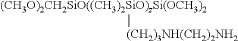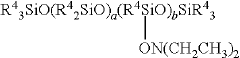How to Determine the Optimum pH for Colloidal Silica Stability
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Colloidal Silica pH Stability Background and Objectives
Colloidal silica systems have evolved significantly over the past century, with their stability mechanisms becoming increasingly understood since the pioneering work of DLVO theory in the 1940s. The stability of colloidal silica dispersions is fundamentally governed by the balance between attractive van der Waals forces and repulsive electrostatic forces, with pH playing a critical role in this equilibrium. Historical developments in this field have progressed from basic empirical observations to sophisticated surface chemistry models that can predict stability behavior across various environmental conditions.
The technological evolution of colloidal silica has been marked by significant breakthroughs in particle synthesis, characterization techniques, and stability control methods. Early research focused primarily on understanding basic silica chemistry, while recent advances have enabled precise manipulation of surface properties through pH adjustment and surface modification. This progression has transformed colloidal silica from a scientific curiosity to an industrial material with applications spanning numerous industries.
Current technological trends indicate a growing emphasis on developing robust methods for determining and maintaining optimal pH conditions for specific colloidal silica applications. The industry is moving toward more precise control systems that can adapt to changing environmental conditions while maintaining colloidal stability. This trend is driven by increasingly demanding applications in electronics, catalysis, and biomedical fields where even minor instability can compromise product performance.
The primary objective of this technical research is to establish systematic methodologies for determining the optimum pH range for colloidal silica stability across various particle sizes, concentrations, and application environments. This includes developing predictive models that can account for the complex interplay between pH, ionic strength, temperature, and other factors affecting stability.
Secondary objectives include identifying key stability indicators that can serve as early warning systems for potential aggregation or gelation, developing rapid assessment protocols for quality control purposes, and exploring novel approaches to expand the stable pH range of colloidal silica systems through surface modification or additive incorporation.
The expected outcomes of this research include standardized protocols for pH optimization, improved understanding of the relationship between particle characteristics and pH sensitivity, and practical guidelines for maintaining stability in industrial applications. These outcomes will address current technological gaps in predicting and controlling colloidal silica behavior under variable conditions, ultimately enabling more reliable and efficient use of these materials in advanced applications.
The technological evolution of colloidal silica has been marked by significant breakthroughs in particle synthesis, characterization techniques, and stability control methods. Early research focused primarily on understanding basic silica chemistry, while recent advances have enabled precise manipulation of surface properties through pH adjustment and surface modification. This progression has transformed colloidal silica from a scientific curiosity to an industrial material with applications spanning numerous industries.
Current technological trends indicate a growing emphasis on developing robust methods for determining and maintaining optimal pH conditions for specific colloidal silica applications. The industry is moving toward more precise control systems that can adapt to changing environmental conditions while maintaining colloidal stability. This trend is driven by increasingly demanding applications in electronics, catalysis, and biomedical fields where even minor instability can compromise product performance.
The primary objective of this technical research is to establish systematic methodologies for determining the optimum pH range for colloidal silica stability across various particle sizes, concentrations, and application environments. This includes developing predictive models that can account for the complex interplay between pH, ionic strength, temperature, and other factors affecting stability.
Secondary objectives include identifying key stability indicators that can serve as early warning systems for potential aggregation or gelation, developing rapid assessment protocols for quality control purposes, and exploring novel approaches to expand the stable pH range of colloidal silica systems through surface modification or additive incorporation.
The expected outcomes of this research include standardized protocols for pH optimization, improved understanding of the relationship between particle characteristics and pH sensitivity, and practical guidelines for maintaining stability in industrial applications. These outcomes will address current technological gaps in predicting and controlling colloidal silica behavior under variable conditions, ultimately enabling more reliable and efficient use of these materials in advanced applications.
