Ozonation: Microplastics/Antibiotic Residues—Degradation Pathways And QA
SEP 18, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ozonation Technology Background and Objectives
Ozonation technology has evolved significantly over the past century since its initial discovery in the late 19th century. Originally developed for drinking water disinfection, ozone treatment has expanded into various environmental applications due to its powerful oxidizing properties. The technology has progressed from basic bubble diffusion systems to advanced catalytic ozonation processes, with significant improvements in energy efficiency and treatment effectiveness.
The evolution of ozonation technology has been marked by several key milestones, including the development of efficient ozone generators, advanced contacting systems, and hybrid processes combining ozone with other treatment technologies. Recent advancements have focused on optimizing ozone dosage, improving mass transfer efficiency, and developing catalysts to enhance oxidation reactions.
In the context of emerging contaminants, ozonation has gained renewed attention as a potential solution for degrading persistent pollutants that conventional treatment methods fail to address. Microplastics and antibiotic residues represent two such critical challenges facing water treatment systems globally, with significant implications for ecosystem and human health.
Microplastics, defined as plastic particles smaller than 5mm, have become ubiquitous environmental contaminants, found in oceans, freshwater systems, and even drinking water. Their persistence and potential to adsorb other pollutants make them particularly concerning. Similarly, antibiotic residues in water bodies contribute to the development of antimicrobial resistance, recognized by the World Health Organization as one of the top global public health threats.
The primary objective of this research is to comprehensively evaluate ozonation as a treatment technology for the degradation of both microplastics and antibiotic residues in water systems. Specifically, the research aims to elucidate the degradation pathways through which ozone attacks these contaminants, identify intermediate products formed during treatment, and assess the efficiency of complete mineralization.
Additionally, this research seeks to establish robust quality assurance protocols for ozonation processes targeting these contaminants, including optimal operational parameters, monitoring techniques, and performance indicators. The goal is to develop standardized methodologies that can be implemented across different water treatment facilities to ensure consistent and reliable removal of these emerging contaminants.
The research also aims to investigate potential synergistic effects when combining ozonation with other treatment technologies, such as advanced oxidation processes, biological treatment, or membrane filtration, to achieve more complete degradation of these persistent contaminants. Through this comprehensive approach, the study intends to contribute to the development of more effective and sustainable water treatment solutions for addressing the growing challenge of microplastics and antibiotic residues in aquatic environments.
The evolution of ozonation technology has been marked by several key milestones, including the development of efficient ozone generators, advanced contacting systems, and hybrid processes combining ozone with other treatment technologies. Recent advancements have focused on optimizing ozone dosage, improving mass transfer efficiency, and developing catalysts to enhance oxidation reactions.
In the context of emerging contaminants, ozonation has gained renewed attention as a potential solution for degrading persistent pollutants that conventional treatment methods fail to address. Microplastics and antibiotic residues represent two such critical challenges facing water treatment systems globally, with significant implications for ecosystem and human health.
Microplastics, defined as plastic particles smaller than 5mm, have become ubiquitous environmental contaminants, found in oceans, freshwater systems, and even drinking water. Their persistence and potential to adsorb other pollutants make them particularly concerning. Similarly, antibiotic residues in water bodies contribute to the development of antimicrobial resistance, recognized by the World Health Organization as one of the top global public health threats.
The primary objective of this research is to comprehensively evaluate ozonation as a treatment technology for the degradation of both microplastics and antibiotic residues in water systems. Specifically, the research aims to elucidate the degradation pathways through which ozone attacks these contaminants, identify intermediate products formed during treatment, and assess the efficiency of complete mineralization.
Additionally, this research seeks to establish robust quality assurance protocols for ozonation processes targeting these contaminants, including optimal operational parameters, monitoring techniques, and performance indicators. The goal is to develop standardized methodologies that can be implemented across different water treatment facilities to ensure consistent and reliable removal of these emerging contaminants.
The research also aims to investigate potential synergistic effects when combining ozonation with other treatment technologies, such as advanced oxidation processes, biological treatment, or membrane filtration, to achieve more complete degradation of these persistent contaminants. Through this comprehensive approach, the study intends to contribute to the development of more effective and sustainable water treatment solutions for addressing the growing challenge of microplastics and antibiotic residues in aquatic environments.
