Analyzing Impurity Effects on Lithium Bromide Solution Efficacy
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
LiBr Solution Background and Research Objectives
Lithium bromide (LiBr) solutions have been extensively utilized in absorption refrigeration systems since the early 20th century, with significant advancements occurring during the 1950s and 1960s. These solutions function as absorbents for water vapor in absorption chillers, leveraging their hygroscopic properties to enable efficient cooling cycles. The fundamental principle relies on LiBr's ability to absorb water vapor at low pressure, creating a concentration gradient that drives the refrigeration process.
The evolution of LiBr solution technology has been marked by continuous improvements in solution stability, heat transfer efficiency, and corrosion resistance. Early systems suffered from crystallization issues and limited efficiency, while modern applications benefit from enhanced formulations and system designs. Recent technological trends indicate a growing interest in developing more environmentally friendly absorption systems with higher coefficient of performance (COP) values.
Impurities in LiBr solutions represent a critical challenge that has persisted throughout the technology's development. These contaminants can originate from various sources, including manufacturing processes, system corrosion, and operational degradation. Common impurities include metal ions (Fe, Cu, Ni), non-condensable gases, and organic compounds that accumulate during system operation.
The primary objective of this research is to comprehensively analyze how different types and concentrations of impurities affect the thermophysical properties and overall efficacy of LiBr solutions in absorption refrigeration applications. Specifically, we aim to quantify the impact of impurities on critical parameters such as vapor pressure, absorption capacity, thermal conductivity, and viscosity across varying concentration levels and operating temperatures.
Secondary objectives include developing predictive models for solution performance degradation based on impurity profiles, establishing threshold values for impurity concentrations beyond which system performance becomes significantly compromised, and exploring potential mitigation strategies to maintain solution efficacy despite impurity presence.
This research addresses the growing industry demand for more reliable and efficient absorption cooling systems, particularly as energy efficiency and environmental considerations drive increased adoption of heat-driven cooling technologies. By enhancing our understanding of impurity effects, we can contribute to the development of more robust LiBr solutions with extended operational lifespans and improved performance stability.
The findings from this investigation will provide valuable insights for system designers, maintenance engineers, and solution manufacturers, potentially leading to new purification techniques, improved solution formulations, and enhanced system monitoring protocols that can detect and address impurity-related issues before they significantly impact system performance.
The evolution of LiBr solution technology has been marked by continuous improvements in solution stability, heat transfer efficiency, and corrosion resistance. Early systems suffered from crystallization issues and limited efficiency, while modern applications benefit from enhanced formulations and system designs. Recent technological trends indicate a growing interest in developing more environmentally friendly absorption systems with higher coefficient of performance (COP) values.
Impurities in LiBr solutions represent a critical challenge that has persisted throughout the technology's development. These contaminants can originate from various sources, including manufacturing processes, system corrosion, and operational degradation. Common impurities include metal ions (Fe, Cu, Ni), non-condensable gases, and organic compounds that accumulate during system operation.
The primary objective of this research is to comprehensively analyze how different types and concentrations of impurities affect the thermophysical properties and overall efficacy of LiBr solutions in absorption refrigeration applications. Specifically, we aim to quantify the impact of impurities on critical parameters such as vapor pressure, absorption capacity, thermal conductivity, and viscosity across varying concentration levels and operating temperatures.
Secondary objectives include developing predictive models for solution performance degradation based on impurity profiles, establishing threshold values for impurity concentrations beyond which system performance becomes significantly compromised, and exploring potential mitigation strategies to maintain solution efficacy despite impurity presence.
This research addresses the growing industry demand for more reliable and efficient absorption cooling systems, particularly as energy efficiency and environmental considerations drive increased adoption of heat-driven cooling technologies. By enhancing our understanding of impurity effects, we can contribute to the development of more robust LiBr solutions with extended operational lifespans and improved performance stability.
