How to Minimize Lithium Bromide Solution Degradation
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
LiBr Solution Degradation Background and Objectives
Lithium bromide (LiBr) solution has been extensively utilized in absorption refrigeration systems since the early 20th century. The technology gained significant traction in the 1950s and 1960s when energy efficiency became a growing concern in industrial and commercial applications. LiBr absorption systems offer advantages in utilizing low-grade thermal energy sources, making them environmentally friendly alternatives to conventional vapor compression systems that rely on electricity and refrigerants with high global warming potential.
The evolution of LiBr absorption technology has been marked by continuous improvements in system design, efficiency, and reliability. However, solution degradation remains one of the most persistent challenges limiting the widespread adoption and long-term performance of these systems. Degradation occurs through various mechanisms including thermal decomposition, air leakage leading to oxidation, and corrosion of system components, all of which compromise system efficiency and operational lifespan.
Recent technological trends indicate a growing interest in enhancing the stability of LiBr solutions through advanced inhibitor formulations, improved system designs to minimize air infiltration, and novel materials for heat exchangers and other components that come into contact with the solution. The integration of smart monitoring systems to detect early signs of degradation represents another emerging approach in this field.
The primary objective of this technical research is to comprehensively analyze the mechanisms of LiBr solution degradation and identify effective strategies to minimize these effects. Specifically, we aim to evaluate current inhibitor technologies, explore novel additives that can enhance solution stability, and investigate system design modifications that can reduce degradation rates.
Additionally, this research seeks to establish quantitative metrics for assessing degradation rates under various operating conditions, enabling more accurate prediction of system performance over time. By developing a deeper understanding of the chemical and physical processes involved in LiBr solution degradation, we can formulate more effective preventive measures and maintenance protocols.
The ultimate goal is to extend the service life of LiBr absorption systems, improve their reliability, and reduce maintenance requirements and operational costs. This would significantly enhance the competitiveness of absorption cooling technology in the broader HVAC market, particularly in applications where waste heat or renewable energy sources are available, contributing to global energy efficiency and sustainability objectives.
The evolution of LiBr absorption technology has been marked by continuous improvements in system design, efficiency, and reliability. However, solution degradation remains one of the most persistent challenges limiting the widespread adoption and long-term performance of these systems. Degradation occurs through various mechanisms including thermal decomposition, air leakage leading to oxidation, and corrosion of system components, all of which compromise system efficiency and operational lifespan.
Recent technological trends indicate a growing interest in enhancing the stability of LiBr solutions through advanced inhibitor formulations, improved system designs to minimize air infiltration, and novel materials for heat exchangers and other components that come into contact with the solution. The integration of smart monitoring systems to detect early signs of degradation represents another emerging approach in this field.
The primary objective of this technical research is to comprehensively analyze the mechanisms of LiBr solution degradation and identify effective strategies to minimize these effects. Specifically, we aim to evaluate current inhibitor technologies, explore novel additives that can enhance solution stability, and investigate system design modifications that can reduce degradation rates.
Additionally, this research seeks to establish quantitative metrics for assessing degradation rates under various operating conditions, enabling more accurate prediction of system performance over time. By developing a deeper understanding of the chemical and physical processes involved in LiBr solution degradation, we can formulate more effective preventive measures and maintenance protocols.
The ultimate goal is to extend the service life of LiBr absorption systems, improve their reliability, and reduce maintenance requirements and operational costs. This would significantly enhance the competitiveness of absorption cooling technology in the broader HVAC market, particularly in applications where waste heat or renewable energy sources are available, contributing to global energy efficiency and sustainability objectives.
Market Analysis for LiBr Absorption Systems
The global market for Lithium Bromide (LiBr) absorption systems has been experiencing steady growth, primarily driven by increasing energy efficiency requirements and the rising demand for sustainable cooling solutions. The market size was valued at approximately $1.2 billion in 2022 and is projected to reach $1.8 billion by 2028, representing a compound annual growth rate of 7.2% during the forecast period.