Market Demand for Micropollutant Treatment Solutions
The global market for micropollutant treatment solutions has witnessed significant growth in recent years, driven primarily by increasing awareness of environmental contamination and its potential health impacts. The presence of microplastics and antibiotic residues in water bodies represents a critical environmental challenge that demands innovative treatment approaches. Current market valuations indicate that the water treatment chemicals market reached approximately $35 billion in 2022, with advanced oxidation processes like ozonation capturing an expanding segment.
Regulatory frameworks worldwide are increasingly focusing on micropollutants, creating substantial market opportunities. The European Union's Water Framework Directive and the United States EPA's Contaminant Candidate List have established stringent guidelines for water quality, directly influencing market demand for advanced treatment technologies. These regulatory pressures are expected to intensify, with several countries implementing zero-discharge policies for pharmaceutical compounds and microplastics.
Municipal water treatment facilities constitute the largest market segment, accounting for nearly 45% of the total demand for micropollutant treatment solutions. These facilities are increasingly upgrading their infrastructure to address emerging contaminants that conventional treatment methods cannot effectively remove. The pharmaceutical industry represents another significant market segment, driven by requirements to treat manufacturing effluents containing antibiotic residues before discharge.
Consumer awareness regarding water quality has risen dramatically, with surveys indicating that 78% of consumers in developed nations express concern about micropollutants in drinking water. This heightened awareness translates directly into market demand for both industrial-scale solutions and point-of-use treatment systems for residential applications.
The Asia-Pacific region presents the fastest-growing market for micropollutant treatment technologies, with annual growth rates exceeding 9%. This growth is attributed to rapid industrialization, increasing urbanization, and strengthening environmental regulations across countries like China, India, and South Korea. North America and Europe remain mature markets with steady growth, primarily driven by infrastructure upgrades and regulatory compliance requirements.
Economic analyses suggest that the cost-benefit ratio for implementing advanced oxidation processes like ozonation is becoming increasingly favorable. While initial capital investments remain significant, operational costs have decreased by approximately 30% over the past decade due to technological improvements and energy efficiency gains. Market forecasts project that the specific segment for ozonation technologies targeting microplastics and pharmaceutical residues will grow at a compound annual rate of 12% through 2028.
The market landscape is further shaped by increasing research funding from both public and private sectors, with annual investments in micropollutant treatment research exceeding $2 billion globally. This substantial financial commitment underscores the recognized importance and market potential of developing effective solutions for these persistent environmental contaminants.
Regulatory frameworks worldwide are increasingly focusing on micropollutants, creating substantial market opportunities. The European Union's Water Framework Directive and the United States EPA's Contaminant Candidate List have established stringent guidelines for water quality, directly influencing market demand for advanced treatment technologies. These regulatory pressures are expected to intensify, with several countries implementing zero-discharge policies for pharmaceutical compounds and microplastics.
Municipal water treatment facilities constitute the largest market segment, accounting for nearly 45% of the total demand for micropollutant treatment solutions. These facilities are increasingly upgrading their infrastructure to address emerging contaminants that conventional treatment methods cannot effectively remove. The pharmaceutical industry represents another significant market segment, driven by requirements to treat manufacturing effluents containing antibiotic residues before discharge.
Consumer awareness regarding water quality has risen dramatically, with surveys indicating that 78% of consumers in developed nations express concern about micropollutants in drinking water. This heightened awareness translates directly into market demand for both industrial-scale solutions and point-of-use treatment systems for residential applications.
The Asia-Pacific region presents the fastest-growing market for micropollutant treatment technologies, with annual growth rates exceeding 9%. This growth is attributed to rapid industrialization, increasing urbanization, and strengthening environmental regulations across countries like China, India, and South Korea. North America and Europe remain mature markets with steady growth, primarily driven by infrastructure upgrades and regulatory compliance requirements.
Economic analyses suggest that the cost-benefit ratio for implementing advanced oxidation processes like ozonation is becoming increasingly favorable. While initial capital investments remain significant, operational costs have decreased by approximately 30% over the past decade due to technological improvements and energy efficiency gains. Market forecasts project that the specific segment for ozonation technologies targeting microplastics and pharmaceutical residues will grow at a compound annual rate of 12% through 2028.
