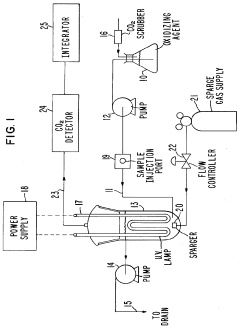
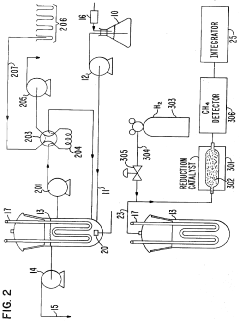
Ozonation: Bromate Formation—Kinetics, TOC/pH Dependence And Mitigation Windows
SEP 18, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ozonation Technology Background and Objectives
Ozonation technology has evolved significantly since its first application in water treatment in the late 19th century. Initially used primarily for disinfection purposes, ozonation has expanded to address multiple water quality challenges including taste and odor control, color removal, micropollutant elimination, and oxidation of inorganic compounds. The technology leverages ozone's powerful oxidizing properties (E° = 2.07V) to break down complex organic molecules and inactivate pathogens through direct and indirect oxidation pathways.
The evolution of ozonation technology has been marked by several key developments, including advanced gas-liquid contacting systems, improved ozone generation efficiency, and enhanced process control mechanisms. Modern ozone generators have achieved significant improvements in energy efficiency, reducing the historically high energy costs associated with ozone production. Additionally, the integration of ozonation with other treatment processes, such as biological filtration in Biological Activated Carbon (BAC) systems, has expanded its application scope.
Despite these advancements, bromate formation during ozonation represents a significant challenge for water treatment facilities. Bromate (BrO3-), a regulated disinfection by-product with carcinogenic properties, forms when ozone oxidizes bromide (Br-) naturally present in source waters. The USEPA and WHO have established strict regulatory limits for bromate at 10 μg/L, necessitating careful process control and mitigation strategies.
The primary objective of this technical research is to comprehensively understand the kinetics governing bromate formation during ozonation processes. Specifically, we aim to elucidate the complex relationships between bromate formation and key water quality parameters, particularly Total Organic Carbon (TOC) content and pH levels. These parameters significantly influence reaction pathways and ultimately determine bromate formation potential.
Furthermore, this research seeks to identify and evaluate practical "mitigation windows" - specific operational conditions where effective disinfection and oxidation can be achieved while minimizing bromate formation. By mapping these operational boundaries, water utilities can optimize treatment processes to balance disinfection requirements with regulatory compliance for bromate.
The technological trajectory suggests increasing integration of real-time monitoring systems, predictive modeling tools, and automated control strategies to dynamically adjust ozonation parameters based on incoming water quality. Advanced oxidation processes (AOPs) combining ozone with hydrogen peroxide, UV radiation, or catalysts represent promising directions for enhancing treatment efficacy while potentially reducing bromate formation under certain conditions.
The evolution of ozonation technology has been marked by several key developments, including advanced gas-liquid contacting systems, improved ozone generation efficiency, and enhanced process control mechanisms. Modern ozone generators have achieved significant improvements in energy efficiency, reducing the historically high energy costs associated with ozone production. Additionally, the integration of ozonation with other treatment processes, such as biological filtration in Biological Activated Carbon (BAC) systems, has expanded its application scope.
Despite these advancements, bromate formation during ozonation represents a significant challenge for water treatment facilities. Bromate (BrO3-), a regulated disinfection by-product with carcinogenic properties, forms when ozone oxidizes bromide (Br-) naturally present in source waters. The USEPA and WHO have established strict regulatory limits for bromate at 10 μg/L, necessitating careful process control and mitigation strategies.
The primary objective of this technical research is to comprehensively understand the kinetics governing bromate formation during ozonation processes. Specifically, we aim to elucidate the complex relationships between bromate formation and key water quality parameters, particularly Total Organic Carbon (TOC) content and pH levels. These parameters significantly influence reaction pathways and ultimately determine bromate formation potential.
Furthermore, this research seeks to identify and evaluate practical "mitigation windows" - specific operational conditions where effective disinfection and oxidation can be achieved while minimizing bromate formation. By mapping these operational boundaries, water utilities can optimize treatment processes to balance disinfection requirements with regulatory compliance for bromate.