Market Applications and Demand Analysis for Stable Colloidal Silica
The global market for colloidal silica has been experiencing significant growth, driven by its versatile applications across multiple industries. The current market size is estimated at approximately 4.2 billion USD, with projections indicating a compound annual growth rate of 5.7% through 2028. This growth trajectory underscores the increasing demand for stable colloidal silica formulations across diverse sectors.
In the semiconductor industry, colloidal silica serves as a critical component in chemical mechanical planarization (CMP) slurries, where pH stability directly impacts polishing performance and defect rates. Market analysis reveals that semiconductor manufacturers are willing to pay premium prices for colloidal silica products with guaranteed pH stability, as this translates to higher yields and reduced manufacturing costs.
The construction sector represents another substantial market, where colloidal silica is utilized in concrete admixtures to enhance strength and durability. Recent market surveys indicate that construction companies increasingly specify pH-stable colloidal silica to ensure consistent performance across varying environmental conditions, particularly in infrastructure projects with extended lifespans.
Paper manufacturing constitutes a traditional yet evolving market for colloidal silica, where it functions as a retention aid and drainage enhancer. Industry reports highlight that paper mills are transitioning toward more environmentally friendly processes, creating demand for colloidal silica formulations that maintain stability at specific pH ranges compatible with these greener manufacturing methods.
The coatings industry demonstrates growing demand for stable colloidal silica as a rheology modifier and anti-settling agent. Market research indicates that coating manufacturers prioritize products with documented pH stability profiles to ensure batch-to-batch consistency and extended shelf life of their formulations.
Emerging applications in battery technology, particularly for electric vehicles, represent a rapidly expanding market segment. Colloidal silica serves as a component in separator materials and electrode coatings, where precise pH control directly correlates with battery performance and safety characteristics.
Healthcare applications, including diagnostic assays and drug delivery systems, constitute a premium market segment with stringent stability requirements. The pharmaceutical industry's demand for colloidal silica with validated pH stability profiles continues to grow at double-digit rates, driven by expanding applications in advanced therapeutic delivery systems.
Market analysis reveals regional variations in demand patterns, with Asia-Pacific showing the strongest growth trajectory due to rapid industrialization and infrastructure development. North American and European markets demonstrate more mature demand profiles, with emphasis on specialized, high-performance applications requiring precise pH stability across extended timeframes.
In the semiconductor industry, colloidal silica serves as a critical component in chemical mechanical planarization (CMP) slurries, where pH stability directly impacts polishing performance and defect rates. Market analysis reveals that semiconductor manufacturers are willing to pay premium prices for colloidal silica products with guaranteed pH stability, as this translates to higher yields and reduced manufacturing costs.
The construction sector represents another substantial market, where colloidal silica is utilized in concrete admixtures to enhance strength and durability. Recent market surveys indicate that construction companies increasingly specify pH-stable colloidal silica to ensure consistent performance across varying environmental conditions, particularly in infrastructure projects with extended lifespans.
Paper manufacturing constitutes a traditional yet evolving market for colloidal silica, where it functions as a retention aid and drainage enhancer. Industry reports highlight that paper mills are transitioning toward more environmentally friendly processes, creating demand for colloidal silica formulations that maintain stability at specific pH ranges compatible with these greener manufacturing methods.
The coatings industry demonstrates growing demand for stable colloidal silica as a rheology modifier and anti-settling agent. Market research indicates that coating manufacturers prioritize products with documented pH stability profiles to ensure batch-to-batch consistency and extended shelf life of their formulations.
Emerging applications in battery technology, particularly for electric vehicles, represent a rapidly expanding market segment. Colloidal silica serves as a component in separator materials and electrode coatings, where precise pH control directly correlates with battery performance and safety characteristics.
Healthcare applications, including diagnostic assays and drug delivery systems, constitute a premium market segment with stringent stability requirements. The pharmaceutical industry's demand for colloidal silica with validated pH stability profiles continues to grow at double-digit rates, driven by expanding applications in advanced therapeutic delivery systems.