The market landscape is further shaped by increasing research funding from both public and private sectors, with annual investments in micropollutant treatment research exceeding $2 billion globally. This substantial financial commitment underscores the recognized importance and market potential of developing effective solutions for these persistent environmental contaminants.
Current Challenges in Microplastic and Antibiotic Degradation
The global presence of microplastics and antibiotic residues in aquatic environments represents one of the most pressing environmental challenges of our time. Despite increasing research efforts, the degradation of these pollutants remains problematic due to their persistent nature and complex molecular structures. Conventional wastewater treatment processes have demonstrated limited efficacy in removing these contaminants, with removal rates typically below 50% for many antibiotics and even lower for microplastics.
Microplastics present unique challenges due to their heterogeneous composition, varying sizes (from nanometers to millimeters), and diverse polymer types. Current degradation methods often result in incomplete breakdown, potentially generating smaller particles that may pose even greater environmental risks. The hydrophobic surface of many microplastics also attracts and concentrates other pollutants, creating complex contaminant mixtures that are difficult to treat effectively.
Antibiotic residues pose different but equally significant challenges. Their molecular stability, designed to ensure therapeutic efficacy, makes them resistant to conventional biodegradation processes. Many antibiotics contain complex ring structures and functional groups that are recalcitrant to oxidation under normal environmental conditions. Additionally, the concentration of these compounds in water bodies can vary seasonally and geographically, complicating treatment strategies.
Ozonation has emerged as a promising advanced oxidation process for degrading both microplastics and antibiotics. However, several technical barriers limit its widespread implementation. The energy intensity of ozone generation remains a significant economic constraint, with current systems requiring 10-15 kWh per kg of ozone produced. Ozone's short half-life (typically minutes in water) necessitates on-site generation and immediate application, adding complexity to treatment systems.
The formation of potentially harmful by-products during ozonation represents another critical challenge. Partial oxidation of antibiotics can create transformation products that may retain antimicrobial activity or exhibit new toxicological properties. Similarly, ozonation of microplastics may release additives such as plasticizers or flame retardants into the water phase.
Quality assurance in ozonation processes faces significant hurdles due to the lack of standardized protocols for monitoring degradation efficiency. Current analytical methods struggle to detect and quantify the diverse range of transformation products generated during treatment. The absence of regulatory frameworks specifically addressing these emerging contaminants further complicates the establishment of treatment targets and performance metrics.
Scaling ozonation technologies from laboratory to industrial applications introduces additional challenges related to reactor design, contact time optimization, and integration with existing water treatment infrastructure. The variability in water matrix composition across different sources significantly impacts ozone demand and treatment efficacy, necessitating site-specific optimization.
Microplastics present unique challenges due to their heterogeneous composition, varying sizes (from nanometers to millimeters), and diverse polymer types. Current degradation methods often result in incomplete breakdown, potentially generating smaller particles that may pose even greater environmental risks. The hydrophobic surface of many microplastics also attracts and concentrates other pollutants, creating complex contaminant mixtures that are difficult to treat effectively.
Antibiotic residues pose different but equally significant challenges. Their molecular stability, designed to ensure therapeutic efficacy, makes them resistant to conventional biodegradation processes. Many antibiotics contain complex ring structures and functional groups that are recalcitrant to oxidation under normal environmental conditions. Additionally, the concentration of these compounds in water bodies can vary seasonally and geographically, complicating treatment strategies.
Ozonation has emerged as a promising advanced oxidation process for degrading both microplastics and antibiotics. However, several technical barriers limit its widespread implementation. The energy intensity of ozone generation remains a significant economic constraint, with current systems requiring 10-15 kWh per kg of ozone produced. Ozone's short half-life (typically minutes in water) necessitates on-site generation and immediate application, adding complexity to treatment systems.
The formation of potentially harmful by-products during ozonation represents another critical challenge. Partial oxidation of antibiotics can create transformation products that may retain antimicrobial activity or exhibit new toxicological properties. Similarly, ozonation of microplastics may release additives such as plasticizers or flame retardants into the water phase.
Quality assurance in ozonation processes faces significant hurdles due to the lack of standardized protocols for monitoring degradation efficiency. Current analytical methods struggle to detect and quantify the diverse range of transformation products generated during treatment. The absence of regulatory frameworks specifically addressing these emerging contaminants further complicates the establishment of treatment targets and performance metrics.