Asia-Pacific currently dominates the market, accounting for over 40% of the global share, with China and Japan being the leading contributors. This regional dominance can be attributed to rapid industrialization, favorable government policies promoting energy-efficient technologies, and increasing investments in sustainable infrastructure development. North America and Europe follow closely, collectively representing about 35% of the market share.
The commercial building sector remains the largest end-user segment for LiBr absorption systems, particularly in applications such as large-scale air conditioning for hotels, hospitals, and office complexes. Industrial applications, especially in process cooling and refrigeration, constitute the second-largest market segment with growing adoption in pharmaceutical manufacturing, food processing, and chemical industries.
A significant market driver is the increasing focus on reducing carbon emissions and meeting stringent environmental regulations. LiBr absorption systems, which can utilize waste heat or solar energy as primary energy sources, align perfectly with global sustainability goals. The rising cost of conventional energy sources further enhances the economic attractiveness of these systems.
However, solution degradation remains a critical challenge affecting market growth. End-users report that maintenance costs associated with LiBr solution degradation can account for up to 15% of the total operational expenses. This issue creates a substantial market opportunity for companies that can develop effective degradation minimization technologies.
Emerging markets in Southeast Asia, Middle East, and Latin America present significant growth opportunities, with projected adoption rates increasing by 9-10% annually. These regions are experiencing rapid urbanization and industrial development, creating demand for efficient cooling solutions in both commercial and industrial sectors.
The competitive landscape features established players like Carrier Corporation, Trane Technologies, and Johnson Controls dominating with approximately 45% combined market share. However, Asian manufacturers, particularly from China and South Korea, are rapidly gaining ground by offering cost-competitive solutions with improving technological capabilities.
Asia-Pacific currently dominates the market, accounting for over 40% of the global share, with China and Japan being the leading contributors. This regional dominance can be attributed to rapid industrialization, favorable government policies promoting energy-efficient technologies, and increasing investments in sustainable infrastructure development. North America and Europe follow closely, collectively representing about 35% of the market share.
The commercial building sector remains the largest end-user segment for LiBr absorption systems, particularly in applications such as large-scale air conditioning for hotels, hospitals, and office complexes. Industrial applications, especially in process cooling and refrigeration, constitute the second-largest market segment with growing adoption in pharmaceutical manufacturing, food processing, and chemical industries.
A significant market driver is the increasing focus on reducing carbon emissions and meeting stringent environmental regulations. LiBr absorption systems, which can utilize waste heat or solar energy as primary energy sources, align perfectly with global sustainability goals. The rising cost of conventional energy sources further enhances the economic attractiveness of these systems.
However, solution degradation remains a critical challenge affecting market growth. End-users report that maintenance costs associated with LiBr solution degradation can account for up to 15% of the total operational expenses. This issue creates a substantial market opportunity for companies that can develop effective degradation minimization technologies.
Emerging markets in Southeast Asia, Middle East, and Latin America present significant growth opportunities, with projected adoption rates increasing by 9-10% annually. These regions are experiencing rapid urbanization and industrial development, creating demand for efficient cooling solutions in both commercial and industrial sectors.
The competitive landscape features established players like Carrier Corporation, Trane Technologies, and Johnson Controls dominating with approximately 45% combined market share. However, Asian manufacturers, particularly from China and South Korea, are rapidly gaining ground by offering cost-competitive solutions with improving technological capabilities.
Current Challenges in LiBr Solution Stability
Lithium bromide (LiBr) solution degradation presents significant challenges in absorption refrigeration systems, impacting both operational efficiency and system longevity. The primary stability issues stem from several interconnected factors that accelerate degradation processes under typical operating conditions.
Corrosion remains the most persistent challenge, as LiBr solutions are inherently corrosive to many common system materials, particularly metals. This corrosivity increases dramatically at higher concentrations and temperatures, conditions frequently encountered in absorption chillers. The corrosion process not only damages critical system components but also introduces metal ions into the solution, which catalyze further degradation reactions and reduce heat transfer efficiency.
