly favors ozone technology when considering operational efficiency, reduced chemical handling, and minimized disinfection byproducts management.
Municipal water treatment represents the largest application segment, accounting for roughly 42% of the total market share. This dominance stems from stringent regulations regarding drinking water quality and the growing adoption of ozone technology as a primary or supplementary disinfection method in urban water systems. The industrial sector follows closely, comprising about 35% of the market, with particular strength in food and beverage, pharmaceutical, and electronics manufacturing applications.
Geographically, North America and Europe currently lead the market with a combined share of 58%, attributed to their advanced water infrastructure and strict regulatory frameworks. However, the Asia-Pacific region is emerging as the fastest-growing market with a projected growth rate of 8.5% annually, driven by rapid industrialization, urbanization, and increasing government investments in water treatment infrastructure.
A key market trend is the growing demand for integrated water treatment solutions that combine ozonation with other technologies such as UV treatment, advanced oxidation processes, and biological filtration. This integration helps water utilities achieve comprehensive disinfection while maintaining bromate levels below regulatory limits, typically 10 μg/L in most developed countries.
The competitive landscape features both established players and innovative startups. Major companies like Xylem, Suez, Evoqua Water Technologies, and Ozonia (Suez) control approximately 65% of the global market. These industry leaders are increasingly focusing on developing smart ozonation systems with precise dosing controls and real-time monitoring capabilities to address the bromate formation challenge.
Customer segmentation reveals distinct needs across different sectors. Municipal utilities prioritize compliance with regulatory standards and operational efficiency, while industrial users focus on application-specific performance and return on investment. The healthcare sector, representing a smaller but growing segment at 8% of the market, demands ultra-pure water with zero tolerance for microbial contamination.
Price sensitivity varies significantly by region and application. While initial capital costs for ozone systems remain higher than conventional chlorination, the total cost of ownership analysis increasingly favors ozone technology when considering operational efficiency, reduced chemical handling, and minimized disinfection byproducts management.
Current Challenges in Bromate Formation Control
Despite significant advancements in ozonation technology for water treatment, controlling bromate formation remains one of the most challenging aspects of this disinfection method. Bromate (BrO3-), a regulated disinfection by-product, forms when ozone oxidizes bromide (Br-) naturally present in source waters. The World Health Organization and many regulatory bodies worldwide have established a maximum contaminant level (MCL) of 10 μg/L for bromate due to its classification as a potential human carcinogen.
The fundamental challenge lies in the competing objectives of achieving adequate disinfection while simultaneously preventing bromate formation from exceeding regulatory limits. This balancing act becomes particularly difficult when treating waters with elevated bromide concentrations, which are increasingly common due to saltwater intrusion, industrial discharges, and the use of hypochlorite solutions containing bromide impurities.
Current water treatment facilities face operational difficulties in real-time monitoring of bromate formation. Unlike residual ozone, which can be continuously measured, bromate analysis typically requires sophisticated laboratory techniques such as ion chromatography. This analytical gap creates a significant delay between treatment and confirmation of regulatory compliance, forcing operators to make conservative decisions that may compromise disinfection efficacy.
Another critical challenge is the variability in source water quality parameters that influence bromate formation. Seasonal fluctuations in temperature, pH, natural organic matter (NOM), and bromide concentrations create a dynamic treatment environment where optimal ozone dosing strategies must constantly evolve. Research has shown that bromate formation increases significantly at higher pH values, warmer temperatures, and longer contact times, complicating the design of year-round treatment protocols.
The presence of natural organic matter further complicates bromate control strategies. While NOM can serve as an ozone demand that reduces bromate formation potential, it also creates competition for disinfection capacity and may form other disinfection by-products. Additionally, the specific character and reactivity of NOM vary widely across water sources and seasons, making standardized approaches difficult to implement.
Emerging contaminants of concern, including pharmaceuticals and personal care products, often require higher ozone doses for effective removal, which consequently increases bromate formation risk. This creates a technological dilemma where addressing one water quality concern potentially exacerbates another, forcing difficult risk management decisions by water utilities.
Finally, existing bromate control strategies such as pH depression, ammonia addition, and chlorine-ammonia processes each come with their own operational complexities, cost implications, and potential secondary effects on water quality. The lack of a universally applicable, cost-effective solution for bromate control continues to limit the broader adoption of ozonation despite its superior disinfection capabilities.
