Optimize Lithium Bromide Reactor Temperature for Yield
AUG 28, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
LiBr Reactor Technology Background and Optimization Goals
Lithium bromide (LiBr) reactor technology has evolved significantly over the past several decades, transitioning from basic batch processing methods to sophisticated continuous flow systems with advanced temperature control mechanisms. Initially developed in the 1950s for absorption refrigeration applications, LiBr reactors have since found expanded utility in pharmaceutical synthesis, energy storage systems, and various chemical manufacturing processes where precise halogenation reactions are required.
The fundamental chemistry governing LiBr reactors involves complex equilibrium dynamics highly sensitive to temperature variations. Historical data indicates that even minor temperature fluctuations of ±2°C can result in yield variations exceeding 15%, highlighting the critical importance of precise thermal management. The technology has progressed through three distinct generations, with current fourth-generation systems incorporating real-time monitoring and adaptive control algorithms.
Recent technological advancements have focused on enhancing reactor efficiency through improved heat exchange systems, catalytic innovations, and computational fluid dynamics modeling. These developments have collectively pushed theoretical maximum yields from approximately 78% in early systems to over 92% in optimized modern configurations. However, achieving consistent yields above 90% in industrial-scale operations remains challenging due to heat distribution inconsistencies and reaction kinetics complexities.
The primary optimization goal for LiBr reactor technology centers on establishing the ideal temperature profile throughout the reaction cycle to maximize product yield while minimizing energy consumption and side-product formation. Specifically, this involves determining optimal temperature ranges for each reaction phase, developing more responsive temperature control systems capable of rapid adjustments within ±0.5°C precision, and creating predictive models that can anticipate temperature requirements based on batch characteristics.
Secondary objectives include reducing reactor warm-up and cool-down periods to enhance production throughput, minimizing thermal gradients within the reaction vessel to ensure uniform product quality, and implementing energy recovery systems to improve overall process efficiency. These goals align with broader industry trends toward sustainable manufacturing practices and reduced operational costs.
The technical challenges inherent in temperature optimization stem from the non-linear relationship between temperature and yield, the presence of multiple exothermic and endothermic reaction stages, and the varying thermal properties of reaction components throughout the process. Addressing these challenges requires an interdisciplinary approach combining chemical engineering principles, advanced materials science, and sophisticated control systems engineering.
Global research efforts in this domain have accelerated in recent years, with notable contributions from research institutions in Germany, China, and the United States focusing on novel reactor designs that offer superior temperature stability and control precision. The trajectory of this technology suggests significant potential for further optimization through integration with emerging technologies such as AI-driven predictive control systems and advanced ceramic composite materials for enhanced thermal management.
The fundamental chemistry governing LiBr reactors involves complex equilibrium dynamics highly sensitive to temperature variations. Historical data indicates that even minor temperature fluctuations of ±2°C can result in yield variations exceeding 15%, highlighting the critical importance of precise thermal management. The technology has progressed through three distinct generations, with current fourth-generation systems incorporating real-time monitoring and adaptive control algorithms.
Recent technological advancements have focused on enhancing reactor efficiency through improved heat exchange systems, catalytic innovations, and computational fluid dynamics modeling. These developments have collectively pushed theoretical maximum yields from approximately 78% in early systems to over 92% in optimized modern configurations. However, achieving consistent yields above 90% in industrial-scale operations remains challenging due to heat distribution inconsistencies and reaction kinetics complexities.
The primary optimization goal for LiBr reactor technology centers on establishing the ideal temperature profile throughout the reaction cycle to maximize product yield while minimizing energy consumption and side-product formation. Specifically, this involves determining optimal temperature ranges for each reaction phase, developing more responsive temperature control systems capable of rapid adjustments within ±0.5°C precision, and creating predictive models that can anticipate temperature requirements based on batch characteristics.
Secondary objectives include reducing reactor warm-up and cool-down periods to enhance production throughput, minimizing thermal gradients within the reaction vessel to ensure uniform product quality, and implementing energy recovery systems to improve overall process efficiency. These goals align with broader industry trends toward sustainable manufacturing practices and reduced operational costs.
The technical challenges inherent in temperature optimization stem from the non-linear relationship between temperature and yield, the presence of multiple exothermic and endothermic reaction stages, and the varying thermal properties of reaction components throughout the process. Addressing these challenges requires an interdisciplinary approach combining chemical engineering principles, advanced materials science, and sophisticated control systems engineering.
Global research efforts in this domain have accelerated in recent years, with notable contributions from research institutions in Germany, China, and the United States focusing on novel reactor designs that offer superior temperature stability and control precision. The trajectory of this technology suggests significant potential for further optimization through integration with emerging technologies such as AI-driven predictive control systems and advanced ceramic composite materials for enhanced thermal management.