Scaling ozonation technologies from laboratory to industrial applications introduces additional challenges related to reactor design, contact time optimization, and integration with existing water treatment infrastructure. The variability in water matrix composition across different sources significantly impacts ozone demand and treatment efficacy, necessitating site-specific optimization.
Established Ozonation Methodologies and Protocols
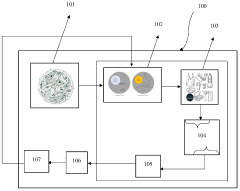
01 Ozonation for water treatment and pollutant degradation
Ozonation processes are widely used for water treatment to degrade various pollutants through oxidation pathways. These systems typically involve the generation of ozone and its application to contaminated water, where it breaks down organic compounds, pharmaceuticals, and other contaminants into less harmful substances. The degradation pathways often include direct ozone reactions and indirect hydroxyl radical-mediated oxidation, which can be optimized for different types of pollutants.- Ozonation for water treatment and pollutant degradation: Ozonation processes are widely used for water treatment to degrade various pollutants. The degradation pathway typically involves the direct reaction of ozone with organic compounds or through the formation of hydroxyl radicals that subsequently attack contaminants. This approach is effective for removing pharmaceuticals, pesticides, and industrial chemicals from wastewater, with the degradation pathways often resulting in the formation of less harmful byproducts that can be further treated or naturally degraded.
- Advanced oxidation processes combining ozone with catalysts: Advanced oxidation processes that combine ozone with various catalysts enhance the degradation efficiency of contaminants. These catalytic systems promote the formation of reactive oxygen species through different degradation pathways, accelerating the breakdown of recalcitrant compounds. The catalysts can include metal oxides, supported metals, or composite materials that facilitate electron transfer and radical formation, leading to more complete mineralization of pollutants compared to ozone alone.
- Ozonation degradation mechanisms for specific organic compounds: Different organic compounds undergo specific degradation pathways when subjected to ozonation. The degradation mechanisms can involve direct ozone attack on electron-rich moieties, such as unsaturated bonds or aromatic rings, or indirect oxidation through hydroxyl radicals. These pathways often lead to the formation of aldehydes, ketones, carboxylic acids, and other oxidized intermediates before complete mineralization to carbon dioxide and water, with the specific pathway depending on the molecular structure of the target compound.
- Ozonation systems and equipment design for optimized degradation: Specialized ozonation systems and equipment designs can optimize degradation pathways by controlling reaction conditions. These systems may incorporate features such as controlled ozone dosing, optimized contact time, pH adjustment mechanisms, and mixing technologies to enhance mass transfer. Some designs also integrate monitoring capabilities to track degradation progress and adjust parameters in real-time, ensuring efficient pollutant breakdown while minimizing the formation of undesirable byproducts.
- Combined treatment approaches integrating ozonation with other technologies: Integrating ozonation with other treatment technologies creates synergistic degradation pathways for complex pollutants. These combined approaches may include ozonation followed by biological treatment, coupling with ultrasound or UV irradiation, or sequential application with adsorption processes. The integration enhances overall treatment efficiency by targeting different aspects of contaminant degradation, with ozone typically breaking down complex molecules into more biodegradable compounds that can be further processed by subsequent treatment steps.