The fundamental challenge lies in the competing objectives of achieving adequate disinfection while simultaneously preventing bromate formation from exceeding regulatory limits. This balancing act becomes particularly difficult when treating waters with elevated bromide concentrations, which are increasingly common due to saltwater intrusion, industrial discharges, and the use of hypochlorite solutions containing bromide impurities.
Current water treatment facilities face operational difficulties in real-time monitoring of bromate formation. Unlike residual ozone, which can be continuously measured, bromate analysis typically requires sophisticated laboratory techniques such as ion chromatography. This analytical gap creates a significant delay between treatment and confirmation of regulatory compliance, forcing operators to make conservative decisions that may compromise disinfection efficacy.
Another critical challenge is the variability in source water quality parameters that influence bromate formation. Seasonal fluctuations in temperature, pH, natural organic matter (NOM), and bromide concentrations create a dynamic treatment environment where optimal ozone dosing strategies must constantly evolve. Research has shown that bromate formation increases significantly at higher pH values, warmer temperatures, and longer contact times, complicating the design of year-round treatment protocols.
The presence of natural organic matter further complicates bromate control strategies. While NOM can serve as an ozone demand that reduces bromate formation potential, it also creates competition for disinfection capacity and may form other disinfection by-products. Additionally, the specific character and reactivity of NOM vary widely across water sources and seasons, making standardized approaches difficult to implement.
Emerging contaminants of concern, including pharmaceuticals and personal care products, often require higher ozone doses for effective removal, which consequently increases bromate formation risk. This creates a technological dilemma where addressing one water quality concern potentially exacerbates another, forcing difficult risk management decisions by water utilities.
Finally, existing bromate control strategies such as pH depression, ammonia addition, and chlorine-ammonia processes each come with their own operational complexities, cost implications, and potential secondary effects on water quality. The lack of a universally applicable, cost-effective solution for bromate control continues to limit the broader adoption of ozonation despite its superior disinfection capabilities.
Current Strategies for Bromate Minimization
01 Bromate formation control in ozonation processes
Bromate is a disinfection byproduct formed during ozonation of water containing bromide. Various methods can be employed to control bromate formation while maintaining effective disinfection, including pH adjustment, ammonia addition, and controlling ozone dosage. These techniques help water treatment facilities meet regulatory limits for bromate while still achieving pathogen inactivation targets.- Bromate formation control in ozonation processes: During water treatment with ozone, bromate can form as a disinfection by-product when bromide is present in the source water. Various techniques are employed to control bromate formation while maintaining effective disinfection, including pH adjustment, ammonia addition, and controlling ozone dosage. These methods aim to keep bromate levels below regulatory limits while ensuring adequate pathogen inactivation.
- Regulatory limits and monitoring for bromate in drinking water: Regulatory agencies worldwide have established maximum contaminant levels for bromate in drinking water. The most common limit is 10 μg/L (10 ppb), though some regions may have stricter standards. Water treatment facilities must implement monitoring protocols to ensure compliance with these limits. Advanced analytical methods are used to detect and quantify bromate at low concentrations to verify that treated water meets safety standards.
- Advanced oxidation processes for disinfection with reduced bromate formation: Advanced oxidation processes (AOPs) combine ozone with other treatment methods such as UV irradiation, hydrogen peroxide, or catalysts to enhance disinfection efficiency while minimizing bromate formation. These combined approaches can achieve higher pathogen inactivation rates at lower ozone doses, thereby reducing the risk of exceeding bromate limits. AOPs are particularly valuable for treating source waters with high bromide concentrations.
- Ozonation system design and optimization for disinfection targets: The design and optimization of ozonation systems focus on achieving specific disinfection targets for various pathogens while minimizing disinfection by-products. Key parameters include contact time, ozone concentration, and reactor configuration. Modern systems incorporate real-time monitoring and automated control to maintain optimal ozone dosage based on water quality parameters. These systems can be designed to achieve log reductions of specific microorganisms while staying within bromate formation constraints.
- Bromate removal technologies for post-ozonation treatment: When bromate formation cannot be adequately prevented during ozonation, post-treatment technologies can be employed to remove bromate from treated water. These include biological filtration, granular activated carbon adsorption, and chemical reduction processes. Some advanced treatment trains incorporate specific bromate reduction steps to ensure compliance with regulatory limits while still benefiting from ozone's powerful disinfection capabilities.
