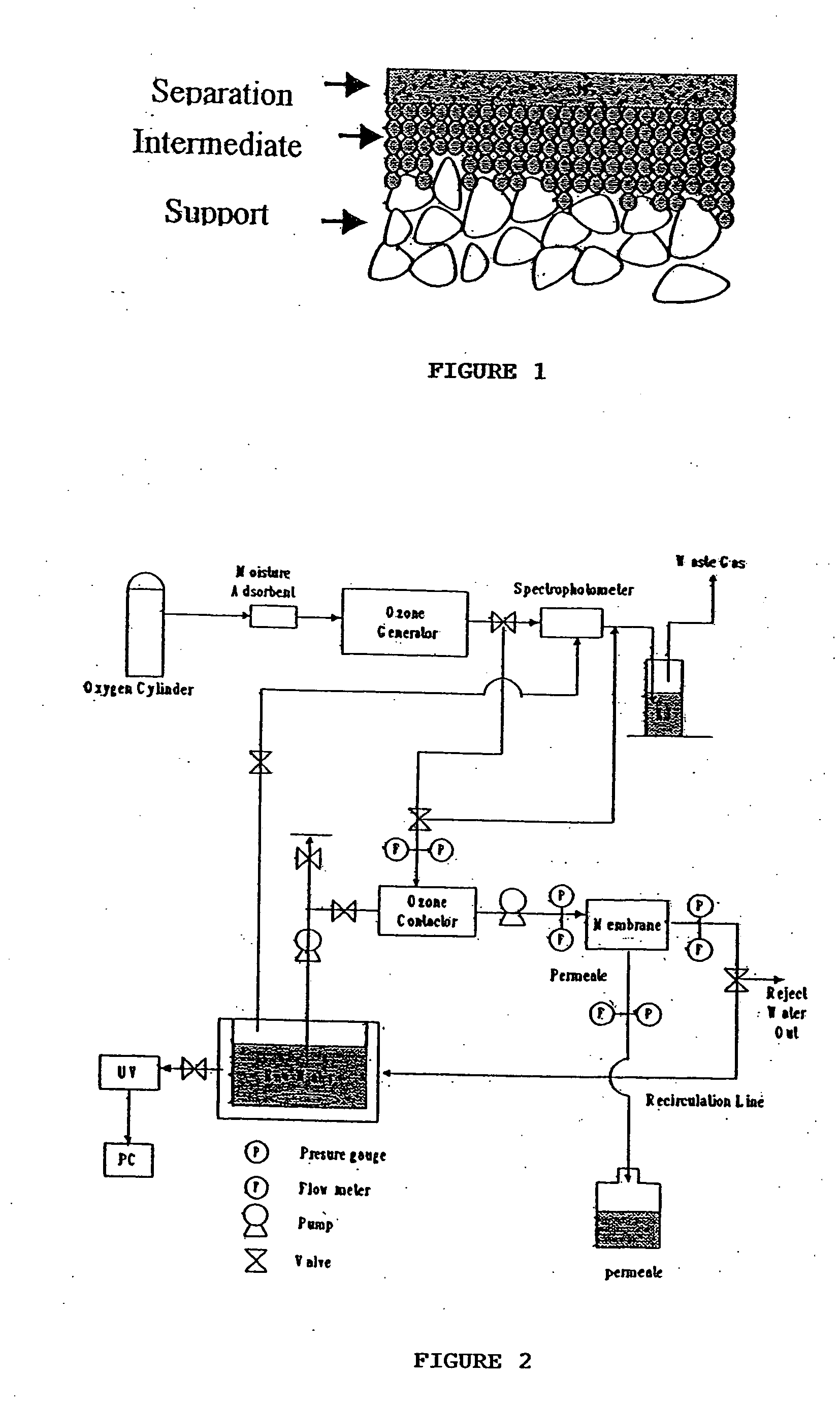
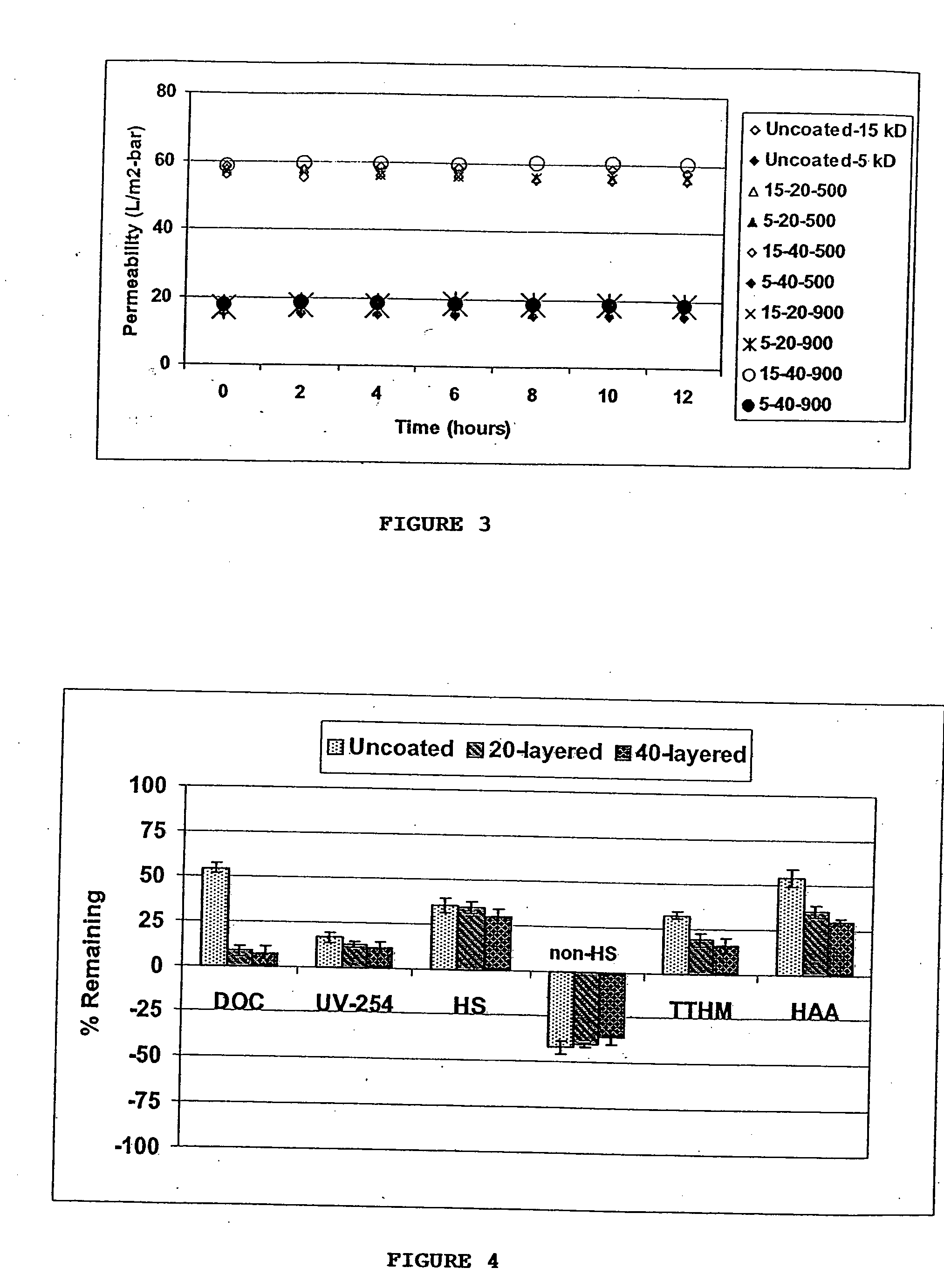
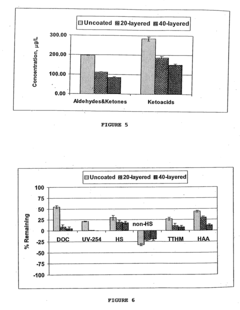
Ozonation: DBP Toxicity Assessment, AOC Formation And Biofilm Risks
SEP 18, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ozonation Technology Background and Objectives
Ozonation technology has evolved significantly since its initial application in water treatment during the late 19th century. Originally implemented for disinfection purposes in Europe, ozonation has expanded into a multifaceted treatment technology addressing various water quality challenges. The evolution of ozonation technology has been marked by continuous improvements in generation efficiency, application methods, and understanding of complex reaction mechanisms in water matrices.
The fundamental principle of ozonation involves the production of ozone (O₃) through electrical discharge in oxygen-containing gas, followed by its dissolution into water where it acts as a powerful oxidant. This process has proven effective against a wide range of contaminants including pathogens, organic compounds, and emerging pollutants. Recent technological advancements have focused on enhancing ozone generation efficiency, optimizing dissolution techniques, and developing hybrid systems that combine ozonation with other treatment processes.
Despite its widespread adoption, ozonation presents several challenges that require comprehensive investigation. The formation of disinfection by-products (DBPs) during ozonation represents a significant concern due to potential toxicity. These DBPs, including bromate, aldehydes, and carboxylic acids, may pose health risks that necessitate thorough toxicity assessment. Additionally, ozonation can increase assimilable organic carbon (AOC) formation through the partial oxidation of complex organic matter, potentially promoting microbial regrowth in distribution systems.
The relationship between ozonation and biofilm formation constitutes another critical area requiring examination. While ozone effectively eliminates planktonic microorganisms, its impact on biofilm development remains complex. The increased AOC resulting from ozonation may serve as a nutrient source for biofilm-forming microorganisms, potentially exacerbating biofilm risks in downstream infrastructure.