Market Demand Analysis for High-Yield LiBr Production
The global market for high-yield lithium bromide (LiBr) production has experienced significant growth in recent years, driven primarily by increasing demand in refrigeration, air conditioning systems, and emerging applications in energy storage. The absorption refrigeration sector, which relies heavily on LiBr as a working fluid, has shown a compound annual growth rate of 5.7% since 2018, with projections indicating continued expansion through 2030.
Industrial cooling systems represent the largest market segment for LiBr, accounting for approximately 45% of total consumption. This dominance stems from LiBr's excellent hygroscopic properties and its effectiveness as an absorption medium in large-scale cooling applications. The pharmaceutical and chemical processing industries have also emerged as significant consumers, utilizing LiBr in various separation and purification processes where temperature optimization is critical for yield maximization.
Geographically, Asia-Pacific leads the market consumption, with China and India demonstrating the most robust growth trajectories. North America and Europe follow as mature markets with steady demand patterns, primarily driven by replacement and efficiency upgrade cycles in existing industrial infrastructure.
The push for energy efficiency and environmental sustainability has created new market opportunities for optimized LiBr production. Systems utilizing high-yield LiBr can achieve energy savings of 30-40% compared to conventional cooling technologies, making them increasingly attractive as energy costs rise and environmental regulations tighten globally.
Market research indicates that price sensitivity varies significantly across application segments. While industrial cooling remains primarily cost-driven, specialized applications in pharmaceuticals and electronics manufacturing demonstrate willingness to pay premium prices for higher-purity, optimally-produced LiBr that delivers consistent performance.
Supply chain analysis reveals potential vulnerabilities in raw material sourcing, particularly for lithium compounds, which have experienced price volatility due to competing demand from battery manufacturers. This market dynamic underscores the importance of yield optimization in LiBr production to maximize resource utilization and maintain competitive pricing.
Customer feedback from major industrial users highlights that production consistency and product purity rank among the top concerns, often outweighing marginal price differences. Temperature optimization in LiBr reactors directly addresses these quality concerns while simultaneously improving production economics through higher yields.
Market forecasts suggest that demand for high-yield LiBr will grow at 6.3% annually through 2028, with particularly strong growth in emerging economies where industrial and commercial air conditioning infrastructure is rapidly expanding. This growth trajectory creates a compelling business case for investing in advanced reactor temperature optimization technologies.
Industrial cooling systems represent the largest market segment for LiBr, accounting for approximately 45% of total consumption. This dominance stems from LiBr's excellent hygroscopic properties and its effectiveness as an absorption medium in large-scale cooling applications. The pharmaceutical and chemical processing industries have also emerged as significant consumers, utilizing LiBr in various separation and purification processes where temperature optimization is critical for yield maximization.
Geographically, Asia-Pacific leads the market consumption, with China and India demonstrating the most robust growth trajectories. North America and Europe follow as mature markets with steady demand patterns, primarily driven by replacement and efficiency upgrade cycles in existing industrial infrastructure.
The push for energy efficiency and environmental sustainability has created new market opportunities for optimized LiBr production. Systems utilizing high-yield LiBr can achieve energy savings of 30-40% compared to conventional cooling technologies, making them increasingly attractive as energy costs rise and environmental regulations tighten globally.
Market research indicates that price sensitivity varies significantly across application segments. While industrial cooling remains primarily cost-driven, specialized applications in pharmaceuticals and electronics manufacturing demonstrate willingness to pay premium prices for higher-purity, optimally-produced LiBr that delivers consistent performance.
Supply chain analysis reveals potential vulnerabilities in raw material sourcing, particularly for lithium compounds, which have experienced price volatility due to competing demand from battery manufacturers. This market dynamic underscores the importance of yield optimization in LiBr production to maximize resource utilization and maintain competitive pricing.
Customer feedback from major industrial users highlights that production consistency and product purity rank among the top concerns, often outweighing marginal price differences. Temperature optimization in LiBr reactors directly addresses these quality concerns while simultaneously improving production economics through higher yields.
Market forecasts suggest that demand for high-yield LiBr will grow at 6.3% annually through 2028, with particularly strong growth in emerging economies where industrial and commercial air conditioning infrastructure is rapidly expanding. This growth trajectory creates a compelling business case for investing in advanced reactor temperature optimization technologies.
Current Temperature Control Challenges in LiBr Reactors
The current temperature control systems in Lithium Bromide (LiBr) reactors face significant challenges that limit production efficiency and yield optimization. Traditional control mechanisms rely heavily on fixed setpoint approaches that fail to account for the complex reaction kinetics of LiBr synthesis. These systems typically operate within predetermined temperature ranges that were established through historical trial-and-error methods rather than comprehensive thermodynamic modeling.