Market analysis reveals regional variations in demand patterns, with Asia-Pacific showing the strongest growth trajectory due to rapid industrialization and infrastructure development. North American and European markets demonstrate more mature demand profiles, with emphasis on specialized, high-performance applications requiring precise pH stability across extended timeframes.
Current pH Control Technologies and Challenges
The current pH control technologies for colloidal silica stability management encompass a range of methodologies, each with specific advantages and limitations. Traditional approaches include manual acid/base addition systems, which require operators to periodically test pH levels and adjust accordingly. While cost-effective for small-scale operations, these systems lack precision and consistency, particularly in industrial applications where minor pH fluctuations can significantly impact colloidal stability.
Automated pH control systems represent a significant advancement, utilizing continuous monitoring through pH probes coupled with programmable logic controllers (PLCs) that trigger precise chemical dosing. These systems maintain tighter pH control but face challenges including probe fouling in silica-rich environments, calibration drift requiring frequent maintenance, and response lag during rapid process changes.
Buffer solution implementation offers another approach, where chemical compounds that resist pH changes when acids or bases are added provide a stabilizing effect. Common buffers for silica applications include phosphate and borate systems. While effective at maintaining pH within narrow ranges, these systems have limited capacity and can introduce unwanted ions that may interfere with colloidal stability through compression of the electrical double layer.
CO2 injection systems have gained popularity for acidification processes, offering precise pH reduction through controlled dissolution of carbon dioxide. This method avoids the handling hazards associated with strong acids but has limited applicability for raising pH and can introduce carbonates that affect silica surface chemistry.
Emerging technologies include microfluidic pH control systems that enable precise manipulation of extremely small fluid volumes, and electrochemical pH modulation that generates acids or bases in-situ through electrolysis of water or salt solutions. These approaches show promise for specialized applications but remain costly for large-scale implementation.
A significant challenge across all technologies is the complex relationship between pH and other parameters affecting colloidal stability, including temperature, ionic strength, and silica concentration. Current systems typically control pH as an isolated parameter without accounting for these interdependencies, leading to suboptimal stability outcomes.
Additionally, real-time monitoring of actual colloidal stability rather than just pH remains technically challenging. Most systems rely on pH as a proxy for stability without direct measurement of zeta potential, particle size distribution, or aggregation kinetics. This disconnect between the controlled parameter (pH) and the desired outcome (stability) represents a fundamental limitation in current approaches.
Automated pH control systems represent a significant advancement, utilizing continuous monitoring through pH probes coupled with programmable logic controllers (PLCs) that trigger precise chemical dosing. These systems maintain tighter pH control but face challenges including probe fouling in silica-rich environments, calibration drift requiring frequent maintenance, and response lag during rapid process changes.
Buffer solution implementation offers another approach, where chemical compounds that resist pH changes when acids or bases are added provide a stabilizing effect. Common buffers for silica applications include phosphate and borate systems. While effective at maintaining pH within narrow ranges, these systems have limited capacity and can introduce unwanted ions that may interfere with colloidal stability through compression of the electrical double layer.
CO2 injection systems have gained popularity for acidification processes, offering precise pH reduction through controlled dissolution of carbon dioxide. This method avoids the handling hazards associated with strong acids but has limited applicability for raising pH and can introduce carbonates that affect silica surface chemistry.
Emerging technologies include microfluidic pH control systems that enable precise manipulation of extremely small fluid volumes, and electrochemical pH modulation that generates acids or bases in-situ through electrolysis of water or salt solutions. These approaches show promise for specialized applications but remain costly for large-scale implementation.
A significant challenge across all technologies is the complex relationship between pH and other parameters affecting colloidal stability, including temperature, ionic strength, and silica concentration. Current systems typically control pH as an isolated parameter without accounting for these interdependencies, leading to suboptimal stability outcomes.