02 Regulatory standards and monitoring for bromate in drinking water
Regulatory agencies worldwide have established maximum contaminant levels for bromate in drinking water, typically ranging from 10 to 25 μg/L. Water treatment facilities must implement monitoring protocols to ensure compliance with these limits. Advanced analytical methods are used to detect bromate at low concentrations, enabling proper control of ozonation processes to maintain levels below regulatory thresholds.Expand Specific Solutions03 Advanced ozonation systems for pathogen inactivation
Modern ozonation systems are designed to achieve specific log reduction values for target pathogens while minimizing bromate formation. These systems incorporate real-time monitoring and control of ozone concentration, contact time, and water quality parameters. The effectiveness of disinfection is measured against various microbial targets including bacteria, viruses, and protozoan parasites like Cryptosporidium and Giardia, with specific CT (concentration × time) values required for each pathogen.Expand Specific Solutions04 Alternative disinfection strategies to complement ozonation
To address bromate formation concerns while maintaining disinfection efficacy, water treatment facilities often implement multi-barrier approaches. These may include combining ozonation with UV treatment, chloramination, or advanced oxidation processes. Such combined strategies allow for lower ozone doses while still achieving disinfection targets, thereby reducing bromate formation potential while ensuring comprehensive pathogen control.Expand Specific Solutions05 Catalytic processes for bromate removal post-ozonation
Various catalytic and reduction processes have been developed to remove bromate after its formation during ozonation. These technologies include granular activated carbon filtration, biological treatment, and chemical reduction using agents such as ferrous iron. Implementation of these post-treatment processes allows water utilities to use effective ozone doses for disinfection while subsequently reducing bromate concentrations to meet regulatory limits.Expand Specific Solutions
Leading Companies in Advanced Oxidation Processes
The ozonation disinfection technology market is in a growth phase, with increasing demand driven by stricter water quality regulations and bromate concerns. The global market is projected to reach significant scale as municipalities and industries seek effective disinfection solutions that minimize disinfection by-products. Leading players demonstrate varying levels of technological maturity: established companies like Mitsubishi Electric, Baker Hughes, and Xylem offer commercial-scale solutions with advanced bromate control, while research institutions such as Harbin Institute of Technology and Sun Yat-Sen University focus on innovative approaches. Specialized firms like 3Oe Scientific, ArcAqua, and SurfPlasma are developing niche applications with proprietary technologies that balance disinfection efficacy with bromate formation prevention through precise ozone dosing, catalytic processes, and real-time monitoring systems.
VA TECH WABAG GmbH
Technical Solution: VA TECH WABAG has engineered an innovative ozonation solution that focuses on ammonia-assisted ozonation to achieve disinfection targets while suppressing bromate formation. Their technology introduces controlled amounts of ammonia prior to ozonation, which reacts with ozone to form chloramines that provide residual disinfection while reducing ozone demand. This approach allows for lower ozone dosages that still achieve pathogen inactivation targets but minimize bromate formation. The system incorporates advanced gas dissolution technology that ensures efficient ozone transfer into water, reducing required ozone doses by up to 30%. WABAG's solution also features a proprietary catalytic ozone decomposition unit that removes residual ozone post-treatment, preventing further bromate formation in distribution systems while maintaining disinfection residuals through secondary disinfectants.
Strengths: Ammonia-assisted approach effectively suppresses bromate formation while maintaining disinfection efficacy; reduced ozone demand lowers operational costs; integrated solution addresses both treatment and distribution concerns. Weaknesses: Ammonia addition requires careful monitoring to prevent nitrification issues; system optimization requires site-specific calibration; potential for increased complexity in treatment train.
Baker Hughes Co.
Technical Solution: Baker Hughes has developed an advanced ozonation technology specifically designed for challenging water treatment applications that require robust disinfection while managing bromate formation. Their solution employs a proprietary gas-liquid contacting system that achieves exceptionally high ozone transfer efficiency (>95%), allowing for effective disinfection with minimal ozone dosage. The system incorporates real-time monitoring of multiple water quality parameters including bromide concentration, organic matter content, and pH, with an adaptive control algorithm that continuously optimizes ozone dosage to maintain disinfection efficacy while preventing bromate formation. Baker Hughes' technology also features an innovative ozone quenching system that rapidly neutralizes residual ozone using catalytic materials, preventing continued oxidation reactions that could lead to bromate formation in distribution systems. Additionally, they've implemented a hybrid approach that combines ozonation with hydrogen peroxide addition at precisely controlled ratios to favor hydroxyl radical formation while minimizing bromate-forming pathways.