Oxidation of the LiBr solution occurs when dissolved oxygen reacts with bromide ions, forming free bromine and other oxidative species. This process is particularly problematic in systems with air leakage or inadequate purging mechanisms. The resulting oxidation products alter solution properties and contribute to increased corrosivity, creating a destructive feedback loop that accelerates overall system deterioration.
Thermal decomposition represents another significant stability challenge, especially in high-temperature generator sections where temperatures can exceed 150°C. Under these conditions, LiBr can undergo chemical transformations that produce hydrogen bromide and other degradation products, compromising solution performance and potentially damaging system components through increased acidity.
Crystallization risk poses operational hazards when solution concentration exceeds solubility limits, typically during temperature fluctuations or improper concentration management. The resulting crystal formation can block flow passages, reduce heat transfer efficiency, and potentially cause mechanical damage to pumps and other components, leading to system failure and costly downtime.
Contamination from external sources, including lubricants, process fluids, and environmental impurities, introduces additional destabilizing factors. These contaminants can trigger unexpected chemical reactions, alter solution properties, and accelerate corrosion processes, making contamination control a critical but challenging aspect of LiBr system management.
The cumulative effect of these degradation mechanisms is reduced coefficient of performance (COP), increased maintenance requirements, and shortened equipment lifespan. Current inhibitor technologies provide only partial protection, with many commercial additives offering effective corrosion inhibition but limited protection against other degradation pathways. Additionally, many inhibitors lose effectiveness over time or under extreme operating conditions, necessitating regular solution monitoring and maintenance.
These interconnected challenges highlight the need for comprehensive approaches to LiBr solution stability that address multiple degradation mechanisms simultaneously while maintaining compatibility with system materials and operational parameters.
Corrosion remains the most persistent challenge, as LiBr solutions are inherently corrosive to many common system materials, particularly metals. This corrosivity increases dramatically at higher concentrations and temperatures, conditions frequently encountered in absorption chillers. The corrosion process not only damages critical system components but also introduces metal ions into the solution, which catalyze further degradation reactions and reduce heat transfer efficiency.
Oxidation of the LiBr solution occurs when dissolved oxygen reacts with bromide ions, forming free bromine and other oxidative species. This process is particularly problematic in systems with air leakage or inadequate purging mechanisms. The resulting oxidation products alter solution properties and contribute to increased corrosivity, creating a destructive feedback loop that accelerates overall system deterioration.
Thermal decomposition represents another significant stability challenge, especially in high-temperature generator sections where temperatures can exceed 150°C. Under these conditions, LiBr can undergo chemical transformations that produce hydrogen bromide and other degradation products, compromising solution performance and potentially damaging system components through increased acidity.
Crystallization risk poses operational hazards when solution concentration exceeds solubility limits, typically during temperature fluctuations or improper concentration management. The resulting crystal formation can block flow passages, reduce heat transfer efficiency, and potentially cause mechanical damage to pumps and other components, leading to system failure and costly downtime.
Contamination from external sources, including lubricants, process fluids, and environmental impurities, introduces additional destabilizing factors. These contaminants can trigger unexpected chemical reactions, alter solution properties, and accelerate corrosion processes, making contamination control a critical but challenging aspect of LiBr system management.
The cumulative effect of these degradation mechanisms is reduced coefficient of performance (COP), increased maintenance requirements, and shortened equipment lifespan. Current inhibitor technologies provide only partial protection, with many commercial additives offering effective corrosion inhibition but limited protection against other degradation pathways. Additionally, many inhibitors lose effectiveness over time or under extreme operating conditions, necessitating regular solution monitoring and maintenance.
These interconnected challenges highlight the need for comprehensive approaches to LiBr solution stability that address multiple degradation mechanisms simultaneously while maintaining compatibility with system materials and operational parameters.