The primary objectives of this technical research include: comprehensively evaluating the toxicity profiles of ozonation-derived DBPs through advanced analytical and toxicological methods; characterizing AOC formation patterns under various ozonation conditions and water qualities; assessing biofilm formation potential following ozonation treatment; and developing strategies to optimize ozonation processes that minimize DBP formation and biofilm risks while maintaining effective disinfection and oxidation capabilities.
This research aims to bridge existing knowledge gaps regarding the complex interplay between ozonation treatment parameters and resulting water quality impacts, ultimately contributing to the development of safer and more effective water treatment protocols. The findings will support evidence-based decision-making for water utilities considering ozonation implementation or seeking to optimize existing ozonation systems.
The fundamental principle of ozonation involves the production of ozone (O₃) through electrical discharge in oxygen-containing gas, followed by its dissolution into water where it acts as a powerful oxidant. This process has proven effective against a wide range of contaminants including pathogens, organic compounds, and emerging pollutants. Recent technological advancements have focused on enhancing ozone generation efficiency, optimizing dissolution techniques, and developing hybrid systems that combine ozonation with other treatment processes.
Despite its widespread adoption, ozonation presents several challenges that require comprehensive investigation. The formation of disinfection by-products (DBPs) during ozonation represents a significant concern due to potential toxicity. These DBPs, including bromate, aldehydes, and carboxylic acids, may pose health risks that necessitate thorough toxicity assessment. Additionally, ozonation can increase assimilable organic carbon (AOC) formation through the partial oxidation of complex organic matter, potentially promoting microbial regrowth in distribution systems.
The relationship between ozonation and biofilm formation constitutes another critical area requiring examination. While ozone effectively eliminates planktonic microorganisms, its impact on biofilm development remains complex. The increased AOC resulting from ozonation may serve as a nutrient source for biofilm-forming microorganisms, potentially exacerbating biofilm risks in downstream infrastructure.
The primary objectives of this technical research include: comprehensively evaluating the toxicity profiles of ozonation-derived DBPs through advanced analytical and toxicological methods; characterizing AOC formation patterns under various ozonation conditions and water qualities; assessing biofilm formation potential following ozonation treatment; and developing strategies to optimize ozonation processes that minimize DBP formation and biofilm risks while maintaining effective disinfection and oxidation capabilities.
This research aims to bridge existing knowledge gaps regarding the complex interplay between ozonation treatment parameters and resulting water quality impacts, ultimately contributing to the development of safer and more effective water treatment protocols. The findings will support evidence-based decision-making for water utilities considering ozonation implementation or seeking to optimize existing ozonation systems.
Market Demand for Advanced Water Treatment Solutions
The global water treatment market is experiencing significant growth driven by increasing concerns over water quality and scarcity. The advanced water treatment solutions sector, particularly those involving ozonation technologies, is projected to reach $38 billion by 2026, growing at a CAGR of 7.2% from 2021. This growth is primarily fueled by stringent water quality regulations across developed nations and emerging economies alike.
Municipal water treatment facilities represent the largest market segment, with approximately 65% of the total demand. These facilities are increasingly adopting advanced oxidation processes, including ozonation, to address emerging contaminants that conventional treatment methods cannot effectively remove. The industrial sector follows closely, contributing about 30% of the market demand, with food and beverage, pharmaceutical, and semiconductor industries leading adoption due to their high-purity water requirements.
Geographically, North America and Europe currently dominate the market for advanced water treatment solutions, accounting for over 60% of global revenue. However, the Asia-Pacific region is witnessing the fastest growth rate at 9.5% annually, driven by rapid industrialization, urbanization, and increasing regulatory pressure in countries like China and India.
The demand for ozonation technology specifically is being propelled by its effectiveness in addressing micropollutants, pharmaceutical residues, and endocrine-disrupting compounds. Market research indicates that over 75% of water utilities surveyed are considering implementing or upgrading ozonation systems within the next five years, citing disinfection efficiency and reduced reliance on chlorination as primary motivators.
Consumer awareness regarding disinfection by-products (DBPs) and their potential health impacts has created a market pull for technologies that minimize these risks. This awareness, coupled with healthcare cost implications of waterborne diseases estimated at $9 billion annually worldwide, is driving investment in advanced treatment solutions.
The biofilm management segment within water treatment systems is growing at 8.3% annually, as utilities and industrial users recognize the operational and health risks associated with biofilm formation in distribution systems. Solutions that address assimilable organic carbon (AOC) formation post-ozonation are particularly sought after, with market research indicating willingness-to-pay premiums of 15-20% for technologies that effectively mitigate these risks.
Climate change impacts on water resources are further accelerating market demand, with 82% of water utilities reporting increased treatment challenges due to changing source water quality. This has created opportunities for integrated treatment solutions that combine ozonation with biological filtration to address both DBP formation and biofilm risks simultaneously.