The findings from this investigation will provide valuable insights for system designers, maintenance engineers, and solution manufacturers, potentially leading to new purification techniques, improved solution formulations, and enhanced system monitoring protocols that can detect and address impurity-related issues before they significantly impact system performance.
Market Analysis of LiBr Absorption Systems
The global market for Lithium Bromide (LiBr) absorption systems has been experiencing steady growth, primarily driven by increasing energy efficiency requirements and the rising demand for sustainable cooling solutions. The market size was valued at approximately $1.2 billion in 2022 and is projected to reach $1.8 billion by 2028, representing a compound annual growth rate (CAGR) of 6.7% during the forecast period.
Asia-Pacific currently dominates the LiBr absorption systems market, accounting for over 40% of the global share. This regional dominance is attributed to rapid industrialization, increasing construction activities, and supportive government policies promoting energy-efficient technologies in countries like China, Japan, and South Korea. Japan, in particular, has been at the forefront of LiBr absorption technology development and implementation.
North America and Europe follow as significant markets, driven by stringent environmental regulations and growing emphasis on reducing carbon footprints across industrial and commercial sectors. The Middle East is emerging as a promising market due to the high cooling demands and abundant waste heat availability from industrial processes.
By application segment, industrial cooling represents the largest market share at 45%, followed by commercial building air conditioning at 35%. The remaining 20% is distributed among specialized applications such as district cooling systems and process cooling in pharmaceutical and food industries.
The market dynamics are significantly influenced by the performance characteristics of LiBr solutions, with solution purity being a critical factor. End-users are increasingly demanding systems with higher coefficient of performance (COP), which directly correlates with the quality and purity of the LiBr solution used.
Recent market trends indicate growing interest in triple-effect absorption chillers that offer higher efficiency compared to traditional single and double-effect systems. Additionally, hybrid systems combining LiBr absorption technology with conventional vapor compression systems are gaining traction for their operational flexibility and enhanced energy efficiency.
The competitive landscape features established players like Carrier Corporation, Trane Technologies, Johnson Controls, and Thermax Limited, alongside emerging companies focusing on technological innovations to improve system efficiency and reduce maintenance requirements related to solution degradation and crystallization issues.
Market challenges include high initial investment costs, technical complexities in maintaining optimal solution concentration, and competition from alternative cooling technologies. However, the increasing focus on reducing electricity consumption and greenhouse gas emissions continues to drive the adoption of LiBr absorption systems across various end-use industries.
Asia-Pacific currently dominates the LiBr absorption systems market, accounting for over 40% of the global share. This regional dominance is attributed to rapid industrialization, increasing construction activities, and supportive government policies promoting energy-efficient technologies in countries like China, Japan, and South Korea. Japan, in particular, has been at the forefront of LiBr absorption technology development and implementation.
North America and Europe follow as significant markets, driven by stringent environmental regulations and growing emphasis on reducing carbon footprints across industrial and commercial sectors. The Middle East is emerging as a promising market due to the high cooling demands and abundant waste heat availability from industrial processes.
By application segment, industrial cooling represents the largest market share at 45%, followed by commercial building air conditioning at 35%. The remaining 20% is distributed among specialized applications such as district cooling systems and process cooling in pharmaceutical and food industries.
The market dynamics are significantly influenced by the performance characteristics of LiBr solutions, with solution purity being a critical factor. End-users are increasingly demanding systems with higher coefficient of performance (COP), which directly correlates with the quality and purity of the LiBr solution used.
Recent market trends indicate growing interest in triple-effect absorption chillers that offer higher efficiency compared to traditional single and double-effect systems. Additionally, hybrid systems combining LiBr absorption technology with conventional vapor compression systems are gaining traction for their operational flexibility and enhanced energy efficiency.
The competitive landscape features established players like Carrier Corporation, Trane Technologies, Johnson Controls, and Thermax Limited, alongside emerging companies focusing on technological innovations to improve system efficiency and reduce maintenance requirements related to solution degradation and crystallization issues.