Existing Degradation Mitigation Strategies
01 Prevention of lithium bromide solution degradation in absorption refrigeration systems
Various methods and devices are employed to prevent degradation of lithium bromide solutions in absorption refrigeration systems. These include specialized purification systems, filtration mechanisms, and circulation techniques that remove impurities and prevent corrosion. By maintaining the solution quality, these systems ensure efficient operation and extended service life of absorption refrigeration equipment.- Prevention of lithium bromide solution degradation in absorption refrigeration systems: Absorption refrigeration systems using lithium bromide solutions can experience degradation over time. Various methods have been developed to prevent this degradation, including the use of corrosion inhibitors, improved system designs, and regular maintenance procedures. These approaches help maintain the efficiency and longevity of the refrigeration system by preventing the breakdown of the lithium bromide solution.
- Purification methods for degraded lithium bromide solutions: When lithium bromide solutions become degraded, various purification methods can be employed to restore their functionality. These methods include filtration systems, chemical treatments, and regeneration processes that remove impurities and contaminants. Purification helps extend the useful life of the solution and maintains the efficiency of absorption refrigeration systems.
- Monitoring and detection systems for lithium bromide solution degradation: Advanced monitoring and detection systems have been developed to identify early signs of lithium bromide solution degradation. These systems use sensors, analytical techniques, and automated monitoring to measure solution properties such as concentration, pH, and contaminant levels. Early detection allows for timely intervention before significant degradation occurs.
- Additives to stabilize lithium bromide solutions: Various additives can be incorporated into lithium bromide solutions to enhance their stability and prevent degradation. These additives include corrosion inhibitors, pH stabilizers, and anti-oxidants that protect the solution from chemical breakdown. The use of appropriate additives can significantly extend the operational life of lithium bromide solutions in absorption refrigeration systems.
- Equipment design improvements to minimize lithium bromide solution degradation: Innovative equipment designs have been developed to minimize the degradation of lithium bromide solutions. These designs include improved heat exchangers, specialized materials that resist corrosion, and optimized flow patterns that reduce thermal stress on the solution. Such design improvements help maintain solution integrity and system efficiency over extended periods of operation.
02 Additives for stabilizing lithium bromide solutions
Chemical additives are incorporated into lithium bromide solutions to enhance stability and prevent degradation. These additives include corrosion inhibitors, pH stabilizers, and oxygen scavengers that protect the solution from oxidation and other degradation mechanisms. The stabilized solutions demonstrate improved performance and longevity in absorption refrigeration and heat pump applications.Expand Specific Solutions03 Monitoring and control systems for lithium bromide solution quality
Advanced monitoring and control systems are implemented to continuously assess lithium bromide solution quality and detect early signs of degradation. These systems utilize sensors, analyzers, and automated control mechanisms to maintain optimal solution parameters and trigger corrective actions when degradation is detected. Real-time monitoring ensures system reliability and prevents catastrophic failures due to solution degradation.Expand Specific Solutions04 Regeneration and purification methods for degraded lithium bromide solutions
Various techniques are employed to regenerate and purify degraded lithium bromide solutions, extending their useful life and restoring performance. These methods include filtration, distillation, ion exchange, and chemical treatments that remove contaminants and breakdown products. Regeneration processes can be performed either in-situ or by extracting the solution for external treatment.Expand Specific Solutions05 Heat exchange system designs to minimize lithium bromide degradation
Specialized heat exchange system designs are developed to minimize thermal stress and degradation of lithium bromide solutions. These designs incorporate materials resistant to corrosion, optimized flow patterns, and temperature control mechanisms that prevent localized overheating. By maintaining appropriate operating conditions, these systems significantly reduce solution degradation rates and extend equipment service life.Expand Specific Solutions
Key Industry Players and Solution Providers
The lithium bromide solution degradation minimization market is in a growth phase, driven by increasing demand for absorption refrigeration systems in HVAC applications. The global market size is expanding steadily, with projections indicating significant growth as energy efficiency concerns rise. Technologically, the field is moderately mature but evolving, with companies like LANXESS and Ganfeng Lithium leading innovation in stabilization additives and corrosion inhibitors. Bromine Compounds Ltd. and Chemetall GmbH have developed proprietary formulations to extend solution life, while academic institutions like Beijing University of Technology and Central South University are advancing fundamental research. Samsung Electronics and Toyota Industries are integrating these technologies into commercial cooling systems, creating a competitive landscape balanced between chemical specialists and end-product manufacturers.