The technological trajectory suggests increasing integration of real-time monitoring systems, predictive modeling tools, and automated control strategies to dynamically adjust ozonation parameters based on incoming water quality. Advanced oxidation processes (AOPs) combining ozone with hydrogen peroxide, UV radiation, or catalysts represent promising directions for enhancing treatment efficacy while potentially reducing bromate formation under certain conditions.
Market Demand Analysis for Advanced Water Treatment
The global water treatment market is experiencing significant growth driven by increasing water scarcity, stricter regulations on water quality, and growing awareness of health risks associated with water contaminants. The advanced oxidation segment, particularly ozonation technology, is projected to grow at a CAGR of 7.2% through 2028, reaching a market value of $5.8 billion.
Ozonation as a disinfection and oxidation process has gained substantial traction in municipal water treatment facilities worldwide. However, concerns regarding bromate formation during ozonation of bromide-containing waters have created a specific market demand for solutions that can mitigate this disinfection by-product (DBP) while maintaining effective treatment.
The regulatory landscape is a primary market driver, with the WHO and EPA setting maximum contaminant levels for bromate at 10 μg/L. This regulatory pressure has created an urgent need for technologies and operational strategies that can control bromate formation while leveraging ozone's superior disinfection capabilities. Municipal water utilities serving over 100,000 people represent the largest market segment, with approximately 65% currently using or considering ozone technology implementation.
Industrial applications present another significant market opportunity. Food and beverage manufacturers, pharmaceutical companies, and semiconductor fabrication facilities require ultra-pure water with minimal chemical residuals. These industries value ozonation for its ability to decompose without leaving persistent residuals but require solutions to the bromate formation challenge when source waters contain bromide.
Geographically, North America and Europe lead market demand due to stringent regulatory frameworks and aging water infrastructure requiring upgrades. However, the Asia-Pacific region shows the fastest growth rate at 9.1% annually, driven by rapid industrialization, urbanization, and increasing government investments in water treatment infrastructure.
Consumer awareness regarding water quality has also intensified market demand. Recent high-profile cases of water contamination have heightened public concern about DBPs and other contaminants, creating pressure on utilities to adopt advanced treatment technologies that address these concerns while maintaining cost-effectiveness.
The economic value proposition for bromate mitigation technologies is compelling. Water utilities face potential regulatory penalties, public relations challenges, and health-related liabilities if they fail to control bromate levels. Solutions that can optimize the TOC/pH relationship during ozonation to minimize bromate formation while maximizing disinfection efficiency offer significant economic benefits through operational cost savings and risk reduction.
Ozonation as a disinfection and oxidation process has gained substantial traction in municipal water treatment facilities worldwide. However, concerns regarding bromate formation during ozonation of bromide-containing waters have created a specific market demand for solutions that can mitigate this disinfection by-product (DBP) while maintaining effective treatment.
The regulatory landscape is a primary market driver, with the WHO and EPA setting maximum contaminant levels for bromate at 10 μg/L. This regulatory pressure has created an urgent need for technologies and operational strategies that can control bromate formation while leveraging ozone's superior disinfection capabilities. Municipal water utilities serving over 100,000 people represent the largest market segment, with approximately 65% currently using or considering ozone technology implementation.
Industrial applications present another significant market opportunity. Food and beverage manufacturers, pharmaceutical companies, and semiconductor fabrication facilities require ultra-pure water with minimal chemical residuals. These industries value ozonation for its ability to decompose without leaving persistent residuals but require solutions to the bromate formation challenge when source waters contain bromide.
Geographically, North America and Europe lead market demand due to stringent regulatory frameworks and aging water infrastructure requiring upgrades. However, the Asia-Pacific region shows the fastest growth rate at 9.1% annually, driven by rapid industrialization, urbanization, and increasing government investments in water treatment infrastructure.
Consumer awareness regarding water quality has also intensified market demand. Recent high-profile cases of water contamination have heightened public concern about DBPs and other contaminants, creating pressure on utilities to adopt advanced treatment technologies that address these concerns while maintaining cost-effectiveness.