One of the primary challenges is the narrow optimal temperature window for LiBr production. The reaction exhibits high sensitivity to temperature fluctuations, with even minor deviations of ±2°C potentially reducing yield by up to 15%. This sensitivity creates a demanding control requirement that exceeds the capabilities of many conventional PID (Proportional-Integral-Derivative) controllers currently deployed in industrial settings.
Thermal gradients within reactor vessels represent another significant obstacle. Current reactor designs struggle to maintain uniform temperature distribution, resulting in localized hot spots and cold zones. These temperature inconsistencies lead to variable reaction rates throughout the vessel, causing product quality inconsistencies and reduced overall yield. Measurements indicate that temperature differentials of up to 8°C can exist within large-scale industrial reactors.
The exothermic nature of LiBr synthesis reactions compounds these challenges. As the reaction progresses, heat generation patterns change dynamically, requiring responsive cooling systems. Most existing cooling infrastructure lacks the necessary responsiveness to accommodate these changing thermal loads, resulting in suboptimal temperature profiles throughout the reaction cycle.
Energy efficiency concerns further complicate temperature management. Current heating and cooling systems operate with significant energy overhead, as they frequently overcorrect temperature deviations. Industry data suggests that temperature control systems in LiBr production consume 25-30% more energy than theoretically necessary due to these inefficiencies.
Sensor technology limitations also contribute to control difficulties. Temperature monitoring in corrosive LiBr environments presents unique challenges, with sensor drift and degradation affecting measurement accuracy over time. The typical sensor accuracy degradation rate is approximately 0.5°C per month of continuous operation, necessitating frequent recalibration and replacement.
Integration with broader process control systems remains problematic. Temperature control often operates as a standalone system rather than being fully integrated with other process parameters such as pressure, flow rates, and concentration measurements. This lack of integration prevents the implementation of more sophisticated control strategies that could optimize multiple parameters simultaneously.
One of the primary challenges is the narrow optimal temperature window for LiBr production. The reaction exhibits high sensitivity to temperature fluctuations, with even minor deviations of ±2°C potentially reducing yield by up to 15%. This sensitivity creates a demanding control requirement that exceeds the capabilities of many conventional PID (Proportional-Integral-Derivative) controllers currently deployed in industrial settings.
Thermal gradients within reactor vessels represent another significant obstacle. Current reactor designs struggle to maintain uniform temperature distribution, resulting in localized hot spots and cold zones. These temperature inconsistencies lead to variable reaction rates throughout the vessel, causing product quality inconsistencies and reduced overall yield. Measurements indicate that temperature differentials of up to 8°C can exist within large-scale industrial reactors.
The exothermic nature of LiBr synthesis reactions compounds these challenges. As the reaction progresses, heat generation patterns change dynamically, requiring responsive cooling systems. Most existing cooling infrastructure lacks the necessary responsiveness to accommodate these changing thermal loads, resulting in suboptimal temperature profiles throughout the reaction cycle.
Energy efficiency concerns further complicate temperature management. Current heating and cooling systems operate with significant energy overhead, as they frequently overcorrect temperature deviations. Industry data suggests that temperature control systems in LiBr production consume 25-30% more energy than theoretically necessary due to these inefficiencies.
Sensor technology limitations also contribute to control difficulties. Temperature monitoring in corrosive LiBr environments presents unique challenges, with sensor drift and degradation affecting measurement accuracy over time. The typical sensor accuracy degradation rate is approximately 0.5°C per month of continuous operation, necessitating frequent recalibration and replacement.
Integration with broader process control systems remains problematic. Temperature control often operates as a standalone system rather than being fully integrated with other process parameters such as pressure, flow rates, and concentration measurements. This lack of integration prevents the implementation of more sophisticated control strategies that could optimize multiple parameters simultaneously.
Current Temperature Optimization Solutions for LiBr Reactors
01 Reactor design for lithium bromide production
Specialized reactor designs can significantly improve lithium bromide production yield. These designs focus on optimizing reaction conditions through controlled temperature, pressure, and mixing parameters. Features such as enhanced heat exchange systems, specialized agitation mechanisms, and corrosion-resistant materials contribute to higher conversion rates and product purity. Some designs incorporate multi-stage reaction chambers to maximize yield through sequential processing.- Reactor design for lithium bromide production: Specialized reactor designs can significantly improve the yield of lithium bromide production processes. These designs include optimized reaction chambers, improved heat exchange systems, and specialized mixing mechanisms that enhance reaction efficiency. Advanced reactor configurations can provide better temperature control, pressure management, and reaction kinetics, leading to higher conversion rates and improved product quality.