Bromine Compounds Ltd.
Technical Solution: Bromine Compounds Ltd. has developed a proprietary stabilization system for lithium bromide (LiBr) solutions used in absorption refrigeration systems. Their approach involves the addition of specific corrosion inhibitors including lithium chromate and lithium molybdate at controlled concentrations (500-1500 ppm) to prevent metal surface degradation. The company has also pioneered an oxygen scavenging mechanism using carefully selected reducing agents that react with dissolved oxygen before it can participate in oxidation reactions with bromide ions. Their solution maintains pH levels between 9.5-11.0 through automated buffer systems, significantly reducing hydrolysis reactions that lead to the formation of hydrobromic acid. Additionally, they've implemented proprietary metal passivation techniques that form protective layers on heat exchanger surfaces, reducing metal ion catalyzed degradation reactions.
Strengths: Comprehensive multi-component approach addressing multiple degradation pathways simultaneously; proven effectiveness in extending solution life by up to 40% in field applications. Weaknesses: Requires precise chemical balance maintenance; some inhibitors used may face environmental restrictions in certain markets; higher initial cost compared to basic LiBr solutions.
LANXESS Deutschland GmbH
Technical Solution: LANXESS has developed an advanced stabilization technology for lithium bromide solutions called LiBr-STABIL™. This system employs a combination of organic and inorganic stabilizers that work synergistically to prevent degradation. Their approach focuses on three key mechanisms: 1) Metal ion chelation using proprietary organic compounds that sequester metal ions like iron and copper which catalyze degradation reactions; 2) Alkalinity control through buffering agents that maintain optimal pH between 10.0-11.5, preventing hydrolysis reactions; and 3) Oxidation inhibition using selected antioxidants that neutralize reactive oxygen species. LANXESS has also developed specialized analytical tools for monitoring solution health, including spectroscopic methods that can detect early signs of degradation before system performance is affected. Their technology has been validated in industrial absorption chillers operating under various conditions, demonstrating significant improvements in solution longevity and system efficiency maintenance over time.
Strengths: Comprehensive solution addressing multiple degradation pathways; includes monitoring technology for preventive maintenance; compatible with existing absorption chiller systems without major modifications. Weaknesses: Requires periodic testing and additive replenishment; higher initial cost compared to conventional solutions; performance may vary depending on specific operating conditions and system materials.
Critical Patents and Research on LiBr Stabilization
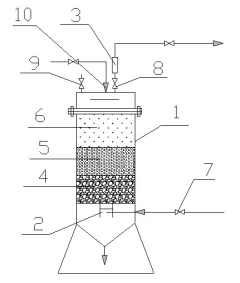
Lithium bromide solution regeneration device
PatentInactiveCN201858825U
Innovation
- Design a lithium bromide solution regeneration device that includes at least two layers of sand filters with different particle sizes. It is installed between the solution pump and the automatic air pumping device. Mechanical impurities are removed through the sand filters that decrease in sequence from bottom to top to ensure that lithium bromide Cleanliness of the solution.
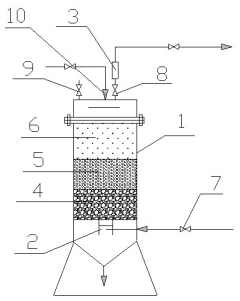
Lithium bromide solution regeneration device of refrigerating machine
PatentInactiveCN213955698U
Innovation
- Design a lithium bromide solution regeneration device for refrigerators, including a tank, a partition, a filter rod and a stirring and adsorption mechanism. The filter rod filters impurities through the filter element and connecting pipe. The first stirring rod and adsorption piece perform stirring and adsorption, and the driving mechanism realizes stirring. The circumferential rotation of the rod effectively removes copper ions, iron ions and organic impurities in the solution.