The economic value proposition for bromate mitigation technologies is compelling. Water utilities face potential regulatory penalties, public relations challenges, and health-related liabilities if they fail to control bromate levels. Solutions that can optimize the TOC/pH relationship during ozonation to minimize bromate formation while maximizing disinfection efficiency offer significant economic benefits through operational cost savings and risk reduction.
Bromate Formation Challenges in Ozonation Processes
Bromate formation during ozonation processes represents one of the most significant challenges in water treatment technologies. When ozone is applied to water containing bromide ions, bromate (BrO3-) can form as a disinfection by-product (DBP). This poses a serious concern as bromate is classified as a potential human carcinogen by the International Agency for Research on Cancer, with regulatory limits set at 10 μg/L in many countries including the United States and European Union.
The formation of bromate follows complex reaction pathways involving multiple intermediates. The primary mechanism involves the oxidation of bromide (Br-) to hypobromite (BrO-), followed by further oxidation to bromate. This process is influenced by several key factors, with reaction kinetics playing a crucial role. Studies have shown that bromate formation exhibits pseudo-first-order kinetics with respect to bromide concentration, while the relationship with ozone exposure follows a non-linear pattern.
The dependence of bromate formation on Total Organic Carbon (TOC) levels presents a paradoxical challenge. Higher TOC concentrations can reduce bromate formation by consuming ozone and hydroxyl radicals that would otherwise oxidize bromide. However, this beneficial effect must be balanced against the primary purpose of ozonation—effective disinfection and oxidation of organic contaminants. This creates a technical dilemma where conditions optimal for disinfection may simultaneously increase bromate formation risk.
pH dependency represents another critical factor, with bromate formation rates increasing significantly at higher pH values. This occurs because at elevated pH, the hypobromite ion (BrO-) becomes more stable, facilitating its further oxidation to bromate. Research indicates that bromate formation can increase by factors of 2-3 for each pH unit increase above neutral conditions, creating a narrow operational window for water utilities.
Water temperature also influences bromate formation kinetics, with higher temperatures accelerating the reaction rates. This seasonal variability complicates treatment strategies, particularly in regions experiencing significant temperature fluctuations throughout the year. Additionally, the presence of natural organic matter (NOM) affects bromate formation through complex interactions with the ozonation process.
The ammonia concentration in source waters presents another variable, as ammonia can react with hypobromous acid to form bromamines, effectively reducing bromate formation. This relationship has led to the development of ammonia addition as a mitigation strategy, though its effectiveness varies with water quality parameters and treatment conditions.
These multifaceted challenges necessitate sophisticated approaches to water treatment design and operation, balancing disinfection efficacy against bromate formation risk while considering the economic implications of additional treatment requirements.
The formation of bromate follows complex reaction pathways involving multiple intermediates. The primary mechanism involves the oxidation of bromide (Br-) to hypobromite (BrO-), followed by further oxidation to bromate. This process is influenced by several key factors, with reaction kinetics playing a crucial role. Studies have shown that bromate formation exhibits pseudo-first-order kinetics with respect to bromide concentration, while the relationship with ozone exposure follows a non-linear pattern.
The dependence of bromate formation on Total Organic Carbon (TOC) levels presents a paradoxical challenge. Higher TOC concentrations can reduce bromate formation by consuming ozone and hydroxyl radicals that would otherwise oxidize bromide. However, this beneficial effect must be balanced against the primary purpose of ozonation—effective disinfection and oxidation of organic contaminants. This creates a technical dilemma where conditions optimal for disinfection may simultaneously increase bromate formation risk.
pH dependency represents another critical factor, with bromate formation rates increasing significantly at higher pH values. This occurs because at elevated pH, the hypobromite ion (BrO-) becomes more stable, facilitating its further oxidation to bromate. Research indicates that bromate formation can increase by factors of 2-3 for each pH unit increase above neutral conditions, creating a narrow operational window for water utilities.
Water temperature also influences bromate formation kinetics, with higher temperatures accelerating the reaction rates. This seasonal variability complicates treatment strategies, particularly in regions experiencing significant temperature fluctuations throughout the year. Additionally, the presence of natural organic matter (NOM) affects bromate formation through complex interactions with the ozonation process.
The ammonia concentration in source waters presents another variable, as ammonia can react with hypobromous acid to form bromamines, effectively reducing bromate formation. This relationship has led to the development of ammonia addition as a mitigation strategy, though its effectiveness varies with water quality parameters and treatment conditions.