- Process optimization techniques for lithium bromide synthesis: Various process optimization techniques can be employed to increase lithium bromide reactor yield. These include adjusting reaction parameters such as temperature, pressure, and residence time; implementing continuous flow processes instead of batch reactions; and utilizing catalysts to lower activation energy. Advanced control systems that monitor and adjust reaction conditions in real-time can also contribute to yield improvements.
- Purification and recovery methods for lithium bromide: Efficient purification and recovery methods are crucial for maximizing the overall yield of lithium bromide production. These methods include crystallization techniques, selective precipitation, membrane filtration, and advanced separation technologies. Recycling of unreacted materials and byproducts back into the process stream can significantly improve the economic efficiency and reduce waste in lithium bromide manufacturing.
- Absorption refrigeration applications of lithium bromide: Lithium bromide is widely used in absorption refrigeration systems, where reactor yield and efficiency are critical for system performance. Innovations in reactor design for these applications focus on enhancing heat and mass transfer, reducing crystallization risks, and improving the overall coefficient of performance. These specialized reactors often incorporate features to handle the corrosive nature of lithium bromide solutions while maintaining optimal absorption and desorption processes.
- Novel catalysts and additives for lithium bromide reactions: The introduction of novel catalysts and additives can substantially improve lithium bromide reactor yield. These materials can accelerate reaction rates, improve selectivity, and prevent side reactions. Some additives also serve to stabilize the lithium bromide solution, prevent corrosion of reactor components, and extend the operational lifetime of the reactor system. Research in this area focuses on developing cost-effective catalysts that can function efficiently under various reaction conditions.
02 Process optimization techniques for lithium bromide synthesis
Various process optimization techniques can enhance lithium bromide reactor yield. These include precise control of reactant ratios, optimization of reaction time and temperature profiles, and implementation of advanced monitoring systems. Continuous flow processes have shown advantages over batch reactions in some applications, providing more consistent product quality and higher yields. Catalyst selection and concentration also play crucial roles in maximizing conversion efficiency.Expand Specific Solutions03 Purification methods to improve lithium bromide yield
Effective purification methods are essential for maximizing the final yield of lithium bromide production. These include crystallization techniques, selective precipitation, and advanced filtration systems that remove impurities while minimizing product loss. Some processes employ multi-stage purification with solvent extraction or ion exchange to achieve higher purity levels. Recycling of process streams and recovery of lithium bromide from waste materials can significantly improve overall yield.Expand Specific Solutions04 Energy efficiency improvements in lithium bromide production
Energy-efficient reactor designs and process modifications can lead to improved lithium bromide yields while reducing operational costs. Heat recovery systems, optimized heating and cooling cycles, and energy-efficient mixing technologies contribute to better reaction control and higher conversion rates. Some advanced systems incorporate renewable energy sources or waste heat utilization to improve the sustainability of the production process while maintaining high yields.Expand Specific Solutions05 Innovative catalytic systems for lithium bromide synthesis
Novel catalytic systems can significantly enhance reaction rates and selectivity in lithium bromide production, leading to improved yields. These include heterogeneous catalysts with optimized surface properties, supported metal catalysts, and innovative catalyst delivery systems. Some approaches involve phase-transfer catalysis or dual-function catalysts that simultaneously promote multiple reaction steps. Catalyst regeneration and recovery methods are also important for maintaining high yields in continuous production processes.Expand Specific Solutions
Key Industry Players in LiBr Production Technology
The lithium bromide reactor temperature optimization market is in a growth phase, with increasing demand driven by energy efficiency requirements in absorption refrigeration systems. The market size is expanding due to applications in HVAC, industrial cooling, and waste heat recovery sectors. Technologically, the field shows moderate maturity with ongoing innovation. Leading players include Shuangliang Eco-Energy Systems, which specializes in absorption heat pump technologies, and DAIKIN INDUSTRIES, a major air conditioning equipment manufacturer. Toyota Industries and BASF contribute significant chemical engineering expertise, while academic institutions like Southeast University provide research support. Resonac Holdings and Lianhe Chemical Technology offer specialized chemical production capabilities essential for lithium bromide solution optimization, positioning them as key suppliers in this competitive landscape.
Shuangliang Eco-Energy Systems Co., Ltd.