Additionally, real-time monitoring of actual colloidal stability rather than just pH remains technically challenging. Most systems rely on pH as a proxy for stability without direct measurement of zeta potential, particle size distribution, or aggregation kinetics. This disconnect between the controlled parameter (pH) and the desired outcome (stability) represents a fundamental limitation in current approaches.
Existing pH Optimization Methodologies and Protocols
01 pH control in colloidal silica production
The pH level is a critical parameter in the production of colloidal silica, affecting stability, particle size, and overall quality. Manufacturers typically control pH through the addition of alkaline substances like sodium hydroxide or ammonia during the production process. Maintaining specific pH ranges (often between 8-10) helps prevent aggregation and ensures uniform particle distribution in the final colloidal suspension.- pH adjustment of colloidal silica for stability: The pH of colloidal silica solutions significantly affects their stability and shelf life. Adjusting the pH to specific ranges (typically alkaline pH 8-10) prevents aggregation and gelation of silica particles. This pH control is crucial for maintaining the dispersion properties of colloidal silica in various applications, as it influences the surface charge of the particles and prevents them from coming together and forming larger aggregates.
- pH-dependent surface modifications of colloidal silica: The surface properties of colloidal silica can be modified by controlling the pH environment. At different pH levels, silica particles exhibit varying surface charges and functional group behaviors, which can be exploited for specific applications. Surface modifications at controlled pH values enable the creation of specialized colloidal silica products with enhanced properties such as improved binding to organic compounds or better compatibility with other materials in composite formulations.
- Acidic colloidal silica formulations: Acidic colloidal silica formulations (pH 2-5) have specific applications in industries such as electronics, catalysts, and precision polishing. At lower pH values, silica particles tend to have different surface characteristics and reactivity compared to their alkaline counterparts. These acidic formulations are particularly useful in semiconductor processing, where they provide controlled etching and polishing properties while minimizing metal contamination issues.
- pH buffering systems for colloidal silica: Buffer systems are incorporated into colloidal silica formulations to maintain pH stability during storage and application. These buffering agents help resist pH changes that might occur due to dilution, contamination, or reaction with other materials. Effective pH buffering is essential for commercial colloidal silica products to ensure consistent performance across various environmental conditions and throughout their shelf life.
- pH-controlled colloidal silica for specific industrial applications: Different industrial applications require colloidal silica with specific pH values to achieve optimal performance. For example, paper manufacturing may utilize alkaline colloidal silica, while electronics polishing might require acidic formulations. The pH of colloidal silica is carefully controlled during manufacturing to create products tailored for concrete strengthening, textile treatments, metal casting, and various coating applications, where the interaction between the silica particles and the substrate materials is pH-dependent.
02 Surface modification of colloidal silica through pH adjustment
The surface properties of colloidal silica particles can be modified by adjusting the pH of the suspension. At different pH levels, the silanol groups on the silica surface exhibit varying degrees of ionization, which affects surface charge and reactivity. This pH-dependent behavior enables the functionalization of colloidal silica for specific applications, including as binding agents, in coatings, or for composite materials.Expand Specific Solutions03 Stability mechanisms of colloidal silica at different pH values
Colloidal silica exhibits different stability characteristics depending on the pH environment. At neutral to alkaline pH (7-10), electrostatic repulsion between negatively charged silica particles prevents aggregation, resulting in stable suspensions. At lower pH values, the reduced surface charge can lead to gelation or precipitation. Understanding these pH-dependent stability mechanisms is essential for formulating colloidal silica products with appropriate shelf life and performance characteristics.Expand Specific Solutions04 pH-responsive colloidal silica applications
The pH-responsive behavior of colloidal silica enables various specialized applications. By leveraging the changes in surface properties and particle interactions that occur at different pH levels, researchers have developed smart materials that respond to environmental pH changes. These applications include controlled release systems, pH-triggered assembly of nanostructures, and responsive coatings that change properties based on ambient pH conditions.Expand Specific Solutions05 pH effects on colloidal silica in composite materials