These multifaceted challenges necessitate sophisticated approaches to water treatment design and operation, balancing disinfection efficacy against bromate formation risk while considering the economic implications of additional treatment requirements.
Current Mitigation Strategies for Bromate Formation
01 Mechanisms of bromate formation during ozonation
During water treatment with ozone, bromate formation occurs through a complex oxidation pathway where bromide ions present in water are oxidized to bromate. This process typically involves multiple steps including the formation of hypobromite and bromite intermediates. The reaction rate and extent of bromate formation are influenced by factors such as pH, temperature, and the presence of natural organic matter. Understanding these mechanisms is crucial for developing effective control strategies in drinking water treatment systems.- Mechanisms of bromate formation during ozonation: During water treatment with ozone, bromate can form when bromide ions present in the water are oxidized. This process typically occurs through multiple pathways, including direct oxidation by ozone and indirect oxidation by hydroxyl radicals. The formation of bromate is influenced by factors such as pH, temperature, and the concentration of bromide ions in the source water. Understanding these mechanisms is crucial for developing effective strategies to minimize bromate formation while maintaining disinfection efficacy.
- pH control methods to reduce bromate formation: Controlling the pH of water during ozonation is an effective strategy to minimize bromate formation. Lower pH conditions (typically below 6.5) can significantly reduce bromate formation by affecting the speciation of bromide and hypobromite, which are intermediates in the bromate formation pathway. Various pH adjustment techniques and buffer systems can be employed before or during the ozonation process to maintain optimal pH levels that balance disinfection requirements with bromate formation control.
- Addition of inhibiting agents to reduce bromate formation: Various chemical additives can be introduced during the ozonation process to inhibit bromate formation. These include ammonia, hydrogen peroxide, and certain organic compounds that can scavenge hydroxyl radicals or compete with bromide for oxidation. Some additives work by converting hypobromite back to bromide, effectively interrupting the bromate formation pathway. The selection and dosing of these inhibiting agents depend on water quality parameters and treatment objectives.
- Advanced ozonation system designs to minimize bromate: Innovative ozonation system designs can help minimize bromate formation while maintaining effective disinfection. These include two-stage ozonation processes with intermediate pH adjustment, pulsed ozone dosing systems, and hybrid systems that combine ozone with other treatment technologies such as UV or advanced oxidation processes. Some designs incorporate real-time monitoring and control systems to optimize ozone dosage based on water quality parameters, thereby minimizing bromate formation potential.
- Post-treatment methods for bromate removal: After ozonation, various post-treatment methods can be employed to remove bromate from treated water. These include biological filtration using specific bacterial strains that can reduce bromate to bromide, adsorption processes using activated carbon or specialized ion exchange resins, and chemical reduction using reducing agents. Some advanced treatment trains incorporate membrane filtration or electrochemical reduction techniques specifically designed to target bromate removal while preserving other water quality parameters.