02 Advanced oxidation processes combining ozone with catalysts
Enhanced degradation pathways can be achieved by combining ozone with various catalysts to form advanced oxidation processes. These systems typically utilize metal catalysts, activated carbon, or other materials to accelerate ozone decomposition and increase hydroxyl radical formation. The catalytic ozonation pathways provide more efficient degradation of recalcitrant compounds through synergistic effects, allowing for lower ozone dosages and more complete mineralization of contaminants.Expand Specific Solutions03 Ozonation degradation monitoring and pathway analysis techniques
Various analytical techniques and monitoring systems are employed to track ozonation degradation pathways and identify transformation products. These methods include chromatography, mass spectrometry, and real-time monitoring of reaction intermediates. By understanding the degradation pathways and transformation products formed during ozonation, processes can be optimized to ensure complete mineralization of target compounds and prevent the formation of potentially harmful by-products.Expand Specific Solutions04 Ozonation systems for specific industrial applications
Specialized ozonation systems are designed for specific industrial applications with unique degradation pathway requirements. These include treatment of industrial wastewater containing complex organic compounds, pharmaceutical residues, textile dyes, and agricultural runoff. The ozonation degradation pathways are tailored to the specific contaminants present in these industrial streams, often involving multi-stage treatment processes and combination with other treatment technologies to achieve complete degradation.Expand Specific Solutions05 Ozone-based degradation of emerging contaminants
Ozonation processes specifically designed for the degradation of emerging contaminants such as pharmaceuticals, personal care products, and microplastics. These contaminants often require specialized ozonation approaches due to their complex structures and resistance to conventional treatment. The degradation pathways typically involve both direct ozone attack on specific functional groups and hydroxyl radical oxidation, with research focusing on identifying optimal conditions to ensure complete mineralization without forming toxic intermediates.Expand Specific Solutions
Leading Organizations in Advanced Oxidation Research
The ozonation technology for degrading microplastics and antibiotic residues is in an emerging growth phase, with a global market expected to expand significantly due to increasing water treatment concerns. Research institutions like The Regents of the University of California, Tongji University, and Massachusetts Institute of Technology are leading fundamental research on degradation pathways, while companies such as Dow Global Technologies and Air Liquide are developing commercial applications. The technology shows moderate maturity with established oxidation principles but requires further development for optimization and quality assurance protocols. Research collaborations between academic institutions (Caltech, Fraunhofer-Gesellschaft) and industry partners are accelerating practical implementation, particularly in wastewater treatment facilities where antibiotic residue removal is becoming increasingly critical.
Dow Global Technologies LLC
Technical Solution: Dow Global Technologies has developed advanced ozonation systems specifically designed for microplastics and antibiotic residue degradation. Their technology employs a multi-stage ozonation process with controlled ozone dosing mechanisms that optimize degradation efficiency while minimizing byproduct formation. The system incorporates real-time monitoring of ozone concentration and reaction parameters to ensure consistent treatment quality. Their research has identified specific degradation pathways for common microplastics like polyethylene and polypropylene, demonstrating that ozone primarily attacks carbon-carbon double bonds and aromatic structures, leading to chain scission and eventual mineralization[1]. For antibiotics, their process targets functional groups such as amine, thioether, and unsaturated bonds, achieving degradation rates exceeding 90% for many common pharmaceutical compounds[3].
Strengths: Superior process control systems allowing precise ozone dosing; extensive degradation pathway mapping for multiple contaminant classes; integrated quality assurance protocols. Weaknesses: Higher energy consumption compared to conventional treatments; potential formation of toxic intermediates requiring additional treatment steps; limited effectiveness against certain recalcitrant compounds.
Advanced Industrial Science & Technology
Technical Solution: Advanced Industrial Science & Technology (AIST) has pioneered catalytic ozonation technology for microplastics and pharmaceutical degradation. Their approach combines conventional ozonation with specially designed metal oxide catalysts that enhance hydroxyl radical formation, significantly improving degradation efficiency. AIST's system features a proprietary catalyst support structure that maximizes contact between ozone, catalysts, and target pollutants. Their research has mapped comprehensive degradation pathways for microplastics, showing that their catalytic process accelerates the breaking of polymer chains through oxidative mechanisms, reducing microplastics to oligomers and eventually to CO2 and H2O[2]. For antibiotics, they've documented transformation pathways involving hydroxylation, decarboxylation, and ring-opening reactions. Their quality assurance protocol incorporates continuous monitoring of treated water using advanced spectroscopic techniques and toxicity assays to verify complete removal of harmful compounds and transformation products[4].
Strengths: Enhanced degradation efficiency through catalytic processes; reduced energy requirements compared to conventional ozonation; comprehensive monitoring systems for quality assurance. Weaknesses: Catalyst regeneration requirements increase operational complexity; potential catalyst leaching concerns; higher initial capital investment compared to standard ozonation systems.
Critical Degradation Pathway Mechanisms Analysis
Deep learning based approach to study the characteristics of various micro plastic degrading enzymes that are released from microorganisms
PatentPendingIN202241040364A
Innovation
- A deep learning-based framework is designed to analyze the characteristics and importance of enzymes released by microorganisms for degrading microplastics, monitoring the entire degradation process and identifying active enzymes involved, with the aim of predicting their efficacy in degrading microplastics.