Municipal water treatment facilities represent the largest market segment, with approximately 65% of the total demand. These facilities are increasingly adopting advanced oxidation processes, including ozonation, to address emerging contaminants that conventional treatment methods cannot effectively remove. The industrial sector follows closely, contributing about 30% of the market demand, with food and beverage, pharmaceutical, and semiconductor industries leading adoption due to their high-purity water requirements.
Geographically, North America and Europe currently dominate the market for advanced water treatment solutions, accounting for over 60% of global revenue. However, the Asia-Pacific region is witnessing the fastest growth rate at 9.5% annually, driven by rapid industrialization, urbanization, and increasing regulatory pressure in countries like China and India.
The demand for ozonation technology specifically is being propelled by its effectiveness in addressing micropollutants, pharmaceutical residues, and endocrine-disrupting compounds. Market research indicates that over 75% of water utilities surveyed are considering implementing or upgrading ozonation systems within the next five years, citing disinfection efficiency and reduced reliance on chlorination as primary motivators.
Consumer awareness regarding disinfection by-products (DBPs) and their potential health impacts has created a market pull for technologies that minimize these risks. This awareness, coupled with healthcare cost implications of waterborne diseases estimated at $9 billion annually worldwide, is driving investment in advanced treatment solutions.
The biofilm management segment within water treatment systems is growing at 8.3% annually, as utilities and industrial users recognize the operational and health risks associated with biofilm formation in distribution systems. Solutions that address assimilable organic carbon (AOC) formation post-ozonation are particularly sought after, with market research indicating willingness-to-pay premiums of 15-20% for technologies that effectively mitigate these risks.
Climate change impacts on water resources are further accelerating market demand, with 82% of water utilities reporting increased treatment challenges due to changing source water quality. This has created opportunities for integrated treatment solutions that combine ozonation with biological filtration to address both DBP formation and biofilm risks simultaneously.
Current Challenges in DBP Formation During Ozonation
Despite the widespread use of ozonation in water treatment, the formation of disinfection by-products (DBPs) remains a significant challenge. Ozone's high oxidation potential, while effective for disinfection, leads to complex reactions with natural organic matter (NOM), bromide, and other constituents in water, resulting in various DBPs with potential health concerns. Current research indicates that ozonation can form aldehydes, ketones, carboxylic acids, and bromate, with the latter being particularly concerning as a regulated carcinogen.
The variability of source water quality presents a major obstacle in predicting and controlling DBP formation. Seasonal changes in NOM composition, fluctuating bromide levels, and varying pH conditions significantly impact the types and concentrations of DBPs formed during ozonation. This unpredictability makes it difficult to establish universal treatment protocols that consistently minimize DBP formation while maintaining effective disinfection.
Brominated DBPs represent a particular challenge in ozonation processes. When bromide is present in source water, ozone can oxidize it to hypobromous acid and bromate, leading to the formation of brominated organic compounds that often exhibit higher cytotoxicity and genotoxicity than their chlorinated analogs. Recent studies have shown that some brominated DBPs formed during ozonation may pose greater health risks than previously recognized DBPs, yet many remain unidentified or poorly characterized.
The analytical limitations in DBP identification and quantification further complicate the assessment of ozonation risks. Current methods can only account for approximately 50% of total organic halides formed during disinfection processes. The remaining "unknown" DBPs may include compounds with significant toxicological relevance. Advanced analytical techniques such as high-resolution mass spectrometry are being developed but are not yet widely implemented in routine monitoring programs.
Balancing disinfection efficacy with DBP minimization creates a regulatory and operational dilemma. Reducing ozone doses to minimize DBP formation may compromise pathogen inactivation, particularly for chlorine-resistant microorganisms like Cryptosporidium. Conversely, increasing ozone exposure to ensure adequate disinfection can exacerbate DBP formation and associated health risks.
The formation of assimilable organic carbon (AOC) during ozonation presents additional challenges related to biological stability. Ozone transforms complex organic molecules into smaller, more biodegradable compounds that can serve as nutrients for microbial regrowth in distribution systems. This increased AOC can lead to biofilm formation, which may harbor opportunistic pathogens and accelerate infrastructure deterioration, creating a secondary public health concern beyond direct DBP toxicity.
The variability of source water quality presents a major obstacle in predicting and controlling DBP formation. Seasonal changes in NOM composition, fluctuating bromide levels, and varying pH conditions significantly impact the types and concentrations of DBPs formed during ozonation. This unpredictability makes it difficult to establish universal treatment protocols that consistently minimize DBP formation while maintaining effective disinfection.
Brominated DBPs represent a particular challenge in ozonation processes. When bromide is present in source water, ozone can oxidize it to hypobromous acid and bromate, leading to the formation of brominated organic compounds that often exhibit higher cytotoxicity and genotoxicity than their chlorinated analogs. Recent studies have shown that some brominated DBPs formed during ozonation may pose greater health risks than previously recognized DBPs, yet many remain unidentified or poorly characterized.