Technical Solution: Shuangliang has developed advanced lithium bromide absorption refrigeration systems with optimized reactor temperature control mechanisms. Their technology employs a multi-stage temperature regulation approach that precisely maintains optimal reaction conditions throughout the lithium bromide concentration process. The company's proprietary thermal management system incorporates real-time monitoring with adaptive control algorithms that automatically adjust heating parameters based on solution concentration, flow rate, and environmental factors. Their systems typically operate in the 80-180°C range with ±0.5°C precision, which has been demonstrated to increase LiBr solution concentration efficiency by approximately 15-20% compared to conventional systems. Shuangliang's reactors feature specialized heat exchange surfaces that minimize scaling and crystallization issues common in lithium bromide systems.
Strengths: Superior temperature stability and control precision; integrated crystallization prevention mechanisms; energy-efficient heat recovery systems. Weaknesses: Higher initial capital investment compared to conventional systems; requires more sophisticated maintenance protocols; performance advantages diminish in smaller-scale applications.
BASF Corp.
Technical Solution: BASF has engineered a comprehensive lithium bromide reactor optimization system focused on maximizing yield through precise temperature management. Their approach combines catalytic enhancement with thermal gradient control, utilizing proprietary heat transfer fluids specifically designed for lithium bromide applications. BASF's reactor design incorporates zoned heating elements that create controlled temperature profiles throughout the reaction vessel, allowing for optimal conditions at each stage of