When incorporating colloidal silica into composite materials, the pH of the system significantly influences the interaction between silica particles and the matrix material. Proper pH adjustment can enhance dispersion, improve interfacial bonding, and optimize mechanical properties of the resulting composite. This is particularly important in applications such as paper coatings, cement additives, and polymer reinforcement, where the uniform distribution of silica particles throughout the matrix is crucial for performance.Expand Specific Solutions
Leading Manufacturers and Research Institutions in Colloidal Silica
The colloidal silica stability optimization market is currently in a growth phase, with increasing applications across semiconductor, chemical, and construction industries. The global market size for colloidal silica is estimated at $650-700 million annually, expanding at 5-7% CAGR. Leading players like Wacker Chemie, Evonik Operations, and Dow Silicones have developed advanced pH control technologies, while specialty manufacturers such as Fuso Chemical and Fujimi focus on ultra-high purity formulations for electronics applications. Technical maturity varies by application sector, with semiconductor-grade solutions being most advanced. Research organizations like Australian Nuclear Science & Technology Organisation collaborate with companies including Merck Patent GmbH and Clariant International to develop next-generation stabilization methods addressing challenges in extreme pH environments.
Wacker Chemie AG
Technical Solution: Wacker Chemie AG has developed a comprehensive approach to colloidal silica stability optimization through precise pH control. Their technology involves a multi-parameter assessment system that correlates zeta potential measurements with pH adjustments to identify the optimal stability zone. The company utilizes proprietary buffer systems that maintain pH stability even under varying temperature and concentration conditions. Their research has demonstrated that maximum colloidal stability typically occurs at pH values between 8-10 for most applications, where the silica particles exhibit maximum negative surface charge[1]. Wacker's approach includes real-time monitoring systems that can detect early signs of destabilization and automatically adjust pH levels to maintain optimal dispersion. Their technology also incorporates specific surface modifications that extend the pH stability range, allowing their colloidal silica products to remain stable across broader application conditions[3].
Strengths: Superior long-term stability through precise zeta potential control; automated pH adjustment capabilities for industrial-scale applications; extended shelf life of products. Weaknesses: Higher implementation costs compared to basic pH control methods; requires specialized equipment for optimal performance; system complexity may require additional operator training.
Akzo Nobel Chemicals International BV
Technical Solution: Akzo Nobel has pioneered an advanced pH optimization system for colloidal silica that integrates electrochemical analysis with computational modeling. Their approach begins with characterizing the silica surface chemistry across the pH spectrum (2-12) to identify the isoelectric point and zones of maximum stability. The company employs a proprietary "stability mapping" technique that correlates particle size distribution, zeta potential, and viscosity changes over time at different pH values[2]. This generates a comprehensive stability profile specific to each colloidal silica formulation. Akzo Nobel's technology includes specialized pH-responsive additives that create an electrosteric stabilization effect, extending stability beyond what electrostatic repulsion alone can achieve. Their research has shown that optimal stability for their colloidal silica products typically occurs at pH 9.2-10.1, where the combination of high negative surface charge and their proprietary stabilizers provides maximum dispersion stability[4].
Strengths: Highly customizable approach for different silica types and applications; excellent stability in high-ionic-strength environments; predictive modeling capabilities for formulation optimization. Weaknesses: Requires significant analytical infrastructure; stabilizing additives may interfere with some end-use applications; system optimization can be time-consuming.
Critical Patents and Research on Colloidal Silica Stability
Colloidal silica and production method therefor
PatentWO2023119549A1
Innovation
- Colloidal silica with a specific relaxation rate of 0.60 or more, measured by pulsed NMR, and a zeta potential of -10 to 10 mV in a pH range of 2 to 5, along with an average particle diameter of 30 nm or less, ensuring stability and minimizing electrostatic interactions with polishing objects.