02 pH control methods to reduce bromate formation
Controlling the pH during ozonation processes can significantly reduce bromate formation. Lower pH conditions inhibit the conversion of bromide to bromate by suppressing the formation of hydroxyl radicals and altering the ozonation pathway. Various techniques for pH adjustment include the addition of acids prior to ozonation and the implementation of two-stage treatment processes where pH is carefully managed at each stage. These methods can achieve substantial reductions in bromate formation while maintaining effective disinfection capabilities.Expand Specific Solutions03 Catalytic processes for bromate control
Advanced catalytic processes can be employed to control bromate formation during ozonation. These include the use of metal catalysts, activated carbon, and other materials that can selectively promote desired oxidation pathways while suppressing bromate formation. Some catalysts work by decomposing ozone to less reactive species, while others may facilitate the reduction of bromate precursors. These catalytic approaches offer promising solutions for water treatment facilities seeking to minimize bromate formation while maintaining effective disinfection.Expand Specific Solutions04 Ammonia addition techniques
The addition of ammonia or ammonia-based compounds during ozonation has been demonstrated to effectively reduce bromate formation. Ammonia reacts with hypobromous acid, a key intermediate in the bromate formation pathway, to form bromamines which are less likely to be oxidized to bromate. Various methods for ammonia dosing, including pre-treatment addition and controlled release systems, have been developed to optimize this approach. This technique can be particularly effective when combined with other control strategies such as pH adjustment.Expand Specific Solutions05 Monitoring and analytical methods for bromate detection
Advanced monitoring and analytical techniques are essential for detecting and quantifying bromate formation during ozonation processes. These include chromatographic methods, spectroscopic techniques, and electrochemical sensors that can provide real-time or near-real-time measurements of bromate concentrations. Improved analytical capabilities enable water treatment operators to optimize treatment conditions, validate control strategies, and ensure compliance with increasingly stringent regulatory limits for bromate in drinking water. These methods vary in sensitivity, specificity, and practical applicability in treatment plant settings.Expand Specific Solutions
Key Industry Players in Ozonation Treatment Solutions
The ozonation bromate formation technology landscape is currently in a growth phase, with increasing market size driven by water treatment applications. The technology maturity varies across different aspects, with kinetics and pH dependence mechanisms well-established but mitigation strategies still evolving. Academic institutions like Harbin Institute of Technology, Zhejiang University, and University of Liverpool lead fundamental research, while companies such as DuPont, Air Products & Chemicals, and METAWATER are commercializing practical applications. Water treatment specialists including Xylem IP Holdings and Saline Water Conversion Corp. are implementing these technologies in real-world systems. The competitive landscape shows collaboration between academic research and industrial implementation, with increasing focus on TOC-dependent mitigation strategies to address regulatory concerns about bromate as a disinfection byproduct.
Harbin Institute of Technology
Technical Solution: Harbin Institute of Technology has conducted groundbreaking research on the fundamental kinetics of bromate formation during ozonation, developing mathematical models that accurately predict formation rates based on multiple water quality parameters. Their approach incorporates advanced spectroscopic techniques to identify and quantify reaction intermediates in the bromate formation pathway. HIT researchers have established critical pH thresholds (6.8 being particularly significant) where bromate formation kinetics undergo substantial changes, providing a scientific basis for operational mitigation windows. Their work has demonstrated that specific TOC compositions, particularly those rich in aromatic structures, can significantly influence bromate formation through competitive ozone demand mechanisms. The institute has developed a comprehensive reaction pathway model that accounts for the complex interactions between ozone, bromide, natural organic matter, and various inorganic constituents, enabling precise prediction of bromate formation potential under varying treatment conditions.
Strengths: Cutting-edge fundamental research providing theoretical foundations for practical applications; sophisticated analytical capabilities for reaction mechanism elucidation. Weaknesses: Research findings require translation into practical engineering solutions; limited commercial-scale implementation experience compared to industry players.
METAWATER Co., Ltd.
Technical Solution: METAWATER has pioneered a comprehensive ozonation technology specifically addressing bromate formation challenges in drinking water treatment. Their system employs a multi-parameter approach that considers the complex kinetics of bromate formation in relation to varying TOC levels and pH conditions. The technology features proprietary catalysts that selectively inhibit bromate formation pathways while maintaining ozone's oxidation efficiency. METAWATER's solution incorporates ammonia addition strategies at precisely calculated dosages (typically 0.1-0.5 mg/L) to create chloramine species that interfere with the bromate formation mechanism. Their advanced control systems continuously monitor bromide concentrations and adjust treatment parameters to maintain operation within identified mitigation windows, achieving bromate levels consistently below 5 μg/L even in challenging high-bromide source waters.
Strengths: Highly specialized in drinking water applications with proven performance in high-bromide waters; excellent regulatory compliance track record across multiple jurisdictions. Weaknesses: System optimization requires extensive initial water quality characterization; ammonia addition strategy may require additional downstream treatment considerations.
Critical Research on TOC/pH Influence Mechanisms
Process for treating bromide-containing water using ozone
PatentWO1994026671A1
Innovation
- Adjusting the pH of bromide-containing water to less than 6.5 and simultaneously introducing CO2 and ozone for several minutes to minimize bromate formation, using known substances like ozone and carbon dioxide without additional chemicals, and employing filtration with activated carbon or aluminum oxide to remove any formed bromate.