Quality Assurance Frameworks for Ozonation Processes
Quality assurance frameworks for ozonation processes in the degradation of microplastics and antibiotic residues require systematic approaches to ensure consistent and reliable treatment outcomes. These frameworks typically encompass multiple layers of monitoring and control mechanisms designed to maintain process integrity throughout the treatment cycle.
The foundation of any quality assurance system for ozonation begins with standardized protocols for equipment calibration and maintenance. Regular verification of ozone generators, contact chambers, and monitoring instruments ensures that the system delivers the intended ozone concentration consistently. This includes daily operational checks and periodic comprehensive assessments of equipment performance against manufacturer specifications.
Process parameter monitoring forms the second critical component, involving continuous measurement of key variables such as ozone concentration, contact time, pH, temperature, and oxidation-reduction potential. Advanced ozonation facilities implement real-time monitoring systems with automated feedback loops that adjust operational parameters to maintain optimal degradation conditions despite fluctuations in influent characteristics.
Quality control testing protocols constitute another essential element, requiring systematic sampling and analysis before, during, and after treatment. For microplastics degradation, this includes particle size distribution analysis, polymer type identification, and quantification of degradation byproducts. For antibiotic residues, analytical methods such as liquid chromatography-mass spectrometry (LC-MS/MS) are employed to measure concentration reductions and identify transformation products.
Documentation and traceability systems support the framework by maintaining comprehensive records of all operational parameters, maintenance activities, and analytical results. Modern facilities increasingly implement digital management systems that enable trend analysis and facilitate regulatory compliance reporting while providing audit trails for all quality-related activities.
Validation methodologies represent perhaps the most sophisticated aspect of quality assurance frameworks. These include challenge testing with known concentrations of target contaminants, verification of degradation pathways through identification of intermediate compounds, and confirmation of treatment efficacy across varying operational conditions. Multi-barrier validation approaches are particularly important for complex matrices where ozonation may be affected by competing reactions.
Staff competency programs ensure that personnel operating ozonation systems possess the necessary knowledge and skills. This includes formal training on process principles, safety protocols, quality control procedures, and emergency response, supplemented by regular performance assessments and continuing education to address emerging technologies and regulatory requirements.
The foundation of any quality assurance system for ozonation begins with standardized protocols for equipment calibration and maintenance. Regular verification of ozone generators, contact chambers, and monitoring instruments ensures that the system delivers the intended ozone concentration consistently. This includes daily operational checks and periodic comprehensive assessments of equipment performance against manufacturer specifications.
Process parameter monitoring forms the second critical component, involving continuous measurement of key variables such as ozone concentration, contact time, pH, temperature, and oxidation-reduction potential. Advanced ozonation facilities implement real-time monitoring systems with automated feedback loops that adjust operational parameters to maintain optimal degradation conditions despite fluctuations in influent characteristics.
Quality control testing protocols constitute another essential element, requiring systematic sampling and analysis before, during, and after treatment. For microplastics degradation, this includes particle size distribution analysis, polymer type identification, and quantification of degradation byproducts. For antibiotic residues, analytical methods such as liquid chromatography-mass spectrometry (LC-MS/MS) are employed to measure concentration reductions and identify transformation products.
Documentation and traceability systems support the framework by maintaining comprehensive records of all operational parameters, maintenance activities, and analytical results. Modern facilities increasingly implement digital management systems that enable trend analysis and facilitate regulatory compliance reporting while providing audit trails for all quality-related activities.
Validation methodologies represent perhaps the most sophisticated aspect of quality assurance frameworks. These include challenge testing with known concentrations of target contaminants, verification of degradation pathways through identification of intermediate compounds, and confirmation of treatment efficacy across varying operational conditions. Multi-barrier validation approaches are particularly important for complex matrices where ozonation may be affected by competing reactions.
Staff competency programs ensure that personnel operating ozonation systems possess the necessary knowledge and skills. This includes formal training on process principles, safety protocols, quality control procedures, and emergency response, supplemented by regular performance assessments and continuing education to address emerging technologies and regulatory requirements.
Environmental Impact Assessment of Ozonation Byproducts
The ozonation process, while effective for degrading microplastics and antibiotic residues, generates various byproducts that warrant careful environmental consideration. These transformation products result from complex oxidation reactions between ozone and target contaminants, potentially creating compounds with different environmental behaviors than their parent molecules.