Silicone-based barrier compositions
PatentActiveUS20220363946A1
Innovation
- Incorporating acidic pH stable colloidal silica into the coating composition, which extends the shelf life to greater than 15 months by stabilizing the formulation and allowing for point-of-sale or point-of-manufacture tinting while maintaining air and water barrier properties.
Environmental Impact of Colloidal Silica Production
The production of colloidal silica involves several processes that can have significant environmental implications. The manufacturing typically requires substantial energy inputs, particularly during the synthesis phase where high temperatures are often necessary. This energy consumption contributes to greenhouse gas emissions when fossil fuels are the primary energy source. Additionally, the production process may involve the use of chemicals such as sodium silicate, sulfuric acid, and various stabilizing agents, which can lead to potential chemical waste streams if not properly managed.
Water usage represents another critical environmental concern in colloidal silica production. The manufacturing process is water-intensive, requiring large volumes for synthesis, washing, and purification stages. In regions facing water scarcity, this high demand can exacerbate local water stress and potentially impact surrounding ecosystems and communities.
Waste management challenges also emerge from colloidal silica production. The process generates silica-containing wastewater that requires treatment before discharge. If improperly handled, these effluents can cause silica accumulation in water bodies, potentially altering aquatic ecosystems and affecting water quality. The pH adjustment necessary for colloidal stability often involves acids or bases that must be neutralized before discharge to prevent disruption of natural water systems.
Air emissions present additional environmental considerations. Depending on the production method, volatile organic compounds (VOCs) or particulate matter may be released, contributing to air quality degradation if adequate control technologies are not implemented. Fine silica dust can pose respiratory hazards if not properly contained during manufacturing and handling operations.
The environmental footprint extends to raw material extraction as well. Silica mining operations can lead to habitat disruption, soil erosion, and landscape alterations. The transportation of raw materials and finished products further contributes to the carbon footprint through fuel consumption and associated emissions.
However, sustainable practices are increasingly being adopted in the industry. These include closed-loop water systems that minimize freshwater consumption, energy-efficient production technologies, and waste heat recovery systems. Some manufacturers are exploring renewable energy sources to power their operations, significantly reducing the carbon intensity of production. Advanced wastewater treatment technologies are being implemented to recover and reuse process water while ensuring that discharged effluents meet stringent environmental standards.
Life cycle assessment studies indicate that optimizing pH conditions for colloidal silica stability can indirectly benefit environmental performance by reducing waste generation from unstable products and minimizing the need for additional chemical treatments to maintain product quality during storage and transportation.
Water usage represents another critical environmental concern in colloidal silica production. The manufacturing process is water-intensive, requiring large volumes for synthesis, washing, and purification stages. In regions facing water scarcity, this high demand can exacerbate local water stress and potentially impact surrounding ecosystems and communities.
Waste management challenges also emerge from colloidal silica production. The process generates silica-containing wastewater that requires treatment before discharge. If improperly handled, these effluents can cause silica accumulation in water bodies, potentially altering aquatic ecosystems and affecting water quality. The pH adjustment necessary for colloidal stability often involves acids or bases that must be neutralized before discharge to prevent disruption of natural water systems.
Air emissions present additional environmental considerations. Depending on the production method, volatile organic compounds (VOCs) or particulate matter may be released, contributing to air quality degradation if adequate control technologies are not implemented. Fine silica dust can pose respiratory hazards if not properly contained during manufacturing and handling operations.
The environmental footprint extends to raw material extraction as well. Silica mining operations can lead to habitat disruption, soil erosion, and landscape alterations. The transportation of raw materials and finished products further contributes to the carbon footprint through fuel consumption and associated emissions.