Market challenges include high initial investment costs, technical complexities in maintaining optimal solution concentration, and competition from alternative cooling technologies. However, the increasing focus on reducing electricity consumption and greenhouse gas emissions continues to drive the adoption of LiBr absorption systems across various end-use industries.
Current Challenges in LiBr Solution Purity Control
Lithium bromide (LiBr) solutions are widely utilized in absorption refrigeration systems due to their excellent thermodynamic properties. However, the presence of impurities significantly compromises the efficacy of these solutions, presenting a major challenge for industrial applications. Current purity control methods face several critical limitations that hinder optimal system performance and longevity.
The primary challenge lies in identifying and quantifying trace impurities that can dramatically alter solution properties. Even minute concentrations of contaminants such as chromates, iron compounds, and organic materials can catalyze corrosion processes and reduce heat transfer efficiency. Conventional detection methods often lack the sensitivity required to identify these trace elements before they cause significant damage.
Corrosion acceleration represents another substantial challenge, particularly in high-temperature zones of absorption systems. Impurities create localized electrochemical cells on metal surfaces, accelerating material degradation. Current corrosion inhibitors demonstrate limited effectiveness when multiple impurity types coexist in the solution, creating complex chemical interactions that are difficult to mitigate with standard approaches.
Crystallization and precipitation issues emerge as impurity concentrations increase during system operation. These solid formations obstruct flow paths, reduce heat exchange efficiency, and create maintenance challenges. Existing filtration systems struggle to remove precipitates effectively once they form, necessitating costly system shutdowns and cleaning procedures.
Solution stability over extended operational periods presents ongoing difficulties. Temperature cycling and concentration variations can trigger unexpected chemical reactions among impurities, altering solution properties unpredictably. Current stabilizing additives often address single impurity types but fail to maintain comprehensive solution integrity across diverse operating conditions.
Monitoring technologies for real-time impurity detection remain inadequate. Most facilities rely on periodic sampling and laboratory analysis, creating significant lag between contamination events and detection. This reactive approach allows impurities to accumulate to problematic levels before remediation begins, compromising system efficiency and component longevity.
Standardization challenges further complicate purity control efforts. The industry lacks universally accepted purity specifications and testing protocols, leading to inconsistent quality control practices across manufacturers and maintenance providers. This variability makes it difficult to establish reliable performance benchmarks and troubleshooting procedures for impurity-related issues.
Economic constraints also limit implementation of advanced purification technologies. Many existing high-efficiency purification methods carry prohibitive capital and operational costs, particularly for smaller absorption system installations. The cost-benefit analysis often favors accepting reduced efficiency rather than investing in comprehensive purification infrastructure.
The primary challenge lies in identifying and quantifying trace impurities that can dramatically alter solution properties. Even minute concentrations of contaminants such as chromates, iron compounds, and organic materials can catalyze corrosion processes and reduce heat transfer efficiency. Conventional detection methods often lack the sensitivity required to identify these trace elements before they cause significant damage.
Corrosion acceleration represents another substantial challenge, particularly in high-temperature zones of absorption systems. Impurities create localized electrochemical cells on metal surfaces, accelerating material degradation. Current corrosion inhibitors demonstrate limited effectiveness when multiple impurity types coexist in the solution, creating complex chemical interactions that are difficult to mitigate with standard approaches.
Crystallization and precipitation issues emerge as impurity concentrations increase during system operation. These solid formations obstruct flow paths, reduce heat exchange efficiency, and create maintenance challenges. Existing filtration systems struggle to remove precipitates effectively once they form, necessitating costly system shutdowns and cleaning procedures.
Solution stability over extended operational periods presents ongoing difficulties. Temperature cycling and concentration variations can trigger unexpected chemical reactions among impurities, altering solution properties unpredictably. Current stabilizing additives often address single impurity types but fail to maintain comprehensive solution integrity across diverse operating conditions.
Monitoring technologies for real-time impurity detection remain inadequate. Most facilities rely on periodic sampling and laboratory analysis, creating significant lag between contamination events and detection. This reactive approach allows impurities to accumulate to problematic levels before remediation begins, compromising system efficiency and component longevity.