The analytical limitations in DBP identification and quantification further complicate the assessment of ozonation risks. Current methods can only account for approximately 50% of total organic halides formed during disinfection processes. The remaining "unknown" DBPs may include compounds with significant toxicological relevance. Advanced analytical techniques such as high-resolution mass spectrometry are being developed but are not yet widely implemented in routine monitoring programs.
Balancing disinfection efficacy with DBP minimization creates a regulatory and operational dilemma. Reducing ozone doses to minimize DBP formation may compromise pathogen inactivation, particularly for chlorine-resistant microorganisms like Cryptosporidium. Conversely, increasing ozone exposure to ensure adequate disinfection can exacerbate DBP formation and associated health risks.
The formation of assimilable organic carbon (AOC) during ozonation presents additional challenges related to biological stability. Ozone transforms complex organic molecules into smaller, more biodegradable compounds that can serve as nutrients for microbial regrowth in distribution systems. This increased AOC can lead to biofilm formation, which may harbor opportunistic pathogens and accelerate infrastructure deterioration, creating a secondary public health concern beyond direct DBP toxicity.
Current Methodologies for DBP Toxicity Assessment
01 Formation and toxicity of disinfection by-products (DBPs) during ozonation
Ozonation of water can lead to the formation of disinfection by-products (DBPs) that may pose health risks. These DBPs are formed when ozone reacts with natural organic matter, bromide, or other compounds present in water. The toxicity of these DBPs varies depending on their chemical structure and concentration. Understanding the formation mechanisms and toxicity profiles of ozonation DBPs is crucial for developing effective water treatment strategies that minimize potential health risks while maintaining disinfection efficacy.- Formation and toxicity of disinfection by-products (DBPs) during ozonation: Ozonation of water can lead to the formation of disinfection by-products (DBPs) that may pose health risks. These DBPs are formed when ozone reacts with natural organic matter, bromide, or other compounds present in water. The toxicity of these DBPs varies depending on their chemical structure and concentration. Understanding the formation mechanisms and toxicity profiles of ozonation DBPs is crucial for developing effective water treatment strategies that minimize health risks while maintaining disinfection efficacy.
- Assimilable organic carbon (AOC) formation during ozonation processes: Ozonation can break down complex organic compounds into smaller, more biodegradable molecules, leading to increased assimilable organic carbon (AOC) in treated water. This AOC serves as a nutrient source for microorganisms and can promote bacterial regrowth in distribution systems. The formation of AOC during ozonation depends on various factors including ozone dose, contact time, water quality parameters, and the characteristics of organic matter present in the source water. Managing AOC formation is essential for controlling microbial regrowth in water distribution networks.
- Biofilm formation risks in ozonated water systems: Increased levels of AOC resulting from ozonation can promote biofilm formation in water distribution systems. Biofilms provide protection for microorganisms against disinfectants and can harbor potentially pathogenic bacteria. These biofilms can develop on pipe surfaces and other water system components, leading to decreased water quality, increased chlorine demand, and potential health risks. Strategies to mitigate biofilm formation include biological filtration following ozonation, maintaining adequate disinfectant residuals, and implementing effective pipe cleaning protocols.
- Monitoring and analytical methods for ozonation by-products and biofilm assessment: Various analytical techniques have been developed to monitor and quantify ozonation by-products, AOC levels, and biofilm formation in water systems. These methods include chromatographic techniques for DBP identification, bioassays for toxicity assessment, microbiological methods for AOC measurement, and microscopic and molecular techniques for biofilm characterization. Continuous monitoring of these parameters allows water utilities to optimize treatment processes, minimize DBP formation, control AOC levels, and prevent excessive biofilm growth in distribution systems.
- Advanced treatment technologies to mitigate ozonation DBPs, AOC, and biofilm risks: Various advanced treatment technologies have been developed to address the challenges associated with ozonation DBPs, AOC formation, and biofilm risks. These include biological filtration processes that remove AOC following ozonation, advanced oxidation processes that can destroy recalcitrant DBPs, membrane filtration systems that provide additional barriers against microorganisms, and innovative disinfection strategies that minimize DBP formation while maintaining effective microbial control. Implementing these technologies as part of a multi-barrier approach can significantly improve water quality and reduce health risks associated with ozonation by-products and biofilms.