Primary ozonation byproducts include aldehydes, ketones, carboxylic acids, and in some cases, brominated disinfection byproducts when bromide is present in the water matrix. Research indicates that ozonation of antibiotics can produce transformation products with reduced antimicrobial activity, but some intermediates may retain biological effects or exhibit new toxicological properties.
For microplastics, ozonation leads to surface oxidation, chain scission, and eventual fragmentation into smaller particles and soluble organic compounds. These smaller plastic fragments may have increased bioavailability and potentially greater ecological impact due to their enhanced mobility in aquatic environments and ability to penetrate biological membranes.
Ecotoxicological studies reveal varying impacts of ozonation byproducts on aquatic organisms. While many transformation products show reduced toxicity compared to parent compounds, some exhibit increased bioactivity. For instance, ozonation of certain antibiotics like fluoroquinolones can generate byproducts with enhanced phototoxicity under sunlight exposure, potentially affecting shallow aquatic ecosystems.
Persistence of ozonation byproducts in the environment depends on their chemical structure and environmental conditions. Some transformation products demonstrate greater recalcitrance to biodegradation than their precursors, potentially extending their environmental residence time and exposure risk to organisms.
Bioaccumulation potential represents another critical concern, as certain ozonation byproducts may exhibit greater lipophilicity than parent compounds, increasing their tendency to accumulate in aquatic organisms and potentially biomagnify through food chains. This is particularly relevant for byproducts derived from microplastics that may retain polymer characteristics while gaining new functional groups.
Mitigation strategies to address these environmental concerns include optimizing ozonation parameters to minimize harmful byproduct formation, implementing post-ozonation treatment processes like biofiltration or activated carbon adsorption, and developing comprehensive monitoring protocols that target known problematic transformation products. Advanced analytical techniques such as high-resolution mass spectrometry coupled with effect-directed analysis are increasingly employed to identify byproducts of environmental concern.
Regulatory frameworks are gradually evolving to address ozonation byproducts, with some jurisdictions implementing monitoring requirements for specific transformation products and establishing environmental quality standards based on ecotoxicological risk assessments.
Primary ozonation byproducts include aldehydes, ketones, carboxylic acids, and in some cases, brominated disinfection byproducts when bromide is present in the water matrix. Research indicates that ozonation of antibiotics can produce transformation products with reduced antimicrobial activity, but some intermediates may retain biological effects or exhibit new toxicological properties.
For microplastics, ozonation leads to surface oxidation, chain scission, and eventual fragmentation into smaller particles and soluble organic compounds. These smaller plastic fragments may have increased bioavailability and potentially greater ecological impact due to their enhanced mobility in aquatic environments and ability to penetrate biological membranes.
Ecotoxicological studies reveal varying impacts of ozonation byproducts on aquatic organisms. While many transformation products show reduced toxicity compared to parent compounds, some exhibit increased bioactivity. For instance, ozonation of certain antibiotics like fluoroquinolones can generate byproducts with enhanced phototoxicity under sunlight exposure, potentially affecting shallow aquatic ecosystems.
Persistence of ozonation byproducts in the environment depends on their chemical structure and environmental conditions. Some transformation products demonstrate greater recalcitrance to biodegradation than their precursors, potentially extending their environmental residence time and exposure risk to organisms.
Bioaccumulation potential represents another critical concern, as certain ozonation byproducts may exhibit greater lipophilicity than parent compounds, increasing their tendency to accumulate in aquatic organisms and potentially biomagnify through food chains. This is particularly relevant for byproducts derived from microplastics that may retain polymer characteristics while gaining new functional groups.
Mitigation strategies to address these environmental concerns include optimizing ozonation parameters to minimize harmful byproduct formation, implementing post-ozonation treatment processes like biofiltration or activated carbon adsorption, and developing comprehensive monitoring protocols that target known problematic transformation products. Advanced analytical techniques such as high-resolution mass spectrometry coupled with effect-directed analysis are increasingly employed to identify byproducts of environmental concern.
Regulatory frameworks are gradually evolving to address ozonation byproducts, with some jurisdictions implementing monitoring requirements for specific transformation products and establishing environmental quality standards based on ecotoxicological risk assessments.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!