Standardization challenges further complicate purity control efforts. The industry lacks universally accepted purity specifications and testing protocols, leading to inconsistent quality control practices across manufacturers and maintenance providers. This variability makes it difficult to establish reliable performance benchmarks and troubleshooting procedures for impurity-related issues.
Economic constraints also limit implementation of advanced purification technologies. Many existing high-efficiency purification methods carry prohibitive capital and operational costs, particularly for smaller absorption system installations. The cost-benefit analysis often favors accepting reduced efficiency rather than investing in comprehensive purification infrastructure.
Existing Impurity Mitigation Strategies
01 Absorption refrigeration systems using lithium bromide solution
Lithium bromide solution is widely used as an absorbent in absorption refrigeration systems due to its high efficiency in absorbing refrigerant vapor. These systems utilize the hygroscopic properties of lithium bromide to create cooling effects. The solution's concentration and temperature are critical factors affecting the absorption efficiency and overall system performance. Improvements in system design have enhanced the efficacy of lithium bromide solutions in refrigeration applications.- Absorption refrigeration systems using lithium bromide solution: Lithium bromide solution is widely used as an absorbent in absorption refrigeration systems due to its high efficiency in absorbing refrigerant vapor. These systems utilize the hygroscopic properties of lithium bromide to create cooling effects. The solution's concentration and temperature significantly affect the absorption efficiency, with properly maintained lithium bromide solutions providing excellent cooling performance while consuming less energy compared to conventional refrigeration systems.
- Dehumidification applications of lithium bromide solution: Lithium bromide solutions are effective in dehumidification processes due to their strong hygroscopic properties. When air passes through a lithium bromide solution, moisture is absorbed, resulting in drier air output. This property makes lithium bromide solutions valuable in industrial dehumidification systems, air conditioning units, and moisture control applications where maintaining specific humidity levels is critical for process efficiency or product quality.
- Heat pump and energy storage applications: Lithium bromide solutions demonstrate high efficacy in heat pump systems and thermal energy storage applications. The solution can store and release thermal energy efficiently through absorption and desorption processes. This property enables the development of energy-efficient heating and cooling systems that can utilize low-grade heat sources. The thermal storage capacity of lithium bromide solutions also makes them suitable for balancing energy supply and demand in various industrial applications.
- Corrosion inhibition and solution stability improvements: Enhancing the stability and reducing the corrosive properties of lithium bromide solutions is crucial for improving their efficacy and extending the lifespan of associated equipment. Various additives and inhibitors can be incorporated into lithium bromide solutions to minimize corrosion of metal components. Improved formulations also address crystallization issues that can occur at certain concentrations and temperatures, thereby enhancing the overall reliability and performance of systems using lithium bromide solutions.
- Novel system designs for improved lithium bromide solution performance: Innovative system designs can significantly enhance the efficacy of lithium bromide solutions in various applications. These designs include advanced heat exchangers, specialized solution distribution systems, and improved regeneration processes. Some systems incorporate vacuum technology to enhance the absorption properties of lithium bromide solutions. Other innovations focus on solution circulation methods, temperature control mechanisms, and integration with renewable energy sources to maximize efficiency and performance while minimizing environmental impact.