02 Assimilable organic carbon (AOC) formation during ozonation processes
Ozonation can break down complex organic compounds into smaller, more biodegradable molecules, leading to increased assimilable organic carbon (AOC) levels in treated water. This AOC serves as a nutrient source for microorganisms and can promote microbial regrowth in distribution systems. The formation of AOC during ozonation depends on various factors including ozone dose, contact time, water quality parameters, and the characteristics of the organic matter present. Managing AOC formation is essential for controlling biological stability in water treatment and distribution systems.Expand Specific Solutions03 Biofilm development risks in ozonated water systems
Increased levels of AOC resulting from ozonation can promote biofilm formation in water distribution systems. Biofilms can harbor pathogenic microorganisms, contribute to disinfectant decay, cause taste and odor problems, and accelerate pipe corrosion. The risk of biofilm development depends on factors such as residual disinfectant levels, water temperature, pipe materials, and hydraulic conditions. Strategies to mitigate biofilm risks include biological filtration following ozonation, maintaining adequate disinfectant residuals, and implementing effective monitoring and maintenance programs for distribution systems.Expand Specific Solutions04 Advanced monitoring and analytical techniques for ozonation by-products
Advanced analytical techniques are essential for identifying and quantifying ozonation by-products and monitoring biofilm formation. These techniques include chromatography coupled with mass spectrometry, fluorescence spectroscopy, ATP measurements, and molecular biological methods. Real-time monitoring systems can provide early warning of potential issues related to DBP formation, AOC levels, or biofilm development. The implementation of comprehensive monitoring programs enables water utilities to optimize ozonation processes, minimize by-product formation, and ensure the biological stability of treated water.Expand Specific Solutions05 Mitigation strategies for ozonation-related water quality issues
Various strategies can be employed to mitigate the formation of toxic DBPs, reduce AOC levels, and prevent biofilm development in ozonated water systems. These include optimizing ozone dosage and contact time, implementing biological filtration processes after ozonation to remove biodegradable compounds, using advanced oxidation processes to enhance the degradation of recalcitrant contaminants, and maintaining appropriate residual disinfectant levels throughout the distribution system. The selection of appropriate mitigation strategies depends on source water quality, treatment objectives, and the specific challenges associated with the water system.Expand Specific Solutions
Leading Organizations in Ozonation Research and Implementation
The ozonation technology market for disinfection byproduct (DBP) toxicity assessment, assimilable organic carbon (AOC) formation, and biofilm risk management is in a growth phase, with increasing adoption driven by stricter water quality regulations. The global water treatment chemicals market, which includes ozonation technologies, is projected to reach approximately $35-40 billion by 2025. Technologically, ozonation solutions have reached moderate maturity, with established players like Kemira Oyj, Evoqua Water Technologies, and Solenis Technologies leading commercial applications. Research institutions including Shanghai Jiao Tong University, University of Washington, and National University of Singapore are advancing fundamental understanding of ozonation processes. China Petroleum & Chemical Corp. and Clean Chemistry are developing specialized applications for industrial water treatment, while companies like NCH Corp. focus on biofilm control solutions integrating ozonation with complementary technologies.
Clean Chemistry, Inc.
Technical Solution: Clean Chemistry, Inc. has developed innovative ozonation alternatives that address the challenges of DBP formation and biofilm risks. Their patented PeroxyMAX® technology utilizes reactive oxygen species generated through proprietary activation methods rather than traditional ozonation, significantly reducing DBP formation while maintaining effective disinfection. This approach generates oxidants on-demand using readily available precursors, eliminating the need for ozone generation equipment. Clean Chemistry's technology has demonstrated up to 80% reduction in halogenated DBP formation compared to conventional ozonation while achieving comparable disinfection efficacy. Their systems incorporate specialized catalysts that selectively target micropollutants while minimizing reactions with natural organic matter that lead to harmful DBPs. Additionally, their technology includes post-treatment stabilization processes that reduce AOC formation, thereby minimizing biofilm growth potential in distribution systems.
Strengths: Significantly reduced DBP formation compared to conventional ozonation; simpler implementation without ozone generation equipment; effective against a broad spectrum of contaminants. Weaknesses: Relatively new technology with limited long-term operational data; requires chemical precursors; may have higher operational costs in certain applications.
University of Washington
Technical Solution: The University of Washington has conducted extensive research on ozonation processes, focusing specifically on DBP toxicity assessment and biofilm formation mechanisms. Their research team has developed novel analytical methods for comprehensive DBP characterization, identifying previously unknown ozonation byproducts and their toxicological profiles. Their work has established correlations between specific water quality parameters and DBP formation potential during ozonation, enabling predictive modeling for treatment optimization. The university's research has pioneered bioassay-directed fractionation techniques that identify the most toxicologically relevant DBPs formed during ozonation, allowing for targeted mitigation strategies. Additionally, they have developed innovative biofilm monitoring techniques using advanced microscopy and molecular biology tools to assess biofilm formation potential following ozonation. Their research has demonstrated that controlled ozone dosing combined with biological filtration can reduce AOC levels by up to 60% compared to ozonation alone, significantly reducing biofilm risks in distribution systems.
Strengths: Cutting-edge analytical capabilities for DBP identification and toxicity assessment; comprehensive understanding of biofilm formation mechanisms; evidence-based optimization strategies. Weaknesses: Research-focused approach may require adaptation for full-scale implementation; limited commercial deployment experience; solutions may require customization for specific water matrices.