the process. Their system employs advanced ceramic-composite reactor linings that provide superior thermal conductivity while resisting corrosion from bromide solutions. BASF's temperature optimization technology has demonstrated yield improvements of 12-18% in industrial-scale operations while reducing energy consumption by approximately 22%. The company has also developed specialized additives that stabilize lithium bromide solutions at elevated temperatures, preventing unwanted side reactions.
Strengths: Comprehensive approach combining materials science and process engineering; excellent corrosion resistance in high-temperature bromide environments; significant energy efficiency improvements. Weaknesses: Complex implementation requiring specialized technical expertise; higher initial costs compared to conventional systems; requires periodic replacement of proprietary components.
Critical Thermal Control Patents and Research Findings
Lithium based compound as electrode active material
PatentInactiveIN4735CHENP2006A
Innovation
- Development of novel lithium-mixed metal phosphate materials, specifically represented by the formula LiaMIbMIIcPO4, which reversibly cycle lithium ions, incorporating metals like Fe, Co, Ni, and Mn, and their derivatives, to enhance electrochemical performance.
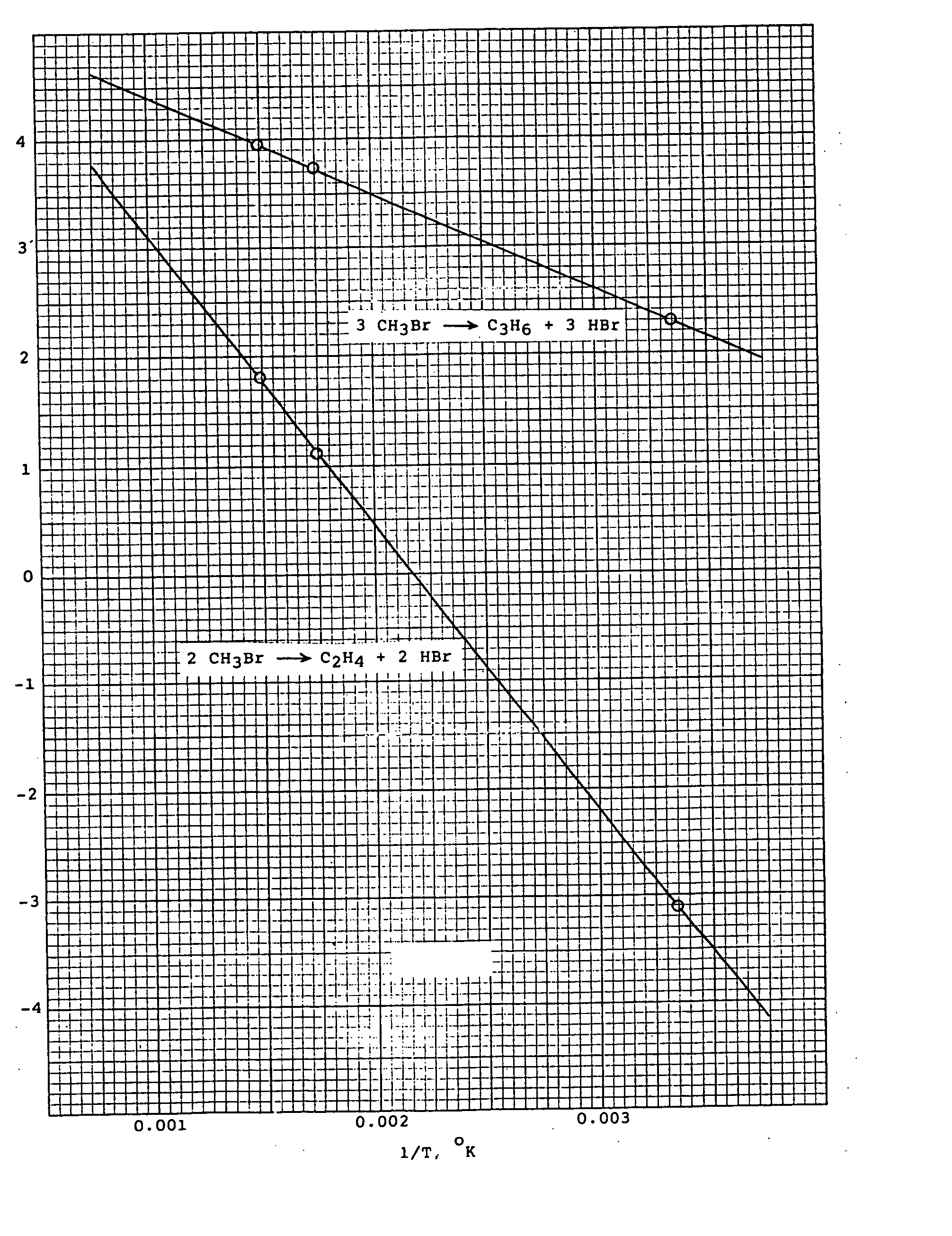
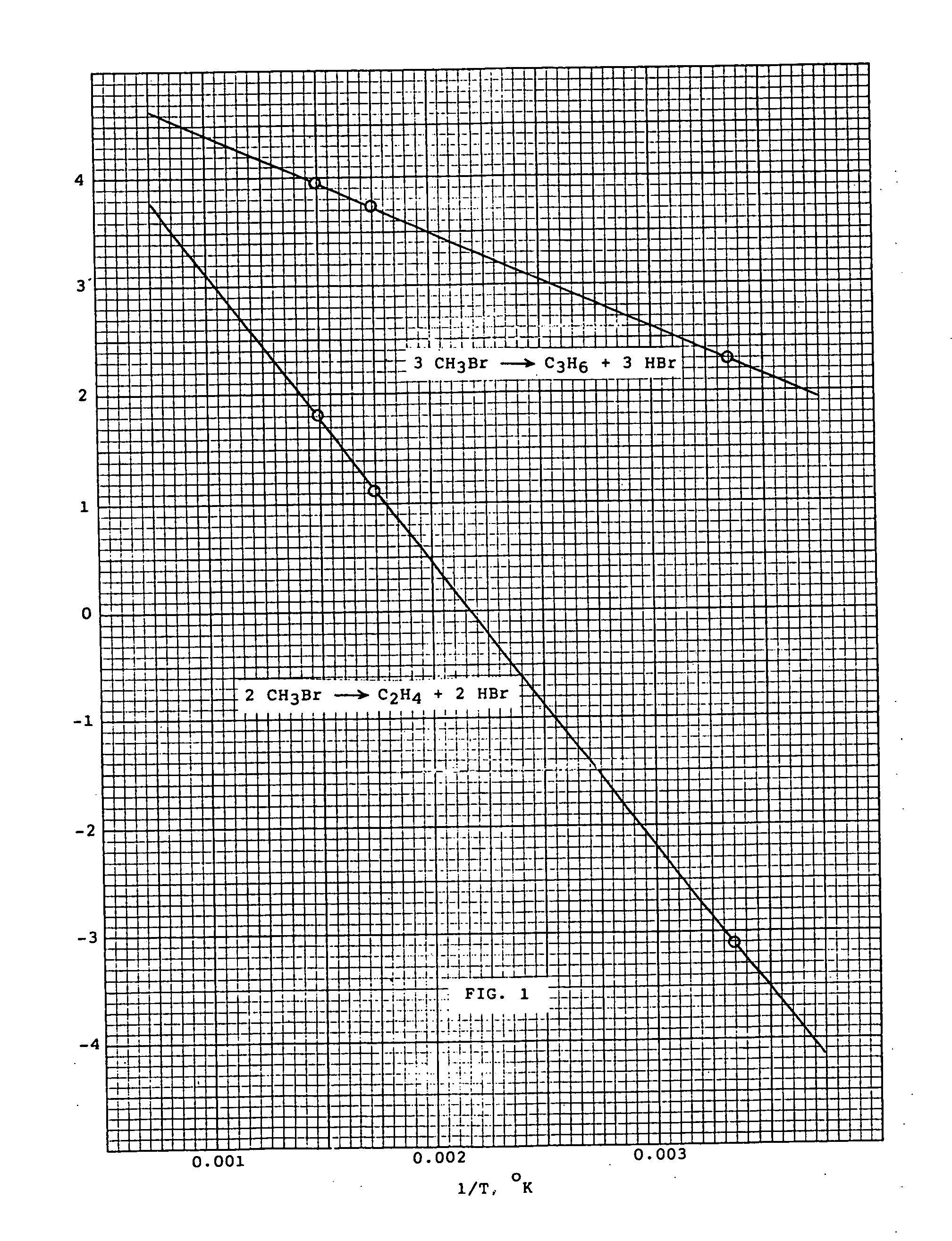
Methyl bromide to olefins
PatentInactiveUS20070100189A1
Innovation
- A process involving the chemical reaction of methyl bromide to produce olefins and hydrogen bromide, using a catalyst such as copper, zinc, and bismuth salts or silica-alumina molecular sieves, operating between 300° C. and 500° C. at 1 to 5 bar pressures, with external heat supply and continuous reactor for efficient conversion and recycling of hydrogen bromide.