Determination of total organic carbon in a plurality of aqueous samples containing halide ion
PatentInactiveUS4288229A
Innovation
- The use of a continuous flow of a precipitate-free aqueous solution containing persulfate ion and mercuric monohalide ion, combined with ultraviolet radiation, ensures complete oxidation of organic matter without halide ion interference, allowing for precise and rapid TOC measurement.
Regulatory Framework for Disinfection Byproducts
The regulatory landscape for disinfection byproducts (DBPs) has evolved significantly in response to growing scientific evidence of their potential health impacts. The United States Environmental Protection Agency (EPA) established the Stage 1 Disinfectants and Disinfection Byproducts Rule in 1998, which specifically addressed bromate formation during ozonation by setting a maximum contaminant level (MCL) of 10 μg/L. This threshold represents a risk-based approach balancing microbial pathogen control with chemical disinfection risks.
The European Union, through its Drinking Water Directive (98/83/EC), adopted similar standards for bromate, also establishing a 10 μg/L limit. However, the EU's revised Drinking Water Directive (2020) maintains this limit while emphasizing a more comprehensive risk assessment approach to water safety management.
The World Health Organization's Guidelines for Drinking-water Quality recommend a provisional guideline value of 10 μg/L for bromate, acknowledging the carcinogenic potential observed in laboratory studies while recognizing practical limitations in treatment technology.
Regulatory frameworks increasingly incorporate the concept of ALARA (As Low As Reasonably Achievable) for bromate formation, recognizing that complete elimination may not be feasible while maintaining effective disinfection. This principle has driven innovation in treatment processes that minimize bromate formation while ensuring pathogen inactivation.
Japan and Australia have adopted more stringent standards for bromate (5 μg/L and 7 μg/L respectively), reflecting regional differences in risk assessment approaches and technological capabilities. These variations highlight the ongoing scientific debate regarding appropriate risk thresholds.
Compliance monitoring requirements vary globally but typically mandate quarterly or monthly sampling at distribution system entry points. Advanced analytical methods, including ion chromatography with conductivity detection and inductively coupled plasma mass spectrometry (ICP-MS), have become standard for regulatory compliance, with detection limits well below regulatory thresholds.
Recent regulatory trends indicate movement toward more holistic approaches that consider cumulative risks from multiple DBPs rather than focusing on individual compounds in isolation. This shift acknowledges the complex chemistry of disinfection processes and the potential for unintended consequences when optimizing for a single parameter.
The regulatory framework continues to evolve as scientific understanding of bromate formation kinetics, pH dependence, and TOC interactions improves, creating a dynamic environment where water utilities must balance disinfection efficacy against increasingly stringent DBP regulations.
The European Union, through its Drinking Water Directive (98/83/EC), adopted similar standards for bromate, also establishing a 10 μg/L limit. However, the EU's revised Drinking Water Directive (2020) maintains this limit while emphasizing a more comprehensive risk assessment approach to water safety management.
The World Health Organization's Guidelines for Drinking-water Quality recommend a provisional guideline value of 10 μg/L for bromate, acknowledging the carcinogenic potential observed in laboratory studies while recognizing practical limitations in treatment technology.
Regulatory frameworks increasingly incorporate the concept of ALARA (As Low As Reasonably Achievable) for bromate formation, recognizing that complete elimination may not be feasible while maintaining effective disinfection. This principle has driven innovation in treatment processes that minimize bromate formation while ensuring pathogen inactivation.
Japan and Australia have adopted more stringent standards for bromate (5 μg/L and 7 μg/L respectively), reflecting regional differences in risk assessment approaches and technological capabilities. These variations highlight the ongoing scientific debate regarding appropriate risk thresholds.
Compliance monitoring requirements vary globally but typically mandate quarterly or monthly sampling at distribution system entry points. Advanced analytical methods, including ion chromatography with conductivity detection and inductively coupled plasma mass spectrometry (ICP-MS), have become standard for regulatory compliance, with detection limits well below regulatory thresholds.
Recent regulatory trends indicate movement toward more holistic approaches that consider cumulative risks from multiple DBPs rather than focusing on individual compounds in isolation. This shift acknowledges the complex chemistry of disinfection processes and the potential for unintended consequences when optimizing for a single parameter.