Key Innovations in AOC Formation Control
Electrochemical device and method for long-term measurement of hypohalites
PatentWO2011005764A1
Innovation
- A four-electrode sensor system with a working electrode, two auxiliary electrodes, and a reference electrode, where the fourth electrode generates ionized water to clean the working electrode, maintaining its surface stability and allowing the use of sensitive metals like gold, while minimizing biofouling and scaling.
Ceramic membrane water filtration
PatentInactiveUS20060175256A1
Innovation
- A composite ceramic filter coated with a multi-layered, nanocrystalline, sintered metal oxide catalyst, such as titanium oxide, manganese oxide, or ferric oxide, that degrades ozone into hydroxyl radicals, which react with organic matter to reduce fouling and improve water quality during filtration.
Regulatory Framework for Ozonation in Water Treatment
The regulatory landscape governing ozonation in water treatment has evolved significantly over the past decades, reflecting growing understanding of both its benefits and potential risks. In the United States, the Environmental Protection Agency (EPA) established the Disinfectants and Disinfection Byproducts Rules (DBPRs) which set maximum contaminant levels for various disinfection byproducts, including those associated with ozonation such as bromate. The current regulatory limit for bromate is 10 μg/L, a standard also adopted by the World Health Organization (WHO) and the European Union.
The Safe Drinking Water Act (SDWA) provides the overarching framework within which ozonation processes must operate, requiring water utilities to balance disinfection efficacy against DBP formation. This balancing act is formalized in the Surface Water Treatment Rules, which mandate specific log reductions of pathogens while simultaneously requiring compliance with DBP regulations.
Internationally, regulatory approaches vary considerably. The European Drinking Water Directive (98/83/EC, updated in 2020) incorporates a precautionary principle approach, setting stringent standards for ozonation byproducts. Japan has established comprehensive guidelines specifically addressing Assimilable Organic Carbon (AOC) formation during ozonation, recognizing its role in biofilm development within distribution systems.
Regulatory frameworks increasingly acknowledge the complex trade-offs inherent in ozonation. While traditional regulations focused primarily on acute toxicity concerns, modern frameworks are evolving to address chronic exposure risks and emerging contaminants. The EPA's Contaminant Candidate List (CCL) now includes several ozonation byproducts for potential future regulation.
A significant regulatory challenge lies in addressing the formation of unknown or uncharacterized DBPs during ozonation. Current analytical methods can identify only approximately 50% of the total organic halogen compounds formed during disinfection processes. This has led regulatory bodies to implement bioassay-based toxicity assessment approaches as complementary tools to chemical-specific regulations.
Recent regulatory trends indicate movement toward performance-based standards rather than prescriptive technology requirements. This shift allows water utilities greater flexibility in designing treatment trains that minimize both pathogen risks and DBP formation while addressing site-specific water quality challenges. Several jurisdictions now require water safety plans that specifically address biofilm formation risks associated with AOC increases following ozonation.
Looking forward, regulatory frameworks are likely to incorporate more comprehensive risk assessment methodologies that consider the entire treatment process and distribution system as an integrated whole, rather than regulating individual treatment steps in isolation.
The Safe Drinking Water Act (SDWA) provides the overarching framework within which ozonation processes must operate, requiring water utilities to balance disinfection efficacy against DBP formation. This balancing act is formalized in the Surface Water Treatment Rules, which mandate specific log reductions of pathogens while simultaneously requiring compliance with DBP regulations.
Internationally, regulatory approaches vary considerably. The European Drinking Water Directive (98/83/EC, updated in 2020) incorporates a precautionary principle approach, setting stringent standards for ozonation byproducts. Japan has established comprehensive guidelines specifically addressing Assimilable Organic Carbon (AOC) formation during ozonation, recognizing its role in biofilm development within distribution systems.
Regulatory frameworks increasingly acknowledge the complex trade-offs inherent in ozonation. While traditional regulations focused primarily on acute toxicity concerns, modern frameworks are evolving to address chronic exposure risks and emerging contaminants. The EPA's Contaminant Candidate List (CCL) now includes several ozonation byproducts for potential future regulation.
A significant regulatory challenge lies in addressing the formation of unknown or uncharacterized DBPs during ozonation. Current analytical methods can identify only approximately 50% of the total organic halogen compounds formed during disinfection processes. This has led regulatory bodies to implement bioassay-based toxicity assessment approaches as complementary tools to chemical-specific regulations.
Recent regulatory trends indicate movement toward performance-based standards rather than prescriptive technology requirements. This shift allows water utilities greater flexibility in designing treatment trains that minimize both pathogen risks and DBP formation while addressing site-specific water quality challenges. Several jurisdictions now require water safety plans that specifically address biofilm formation risks associated with AOC increases following ozonation.