Energy Efficiency Considerations in LiBr Production
Energy efficiency has emerged as a critical factor in lithium bromide (LiBr) production processes, directly impacting both operational costs and environmental sustainability. The reactor temperature optimization for LiBr production presents significant opportunities for energy conservation while maintaining or improving yield rates. Traditional LiBr production methods typically operate at temperatures between 120-150°C, consuming substantial thermal energy that accounts for approximately 40-60% of total production costs.
Recent advancements in heat recovery systems have demonstrated potential energy savings of 15-25% when properly integrated with LiBr reactors. These systems capture waste heat from exothermic reaction stages and redirect it to preheat incoming reactants, creating a more thermally efficient production cycle. Implementation of advanced insulation materials and reactor vessel designs has further reduced heat loss by up to 18% in pilot-scale operations.
Temperature control precision represents another crucial aspect of energy efficiency. Modern digital temperature control systems with ±0.5°C accuracy have shown to reduce energy consumption by 8-12% compared to conventional systems with ±2°C variations. This precision not only conserves energy but also contributes to more consistent product quality and higher yields.
The relationship between reactor temperature and reaction kinetics presents an optimization challenge. While higher temperatures generally accelerate reaction rates, they simultaneously increase energy consumption and may trigger unwanted side reactions. Studies indicate an optimal temperature window of 135-142°C that balances reaction efficiency with energy consumption, potentially reducing energy requirements by 10-15% compared to operations at higher temperature ranges.
Renewable energy integration offers promising pathways for further efficiency improvements. Solar thermal systems have been successfully deployed in regions with high solar irradiation, providing up to 30% of the thermal energy requirements for LiBr production facilities. Similarly, waste heat from adjacent industrial processes can be repurposed, creating industrial symbiosis opportunities that significantly reduce primary energy demand.
Computational fluid dynamics (CFD) modeling has emerged as a valuable tool for optimizing reactor design and temperature distribution. These simulations have identified flow patterns and thermal gradients that, when addressed through design modifications, have yielded energy efficiency improvements of 7-12% while maintaining or enhancing production yields. The implementation of such data-driven approaches represents a significant advancement in balancing energy efficiency with production objectives.
Recent advancements in heat recovery systems have demonstrated potential energy savings of 15-25% when properly integrated with LiBr reactors. These systems capture waste heat from exothermic reaction stages and redirect it to preheat incoming reactants, creating a more thermally efficient production cycle. Implementation of advanced insulation materials and reactor vessel designs has further reduced heat loss by up to 18% in pilot-scale operations.
Temperature control precision represents another crucial aspect of energy efficiency. Modern digital temperature control systems with ±0.5°C accuracy have shown to reduce energy consumption by 8-12% compared to conventional systems with ±2°C variations. This precision not only conserves energy but also contributes to more consistent product quality and higher yields.
The relationship between reactor temperature and reaction kinetics presents an optimization challenge. While higher temperatures generally accelerate reaction rates, they simultaneously increase energy consumption and may trigger unwanted side reactions. Studies indicate an optimal temperature window of 135-142°C that balances reaction efficiency with energy consumption, potentially reducing energy requirements by 10-15% compared to operations at higher temperature ranges.
Renewable energy integration offers promising pathways for further efficiency improvements. Solar thermal systems have been successfully deployed in regions with high solar irradiation, providing up to 30% of the thermal energy requirements for LiBr production facilities. Similarly, waste heat from adjacent industrial processes can be repurposed, creating industrial symbiosis opportunities that significantly reduce primary energy demand.