Environmental Impact of LiBr Systems
Lithium bromide (LiBr) absorption systems, while offering energy-efficient cooling solutions, present significant environmental concerns that warrant careful consideration. The environmental footprint of these systems extends beyond their operational phase to include manufacturing, maintenance, and disposal processes. When LiBr solution degrades, it can lead to the release of corrosive compounds and bromide ions into the environment, potentially contaminating soil and water resources if not properly managed.
Water bodies exposed to LiBr discharge may experience altered pH levels and increased salinity, which can disrupt aquatic ecosystems and harm sensitive species. The bromide ions can also react with naturally occurring organic matter to form brominated disinfection byproducts (DBPs), some of which are classified as potential carcinogens. This poses indirect risks to human health through contaminated drinking water sources.
Air quality concerns arise primarily during system maintenance or in the event of leaks. Hydrogen bromide gas, which can form when LiBr solution interacts with moisture in the air, is a respiratory irritant that can contribute to localized air pollution. While these emissions are typically minimal during normal operation, they represent an environmental consideration in densely populated areas.
From a resource perspective, lithium extraction for LiBr production raises sustainability questions. Lithium mining operations, particularly in water-scarce regions, can deplete groundwater reserves and disrupt local ecosystems. The growing demand for lithium across various industries has intensified these environmental pressures, highlighting the importance of responsible sourcing practices.
Energy consumption associated with LiBr systems must also be evaluated within their environmental context. Although these systems generally offer energy savings compared to conventional cooling technologies, their environmental benefit depends on the carbon intensity of the energy sources powering them. In regions heavily dependent on fossil fuels, the indirect emissions from operating LiBr systems may partially offset their efficiency advantages.
Minimizing LiBr solution degradation directly contributes to environmental protection by extending system lifespan, reducing waste generation, and decreasing the frequency of potentially harmful maintenance procedures. Advanced degradation prevention techniques, such as vacuum maintenance systems and corrosion inhibitors, not only improve system performance but also enhance environmental sustainability by limiting the need for solution replacement and disposal.
Regulatory frameworks governing the use and disposal of LiBr solutions vary globally, with increasingly stringent environmental standards emerging in many jurisdictions. Companies deploying these systems must navigate complex compliance requirements while implementing best practices for environmental stewardship throughout the technology lifecycle.
Water bodies exposed to LiBr discharge may experience altered pH levels and increased salinity, which can disrupt aquatic ecosystems and harm sensitive species. The bromide ions can also react with naturally occurring organic matter to form brominated disinfection byproducts (DBPs), some of which are classified as potential carcinogens. This poses indirect risks to human health through contaminated drinking water sources.
Air quality concerns arise primarily during system maintenance or in the event of leaks. Hydrogen bromide gas, which can form when LiBr solution interacts with moisture in the air, is a respiratory irritant that can contribute to localized air pollution. While these emissions are typically minimal during normal operation, they represent an environmental consideration in densely populated areas.
From a resource perspective, lithium extraction for LiBr production raises sustainability questions. Lithium mining operations, particularly in water-scarce regions, can deplete groundwater reserves and disrupt local ecosystems. The growing demand for lithium across various industries has intensified these environmental pressures, highlighting the importance of responsible sourcing practices.
Energy consumption associated with LiBr systems must also be evaluated within their environmental context. Although these systems generally offer energy savings compared to conventional cooling technologies, their environmental benefit depends on the carbon intensity of the energy sources powering them. In regions heavily dependent on fossil fuels, the indirect emissions from operating LiBr systems may partially offset their efficiency advantages.
Minimizing LiBr solution degradation directly contributes to environmental protection by extending system lifespan, reducing waste generation, and decreasing the frequency of potentially harmful maintenance procedures. Advanced degradation prevention techniques, such as vacuum maintenance systems and corrosion inhibitors, not only improve system performance but also enhance environmental sustainability by limiting the need for solution replacement and disposal.
Regulatory frameworks governing the use and disposal of LiBr solutions vary globally, with increasingly stringent environmental standards emerging in many jurisdictions. Companies deploying these systems must navigate complex compliance requirements while implementing best practices for environmental stewardship throughout the technology lifecycle.