The regulatory framework continues to evolve as scientific understanding of bromate formation kinetics, pH dependence, and TOC interactions improves, creating a dynamic environment where water utilities must balance disinfection efficacy against increasingly stringent DBP regulations.
Environmental Impact Assessment of Ozonation Technologies
Ozonation technologies, while effective for water treatment, present significant environmental considerations that must be thoroughly assessed. The formation of bromate during ozonation represents a critical environmental concern, as this disinfection by-product is classified as a potential human carcinogen by the World Health Organization, with regulatory limits typically set at 10 μg/L in drinking water systems globally.
The environmental impact of bromate formation is heavily influenced by source water characteristics, particularly bromide concentration, pH levels, and total organic carbon (TOC) content. Studies indicate that waters with higher bromide concentrations (>100 μg/L) are at elevated risk for exceeding regulatory bromate thresholds during conventional ozonation processes, creating potential environmental compliance challenges.
Kinetic analyses reveal that bromate formation follows complex pathways involving multiple intermediates, with formation rates accelerating at higher pH values (>7.5) and temperatures. This pH dependence creates a significant environmental trade-off, as higher pH values that might benefit certain treatment objectives simultaneously increase bromate formation risk, potentially introducing regulated contaminants into treated water systems.
TOC concentration demonstrates an inverse relationship with bromate formation, as organic matter can scavenge hydroxyl radicals and reduce ozone exposure. This relationship provides an important environmental mitigation pathway, though it must be balanced against disinfection efficacy requirements and the formation of other organic disinfection by-products.
Environmental impact assessments must consider the energy requirements of various ozonation technologies, as conventional systems typically consume 0.8-1.2 kWh per cubic meter of treated water. Advanced oxidation processes that incorporate hydrogen peroxide or UV radiation to mitigate bromate formation often increase this energy footprint by 15-30%, contributing to indirect environmental impacts through increased carbon emissions.
The environmental persistence of bromate presents additional concerns, as it demonstrates stability in aquatic environments with limited natural degradation pathways. Discharge of bromate-containing waters into natural systems may result in accumulation, with potential impacts on aquatic ecosystems that remain incompletely characterized in current ecological risk assessments.
Mitigation technologies such as pH depression, ammonia addition, and chlorine-ammonia processes offer environmental benefits through bromate reduction but may introduce secondary environmental considerations including increased chemical usage, handling risks, and potential formation of alternative disinfection by-products that require comprehensive life-cycle assessment approaches.
The environmental impact of bromate formation is heavily influenced by source water characteristics, particularly bromide concentration, pH levels, and total organic carbon (TOC) content. Studies indicate that waters with higher bromide concentrations (>100 μg/L) are at elevated risk for exceeding regulatory bromate thresholds during conventional ozonation processes, creating potential environmental compliance challenges.
Kinetic analyses reveal that bromate formation follows complex pathways involving multiple intermediates, with formation rates accelerating at higher pH values (>7.5) and temperatures. This pH dependence creates a significant environmental trade-off, as higher pH values that might benefit certain treatment objectives simultaneously increase bromate formation risk, potentially introducing regulated contaminants into treated water systems.
TOC concentration demonstrates an inverse relationship with bromate formation, as organic matter can scavenge hydroxyl radicals and reduce ozone exposure. This relationship provides an important environmental mitigation pathway, though it must be balanced against disinfection efficacy requirements and the formation of other organic disinfection by-products.
Environmental impact assessments must consider the energy requirements of various ozonation technologies, as conventional systems typically consume 0.8-1.2 kWh per cubic meter of treated water. Advanced oxidation processes that incorporate hydrogen peroxide or UV radiation to mitigate bromate formation often increase this energy footprint by 15-30%, contributing to indirect environmental impacts through increased carbon emissions.
The environmental persistence of bromate presents additional concerns, as it demonstrates stability in aquatic environments with limited natural degradation pathways. Discharge of bromate-containing waters into natural systems may result in accumulation, with potential impacts on aquatic ecosystems that remain incompletely characterized in current ecological risk assessments.
Mitigation technologies such as pH depression, ammonia addition, and chlorine-ammonia processes offer environmental benefits through bromate reduction but may introduce secondary environmental considerations including increased chemical usage, handling risks, and potential formation of alternative disinfection by-products that require comprehensive life-cycle assessment approaches.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!