02 Heat and mass transfer enhancement in lithium bromide systems
Various techniques have been developed to enhance heat and mass transfer in lithium bromide solution systems. These include the use of advanced heat exchangers, improved solution distribution methods, and optimized flow patterns. Enhanced heat and mass transfer leads to better absorption or desorption rates, improving the overall efficacy of lithium bromide solutions in applications such as absorption chillers and heat pumps.Expand Specific Solutions03 Corrosion inhibition in lithium bromide systems
Lithium bromide solutions can be corrosive to metal components in absorption systems. Various corrosion inhibitors and material treatments have been developed to mitigate this issue. These include the addition of specific chemical compounds to the solution, surface treatments of metal components, and the use of corrosion-resistant materials. Effective corrosion inhibition significantly extends the service life of equipment using lithium bromide solutions.Expand Specific Solutions04 Concentration control and crystallization prevention
Maintaining optimal concentration of lithium bromide solution is crucial for system efficacy. Various methods have been developed to prevent crystallization, which can occur at high concentrations and cause system failure. These include precise temperature and concentration control mechanisms, solution additives that modify crystallization behavior, and specialized system designs that minimize the risk of crystallization during operation or shutdown periods.Expand Specific Solutions05 Energy efficiency improvements in lithium bromide systems
Innovations in system design and operation have significantly improved the energy efficiency of processes using lithium bromide solutions. These include multi-stage absorption systems, heat recovery mechanisms, and advanced control strategies. Integration with renewable energy sources and waste heat recovery systems has further enhanced the energy efficiency and environmental sustainability of lithium bromide solution applications.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The lithium bromide solution efficacy market is in a growth phase, driven by increasing demand for absorption refrigeration systems and energy-efficient cooling technologies. The market size is expanding steadily, particularly in industrial and commercial cooling applications, with an estimated compound annual growth rate of 5-7%. Technologically, the field is moderately mature but evolving, with companies focusing on improving solution purity and performance. Key players include LG Chem and Guangzhou Tinci Materials Technology leading in chemical innovation, while Shin-Etsu Chemical and AGC contribute significant advancements in purification techniques. Research institutions like the Institute of Process Engineering (Chinese Academy of Sciences) are collaborating with industry to address impurity challenges, indicating a collaborative ecosystem developing around solution efficacy optimization.
LG Chem Ltd.
Technical Solution: LG Chem has developed advanced purification technologies for lithium bromide solutions used in absorption refrigeration systems. Their approach involves multi-stage filtration processes combined with proprietary chemical treatments to remove critical impurities such as chromates, sulfates, and heavy metals that significantly impact system performance. Their research has demonstrated that controlling iron content below 5 ppm can extend equipment lifespan by up to 40%. LG Chem's solution incorporates corrosion inhibitors specifically designed to counteract the effects of residual impurities, maintaining solution efficacy even when trace contaminants are present. Their patented stabilization technology prevents precipitation of impurities during thermal cycling, addressing one of the key challenges in absorption chiller operations.
Strengths: Superior corrosion resistance in high-temperature applications; comprehensive approach addressing multiple impurity types simultaneously; proven field performance with documented efficiency improvements. Weakness: Higher initial cost compared to conventional solutions; requires periodic monitoring and maintenance to maintain optimal performance levels.
Central Glass Co., Ltd.
Technical Solution: Central Glass has pioneered an innovative approach to managing impurity effects in lithium bromide solutions through their proprietary "Selective Ion Capture" technology. This system employs functionalized polymeric adsorbents that selectively bind to specific ionic impurities while leaving the active lithium bromide molecules unaffected. Their research has shown that controlling hydroxide ion concentration is particularly critical, with optimal performance achieved at pH levels between 8.2-9.0. Central Glass has developed specialized additives that form protective films on metal surfaces, effectively isolating them from corrosive impurities in the solution. Their comprehensive impurity management system includes real-time monitoring capabilities that can detect concentration changes as small as 0.5 ppm for critical contaminants, allowing for preventive maintenance before efficiency degradation occurs.
Strengths: Highly selective impurity removal that maintains solution concentration; minimal solution replacement requirements; effective across a wide range of operating conditions. Weakness: Complex implementation requiring specialized technical expertise; higher upfront investment compared to conventional purification methods.