Strengths: Exceptionally high ozone transfer efficiency reduces required dosage and associated bromate formation; adaptive control system responds to changing water quality conditions; integrated quenching system prevents continued bromate formation. Weaknesses: System complexity requires specialized technical expertise for operation and maintenance; higher capital investment compared to conventional systems; optimization requires extensive site-specific calibration.
Key Patents in Selective Ozonation Technologies
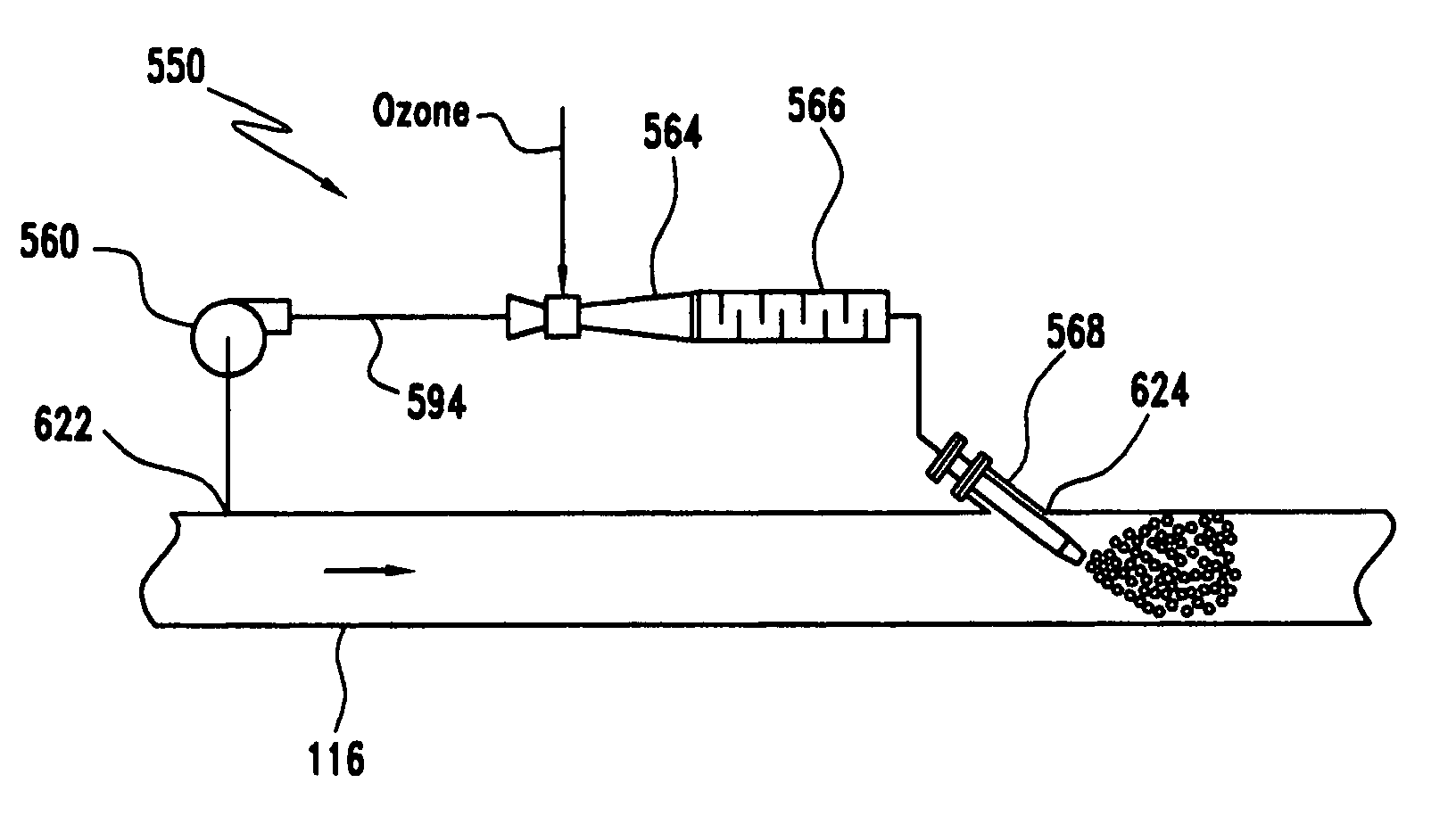
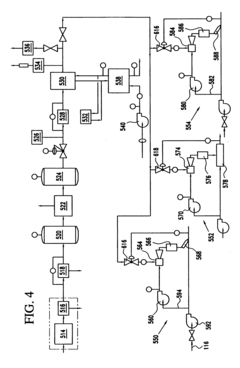
Ozone retention method and system
PatentInactiveUS20060021951A1
Innovation
- A method and system that inject ozone into a portion of the ballast water in a bypass line, allowing for controlled retention time before recombination with the main water flow, minimizing ozone loss and maintaining synergistic disinfection through ozonation and bromination, using a venturi injector to lower pressure and increase velocity for effective mixing.