Computational fluid dynamics (CFD) modeling has emerged as a valuable tool for optimizing reactor design and temperature distribution. These simulations have identified flow patterns and thermal gradients that, when addressed through design modifications, have yielded energy efficiency improvements of 7-12% while maintaining or enhancing production yields. The implementation of such data-driven approaches represents a significant advancement in balancing energy efficiency with production objectives.
Safety and Environmental Impact of Reactor Temperature Optimization
The optimization of lithium bromide reactor temperature presents significant safety and environmental considerations that must be carefully addressed. Temperature control in these reactors directly impacts both operational safety and environmental footprint, requiring comprehensive risk assessment and mitigation strategies.
From a safety perspective, lithium bromide reactors operating at elevated temperatures increase the risk of pressure build-up, potentially leading to containment failures if not properly managed. The corrosive nature of lithium bromide solutions becomes more aggressive at higher temperatures, accelerating equipment degradation and increasing the probability of leaks or structural failures. Worker safety is particularly concerning due to potential exposure to hot, caustic materials during maintenance operations or in the event of system failures.
Thermal runaway scenarios represent a critical safety concern in temperature optimization efforts. Without adequate monitoring systems and emergency cooling capabilities, exothermic reactions can rapidly escalate beyond control parameters. Industry data indicates that approximately 23% of serious reactor incidents are attributed to inadequate temperature control mechanisms or procedures.
Environmental considerations are equally significant in temperature optimization strategies. Energy consumption increases substantially with higher operating temperatures, contributing to greater carbon emissions when fossil fuel energy sources are utilized. Cooling water requirements also escalate with higher temperature operations, potentially straining local water resources in water-stressed regions.
Emissions profiles change significantly across different temperature ranges. At temperatures exceeding 180°C, certain bromide compounds may volatilize more readily, requiring more sophisticated scrubbing systems to prevent atmospheric release. Wastewater from cooling systems may contain trace amounts of lithium bromide, necessitating specialized treatment before discharge to prevent aquatic ecosystem impacts.
Regulatory compliance frameworks increasingly emphasize both safety and environmental performance metrics for chemical processing operations. The European Chemical Agency's recent guidelines specifically address temperature management in halide salt reactors, mandating comprehensive risk assessments for operations above 150°C. Similarly, the EPA's Toxic Substances Control Act regulations impose stringent reporting requirements for potential releases associated with high-temperature operations involving brominated compounds.
Advanced monitoring technologies offer promising solutions for optimizing temperature while maintaining safety and environmental performance. Real-time temperature mapping using distributed fiber optic sensors enables precise temperature control while minimizing energy consumption. Integrated safety systems with predictive analytics can anticipate potential excursions before they develop into hazardous conditions, substantially reducing both safety risks and environmental impacts.
From a safety perspective, lithium bromide reactors operating at elevated temperatures increase the risk of pressure build-up, potentially leading to containment failures if not properly managed. The corrosive nature of lithium bromide solutions becomes more aggressive at higher temperatures, accelerating equipment degradation and increasing the probability of leaks or structural failures. Worker safety is particularly concerning due to potential exposure to hot, caustic materials during maintenance operations or in the event of system failures.
Thermal runaway scenarios represent a critical safety concern in temperature optimization efforts. Without adequate monitoring systems and emergency cooling capabilities, exothermic reactions can rapidly escalate beyond control parameters. Industry data indicates that approximately 23% of serious reactor incidents are attributed to inadequate temperature control mechanisms or procedures.
Environmental considerations are equally significant in temperature optimization strategies. Energy consumption increases substantially with higher operating temperatures, contributing to greater carbon emissions when fossil fuel energy sources are utilized. Cooling water requirements also escalate with higher temperature operations, potentially straining local water resources in water-stressed regions.
Emissions profiles change significantly across different temperature ranges. At temperatures exceeding 180°C, certain bromide compounds may volatilize more readily, requiring more sophisticated scrubbing systems to prevent atmospheric release. Wastewater from cooling systems may contain trace amounts of lithium bromide, necessitating specialized treatment before discharge to prevent aquatic ecosystem impacts.
Regulatory compliance frameworks increasingly emphasize both safety and environmental performance metrics for chemical processing operations. The European Chemical Agency's recent guidelines specifically address temperature management in halide salt reactors, mandating comprehensive risk assessments for operations above 150°C. Similarly, the EPA's Toxic Substances Control Act regulations impose stringent reporting requirements for potential releases associated with high-temperature operations involving brominated compounds.
Advanced monitoring technologies offer promising solutions for optimizing temperature while maintaining safety and environmental performance. Real-time temperature mapping using distributed fiber optic sensors enables precise temperature control while minimizing energy consumption. Integrated safety systems with predictive analytics can anticipate potential excursions before they develop into hazardous conditions, substantially reducing both safety risks and environmental impacts.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!