Material Compatibility and Corrosion Management
Material compatibility represents a critical factor in minimizing lithium bromide (LiBr) solution degradation in absorption refrigeration systems. The interaction between LiBr solution and system components can trigger corrosion processes that not only damage equipment but also accelerate solution degradation through contamination with corrosion products. Research indicates that carbon steel, commonly used in system construction, exhibits particularly high corrosion rates when exposed to LiBr solutions, especially at elevated temperatures and concentrations.
Stainless steel alloys, particularly those with higher molybdenum content such as 316L and 317L grades, demonstrate significantly improved corrosion resistance. However, even these materials can experience pitting and crevice corrosion under certain operating conditions. Titanium and its alloys offer exceptional resistance to LiBr corrosion but present economic challenges due to their higher cost and fabrication complexity.
Copper and copper-nickel alloys occupy an intermediate position in the corrosion resistance spectrum. While they perform better than carbon steel, they remain susceptible to degradation in high-temperature environments or when oxygen contamination occurs. Recent developments in material science have introduced specialized coatings and surface treatments that can enhance the corrosion resistance of less expensive base materials.
Effective corrosion management strategies must address both material selection and operational parameters. The implementation of corrosion inhibitors represents a cost-effective approach to extending equipment life and reducing solution degradation. Molybdate compounds have demonstrated particular effectiveness in LiBr systems, forming protective oxide layers on metal surfaces. Similarly, nitrate-based inhibitors can provide significant protection, though their effectiveness diminishes at higher temperatures.
Regular monitoring of solution pH is essential, as maintaining optimal pH levels (typically between 9.5-11.0) can significantly reduce corrosion rates. The introduction of alkaline additives such as lithium hydroxide helps maintain this protective pH range. Additionally, oxygen scavengers and reducing agents play a crucial role in minimizing oxidation-related degradation by eliminating dissolved oxygen from the solution.
Modern system designs increasingly incorporate materials segregation strategies, utilizing more resistant materials in high-risk areas while employing less expensive options in less vulnerable zones. This approach optimizes both performance and cost-effectiveness. Preventive maintenance protocols, including regular inspection and cleaning to remove deposits that might harbor corrosive microenvironments, further extend system longevity and maintain solution integrity.
Stainless steel alloys, particularly those with higher molybdenum content such as 316L and 317L grades, demonstrate significantly improved corrosion resistance. However, even these materials can experience pitting and crevice corrosion under certain operating conditions. Titanium and its alloys offer exceptional resistance to LiBr corrosion but present economic challenges due to their higher cost and fabrication complexity.
Copper and copper-nickel alloys occupy an intermediate position in the corrosion resistance spectrum. While they perform better than carbon steel, they remain susceptible to degradation in high-temperature environments or when oxygen contamination occurs. Recent developments in material science have introduced specialized coatings and surface treatments that can enhance the corrosion resistance of less expensive base materials.
Effective corrosion management strategies must address both material selection and operational parameters. The implementation of corrosion inhibitors represents a cost-effective approach to extending equipment life and reducing solution degradation. Molybdate compounds have demonstrated particular effectiveness in LiBr systems, forming protective oxide layers on metal surfaces. Similarly, nitrate-based inhibitors can provide significant protection, though their effectiveness diminishes at higher temperatures.
Regular monitoring of solution pH is essential, as maintaining optimal pH levels (typically between 9.5-11.0) can significantly reduce corrosion rates. The introduction of alkaline additives such as lithium hydroxide helps maintain this protective pH range. Additionally, oxygen scavengers and reducing agents play a crucial role in minimizing oxidation-related degradation by eliminating dissolved oxygen from the solution.
Modern system designs increasingly incorporate materials segregation strategies, utilizing more resistant materials in high-risk areas while employing less expensive options in less vulnerable zones. This approach optimizes both performance and cost-effectiveness. Preventive maintenance protocols, including regular inspection and cleaning to remove deposits that might harbor corrosive microenvironments, further extend system longevity and maintain solution integrity.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!