Regulatory Framework for Disinfection Byproducts
The regulatory landscape for disinfection byproducts (DBPs) has evolved significantly over the past few decades, driven by increasing scientific understanding of their health impacts. The United States Environmental Protection Agency (EPA) established the Stage 1 Disinfectants and Disinfection Byproducts Rule in 1998, which set the maximum contaminant level (MCL) for bromate at 10 μg/L on an annual running average basis. This standard has been adopted by many countries worldwide and remains a critical benchmark for water treatment facilities employing ozonation.
The European Union, through its Drinking Water Directive (98/83/EC), similarly established a 10 μg/L limit for bromate, though monitoring requirements and implementation timelines differ from the US approach. In 2020, the EU updated this directive (2020/2184) while maintaining the same bromate limit, emphasizing the continued recognition of this threshold as protective of public health.
The World Health Organization (WHO) Guidelines for Drinking Water Quality recommend a provisional guideline value of 10 μg/L for bromate, classifying it as a possible human carcinogen (Group 2B). This classification has significantly influenced regulatory approaches globally, particularly in developing nations establishing their own water quality standards.
Japan has implemented more stringent regulations, with a bromate limit of 5 μg/L, reflecting their precautionary approach to water quality management. This lower threshold has driven innovation in ozonation technology within the Japanese water treatment sector, particularly in bromide-rich source waters.
Regulatory frameworks also address the balance between microbial disinfection requirements and DBP formation. The Surface Water Treatment Rule (SWTR) in the US mandates specific log reductions of pathogens, while simultaneously requiring compliance with DBP limits. This creates a regulatory tension that water utilities must navigate when implementing ozonation systems.
Compliance monitoring protocols vary by jurisdiction but typically involve quarterly sampling at distribution system points. The analytical methods approved for bromate detection have evolved from ion chromatography to more sensitive techniques including IC-ICP-MS, allowing detection limits as low as 0.5 μg/L. This improved analytical capability has enabled more precise monitoring and control strategies.
Recent regulatory trends indicate a potential future lowering of bromate limits as analytical capabilities improve and epidemiological studies provide more definitive evidence of health impacts at lower concentrations. Several jurisdictions are considering risk-based approaches that account for source water quality variations and seasonal fluctuations in bromide concentrations.
The European Union, through its Drinking Water Directive (98/83/EC), similarly established a 10 μg/L limit for bromate, though monitoring requirements and implementation timelines differ from the US approach. In 2020, the EU updated this directive (2020/2184) while maintaining the same bromate limit, emphasizing the continued recognition of this threshold as protective of public health.
The World Health Organization (WHO) Guidelines for Drinking Water Quality recommend a provisional guideline value of 10 μg/L for bromate, classifying it as a possible human carcinogen (Group 2B). This classification has significantly influenced regulatory approaches globally, particularly in developing nations establishing their own water quality standards.
Japan has implemented more stringent regulations, with a bromate limit of 5 μg/L, reflecting their precautionary approach to water quality management. This lower threshold has driven innovation in ozonation technology within the Japanese water treatment sector, particularly in bromide-rich source waters.
Regulatory frameworks also address the balance between microbial disinfection requirements and DBP formation. The Surface Water Treatment Rule (SWTR) in the US mandates specific log reductions of pathogens, while simultaneously requiring compliance with DBP limits. This creates a regulatory tension that water utilities must navigate when implementing ozonation systems.
Compliance monitoring protocols vary by jurisdiction but typically involve quarterly sampling at distribution system points. The analytical methods approved for bromate detection have evolved from ion chromatography to more sensitive techniques including IC-ICP-MS, allowing detection limits as low as 0.5 μg/L. This improved analytical capability has enabled more precise monitoring and control strategies.