Looking forward, regulatory frameworks are likely to incorporate more comprehensive risk assessment methodologies that consider the entire treatment process and distribution system as an integrated whole, rather than regulating individual treatment steps in isolation.
Public Health Implications of Ozonation Byproducts
The public health implications of ozonation byproducts represent a critical area of concern in water treatment systems. While ozonation effectively eliminates many pathogens and contaminants, the formation of disinfection byproducts (DBPs) introduces potential health risks that require comprehensive assessment. These ozonation-derived DBPs include bromate, aldehydes, ketones, and carboxylic acids, each carrying distinct toxicological profiles that may impact human health through various exposure pathways.
Epidemiological studies have linked certain ozonation byproducts to adverse health outcomes, including potential carcinogenic, mutagenic, and reproductive effects. Bromate, a regulated DBP formed during ozonation of bromide-containing waters, has been classified as a possible human carcinogen by the International Agency for Research on Cancer (IARC). Research indicates that chronic exposure to bromate may increase the risk of renal cell tumors and mesotheliomas in laboratory animals, raising concerns about similar effects in humans.
The formation of assimilable organic carbon (AOC) during ozonation presents another public health challenge. AOC serves as a nutrient source for microbial growth in distribution systems, potentially leading to biofilm formation. These biofilms can harbor opportunistic pathogens such as Legionella pneumophila and Pseudomonas aeruginosa, which pose significant risks to immunocompromised individuals, the elderly, and young children.
Risk assessment frameworks for ozonation byproducts must consider both acute and chronic exposure scenarios. Short-term exposure to high concentrations of certain aldehydes may cause respiratory irritation and sensitization, while long-term exposure to low levels of brominated compounds may contribute to cumulative health risks. The challenge lies in balancing the microbial risk reduction achieved through ozonation against the chemical risks introduced by DBPs.
Vulnerable populations require special consideration in evaluating ozonation byproduct risks. Pregnant women, infants, and individuals with compromised immune systems may experience heightened susceptibility to certain DBPs. For instance, some aldehydes formed during ozonation have demonstrated developmental toxicity in animal studies, suggesting potential risks during critical windows of human development.
Regulatory frameworks worldwide have established maximum contaminant levels for certain ozonation byproducts, particularly bromate, typically set at 10 μg/L. However, emerging research suggests that unregulated DBPs may pose equal or greater health concerns, highlighting the need for expanded monitoring programs and revised risk assessment methodologies that account for mixture effects and exposure variability across populations.
Public health surveillance systems play a crucial role in detecting potential associations between ozonation byproducts and community health outcomes. Integration of biomonitoring data with water quality parameters can provide valuable insights into exposure patterns and help identify populations at elevated risk, ultimately informing more targeted risk management strategies and treatment optimization protocols.
Epidemiological studies have linked certain ozonation byproducts to adverse health outcomes, including potential carcinogenic, mutagenic, and reproductive effects. Bromate, a regulated DBP formed during ozonation of bromide-containing waters, has been classified as a possible human carcinogen by the International Agency for Research on Cancer (IARC). Research indicates that chronic exposure to bromate may increase the risk of renal cell tumors and mesotheliomas in laboratory animals, raising concerns about similar effects in humans.
The formation of assimilable organic carbon (AOC) during ozonation presents another public health challenge. AOC serves as a nutrient source for microbial growth in distribution systems, potentially leading to biofilm formation. These biofilms can harbor opportunistic pathogens such as Legionella pneumophila and Pseudomonas aeruginosa, which pose significant risks to immunocompromised individuals, the elderly, and young children.
Risk assessment frameworks for ozonation byproducts must consider both acute and chronic exposure scenarios. Short-term exposure to high concentrations of certain aldehydes may cause respiratory irritation and sensitization, while long-term exposure to low levels of brominated compounds may contribute to cumulative health risks. The challenge lies in balancing the microbial risk reduction achieved through ozonation against the chemical risks introduced by DBPs.
Vulnerable populations require special consideration in evaluating ozonation byproduct risks. Pregnant women, infants, and individuals with compromised immune systems may experience heightened susceptibility to certain DBPs. For instance, some aldehydes formed during ozonation have demonstrated developmental toxicity in animal studies, suggesting potential risks during critical windows of human development.
Regulatory frameworks worldwide have established maximum contaminant levels for certain ozonation byproducts, particularly bromate, typically set at 10 μg/L. However, emerging research suggests that unregulated DBPs may pose equal or greater health concerns, highlighting the need for expanded monitoring programs and revised risk assessment methodologies that account for mixture effects and exposure variability across populations.
Public health surveillance systems play a crucial role in detecting potential associations between ozonation byproducts and community health outcomes. Integration of biomonitoring data with water quality parameters can provide valuable insights into exposure patterns and help identify populations at elevated risk, ultimately informing more targeted risk management strategies and treatment optimization protocols.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!