However, sustainable practices are increasingly being adopted in the industry. These include closed-loop water systems that minimize freshwater consumption, energy-efficient production technologies, and waste heat recovery systems. Some manufacturers are exploring renewable energy sources to power their operations, significantly reducing the carbon intensity of production. Advanced wastewater treatment technologies are being implemented to recover and reuse process water while ensuring that discharged effluents meet stringent environmental standards.
Life cycle assessment studies indicate that optimizing pH conditions for colloidal silica stability can indirectly benefit environmental performance by reducing waste generation from unstable products and minimizing the need for additional chemical treatments to maintain product quality during storage and transportation.
Quality Control Standards and Testing Procedures
Quality control is essential for ensuring the consistent performance of colloidal silica systems across various pH conditions. Standardized testing procedures must be implemented to accurately determine and maintain optimal pH levels for colloidal stability. These procedures should include regular calibration of pH measurement equipment using certified buffer solutions traceable to national or international standards, with calibration performed at minimum before each testing session and ideally at temperatures matching the intended application environment.
Particle size distribution analysis represents a critical quality control parameter, with dynamic light scattering (DLS) serving as the preferred method for monitoring colloidal stability. Established protocols should specify sample preparation techniques, measurement conditions, and acceptance criteria for size distribution metrics. Zeta potential measurements provide complementary data on electrostatic stability, with values typically exceeding ±30 mV indicating good stability. These measurements should be conducted under controlled temperature conditions with standardized ionic strength parameters.
Turbidity testing offers a rapid assessment of colloidal stability, with specifications for maximum acceptable turbidity values based on application requirements. Accelerated stability testing protocols should subject samples to controlled temperature cycling, mechanical agitation, and extended storage periods to predict long-term stability behavior. Statistical process control methods must be implemented to track stability parameters over time, establishing control limits that trigger corrective actions when deviations occur.
Documentation requirements should include detailed records of all testing parameters, raw data, calculated results, and any deviations from standard procedures. Regular proficiency testing through participation in interlaboratory comparison programs ensures measurement accuracy and consistency across different testing facilities. Quality control charts should be maintained to visualize trends in critical parameters like zeta potential, particle size, and pH drift over time.
For production environments, in-line monitoring systems capable of continuous pH measurement with automated feedback control mechanisms represent best practice. These systems should include redundant measurement capabilities and automated alarms for out-of-specification conditions. Acceptance criteria must be established for each colloidal silica formulation, defining the acceptable pH range, maximum allowable particle size variation, and minimum zeta potential values required for release to production or customers.
Particle size distribution analysis represents a critical quality control parameter, with dynamic light scattering (DLS) serving as the preferred method for monitoring colloidal stability. Established protocols should specify sample preparation techniques, measurement conditions, and acceptance criteria for size distribution metrics. Zeta potential measurements provide complementary data on electrostatic stability, with values typically exceeding ±30 mV indicating good stability. These measurements should be conducted under controlled temperature conditions with standardized ionic strength parameters.
Turbidity testing offers a rapid assessment of colloidal stability, with specifications for maximum acceptable turbidity values based on application requirements. Accelerated stability testing protocols should subject samples to controlled temperature cycling, mechanical agitation, and extended storage periods to predict long-term stability behavior. Statistical process control methods must be implemented to track stability parameters over time, establishing control limits that trigger corrective actions when deviations occur.
Documentation requirements should include detailed records of all testing parameters, raw data, calculated results, and any deviations from standard procedures. Regular proficiency testing through participation in interlaboratory comparison programs ensures measurement accuracy and consistency across different testing facilities. Quality control charts should be maintained to visualize trends in critical parameters like zeta potential, particle size, and pH drift over time.
For production environments, in-line monitoring systems capable of continuous pH measurement with automated feedback control mechanisms represent best practice. These systems should include redundant measurement capabilities and automated alarms for out-of-specification conditions. Acceptance criteria must be established for each colloidal silica formulation, defining the acceptable pH range, maximum allowable particle size variation, and minimum zeta potential values required for release to production or customers.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!