Recent regulatory trends indicate a potential future lowering of bromate limits as analytical capabilities improve and epidemiological studies provide more definitive evidence of health impacts at lower concentrations. Several jurisdictions are considering risk-based approaches that account for source water quality variations and seasonal fluctuations in bromide concentrations.
Environmental Impact Assessment of Ozonation Systems
Ozonation systems, while effective for water disinfection, present several environmental considerations that require thorough assessment. The primary environmental benefit of ozonation is its ability to disinfect water without introducing persistent chemical residuals, as ozone rapidly decomposes to oxygen. This characteristic significantly reduces the long-term ecological impact compared to chlorine-based disinfection methods that can form harmful disinfection by-products persisting in receiving water bodies.
However, the environmental footprint of ozonation systems extends beyond water quality considerations. The energy intensity of ozone generation represents a substantial environmental concern. Conventional ozone generators typically consume between 10-15 kWh per kilogram of ozone produced, contributing to indirect carbon emissions when powered by non-renewable energy sources. This energy requirement necessitates careful evaluation of the carbon footprint associated with ozonation implementation, particularly in regions heavily dependent on fossil fuels for electricity generation.
The production of bromate during ozonation of bromide-containing waters presents another significant environmental challenge. Bromate is classified as a potential human carcinogen, and its formation must be carefully managed to prevent ecological harm in receiving waters. Studies have demonstrated that bromate can exhibit toxicity to aquatic organisms at concentrations as low as 0.5 mg/L, potentially affecting sensitive species in discharge environments.
Air emissions from ozonation facilities also warrant consideration in environmental impact assessments. Ozone is a powerful oxidant and atmospheric pollutant that can contribute to respiratory issues in humans and vegetation damage when released. Modern ozonation systems incorporate ozone destruction units that typically achieve 95-99% destruction efficiency, but residual emissions must still be monitored and managed according to local air quality regulations.
The life cycle assessment of ozonation infrastructure reveals additional environmental considerations. The manufacturing of specialized materials for ozone-resistant components, including stainless steel, PTFE, and other fluoropolymers, carries embedded environmental costs in terms of resource extraction, processing emissions, and end-of-life disposal challenges. These factors contribute to the overall environmental footprint of ozonation technology implementation.
When balancing disinfection targets against bromate formation, the environmental assessment must consider the trade-offs between pathogen reduction benefits and chemical byproduct risks. Optimized ozonation strategies that incorporate pH adjustment, ammonia addition, or two-stage processes can significantly reduce environmental impacts while maintaining disinfection efficacy, demonstrating the importance of system design in environmental performance.
However, the environmental footprint of ozonation systems extends beyond water quality considerations. The energy intensity of ozone generation represents a substantial environmental concern. Conventional ozone generators typically consume between 10-15 kWh per kilogram of ozone produced, contributing to indirect carbon emissions when powered by non-renewable energy sources. This energy requirement necessitates careful evaluation of the carbon footprint associated with ozonation implementation, particularly in regions heavily dependent on fossil fuels for electricity generation.
The production of bromate during ozonation of bromide-containing waters presents another significant environmental challenge. Bromate is classified as a potential human carcinogen, and its formation must be carefully managed to prevent ecological harm in receiving waters. Studies have demonstrated that bromate can exhibit toxicity to aquatic organisms at concentrations as low as 0.5 mg/L, potentially affecting sensitive species in discharge environments.
Air emissions from ozonation facilities also warrant consideration in environmental impact assessments. Ozone is a powerful oxidant and atmospheric pollutant that can contribute to respiratory issues in humans and vegetation damage when released. Modern ozonation systems incorporate ozone destruction units that typically achieve 95-99% destruction efficiency, but residual emissions must still be monitored and managed according to local air quality regulations.
The life cycle assessment of ozonation infrastructure reveals additional environmental considerations. The manufacturing of specialized materials for ozone-resistant components, including stainless steel, PTFE, and other fluoropolymers, carries embedded environmental costs in terms of resource extraction, processing emissions, and end-of-life disposal challenges. These factors contribute to the overall environmental footprint of ozonation technology implementation.
When balancing disinfection targets against bromate formation, the environmental assessment must consider the trade-offs between pathogen reduction benefits and chemical byproduct risks. Optimized ozonation strategies that incorporate pH adjustment, ammonia addition, or two-stage processes can significantly reduce environmental impacts while maintaining disinfection efficacy, demonstrating the importance of system design in environmental performance.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!