Critical Patents and Studies on LiBr Solution Purification
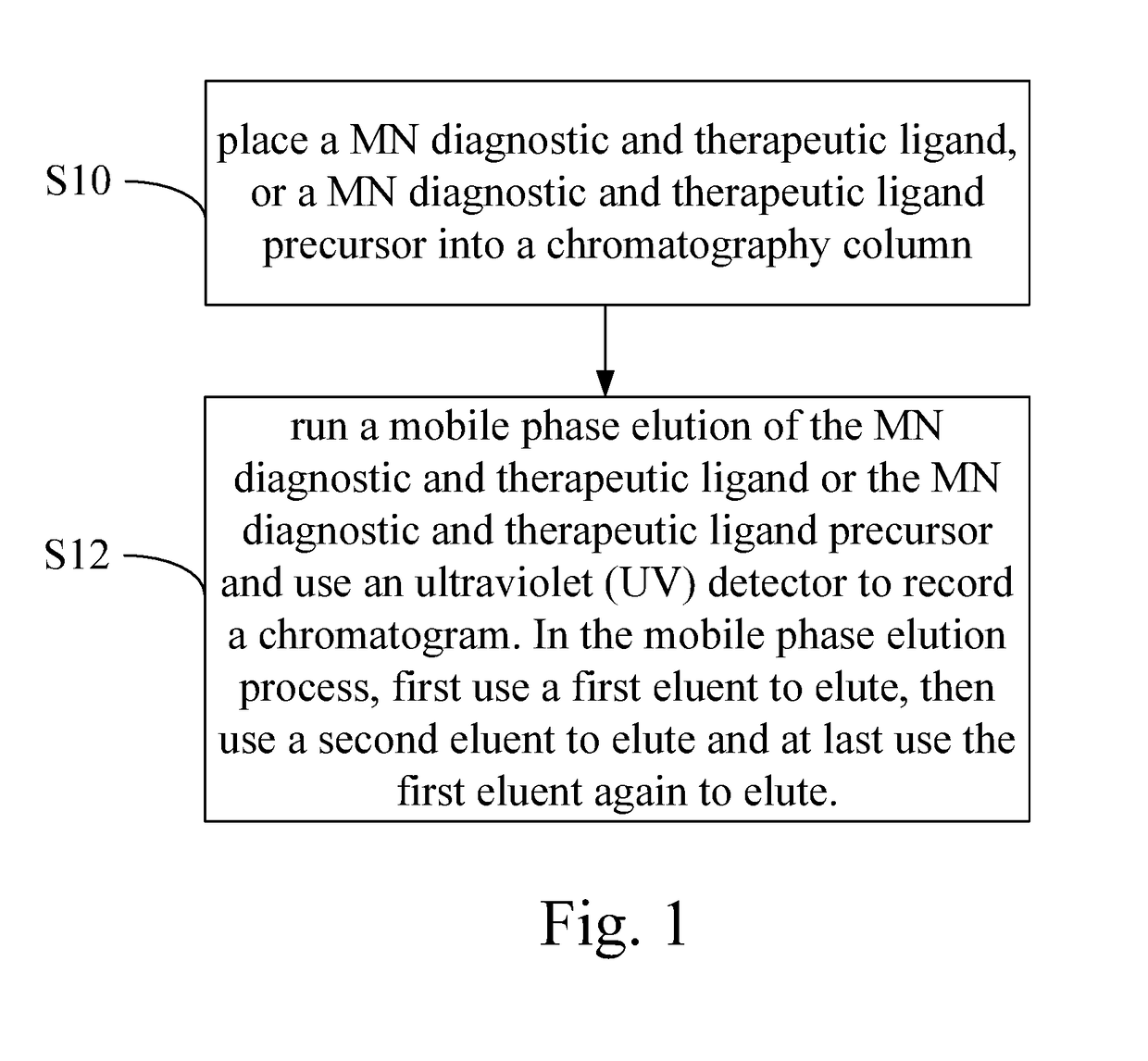
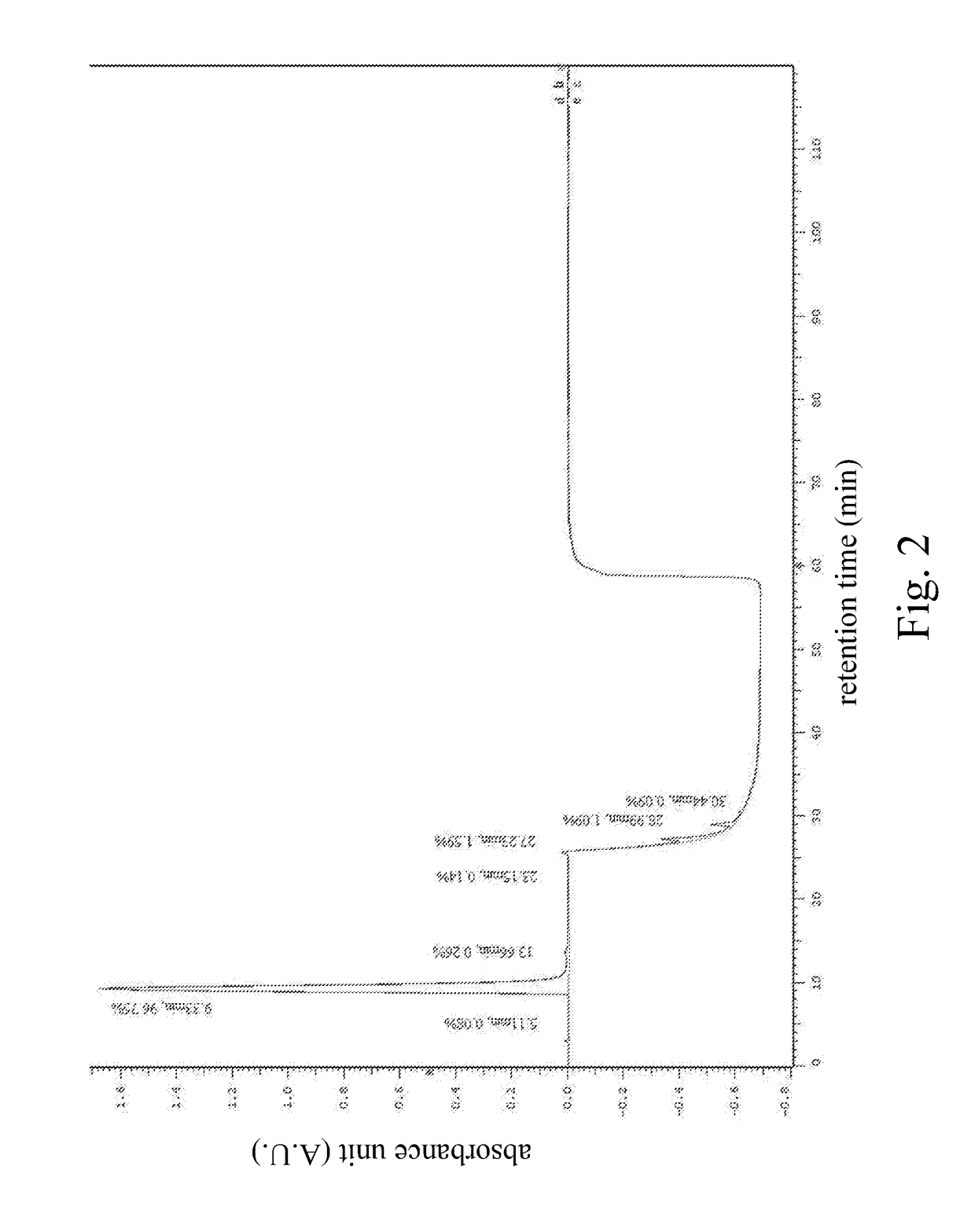
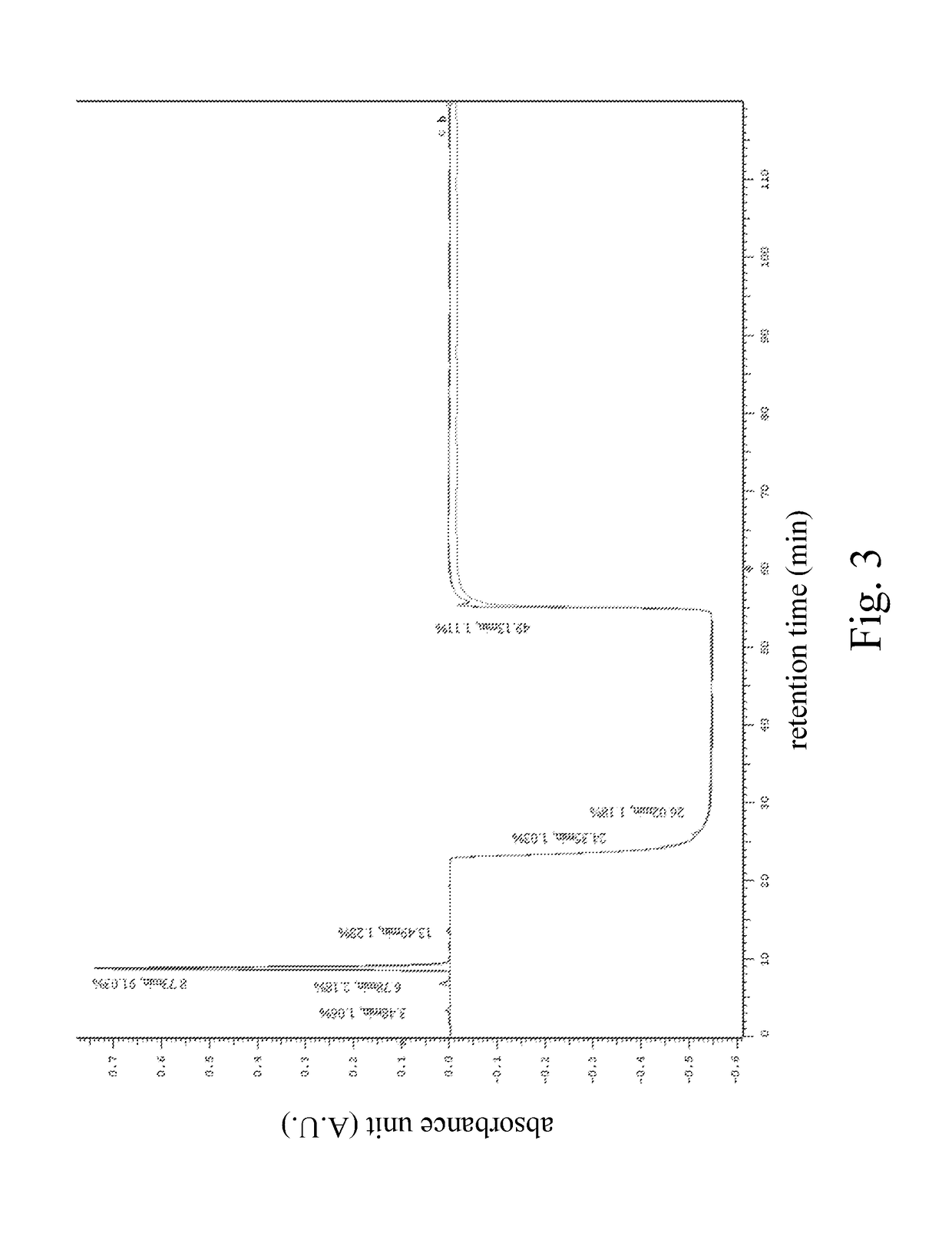
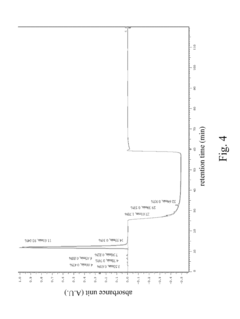
High performance liquid chromatography method for analysis of MN diagnostic and therapeutic ligand and precursor
PatentInactiveUS20180059072A1
Innovation
- A HPLC method utilizing a high ratio of acetonitrile in the mobile phase to wash out low-polarity impurities and employing gradient elution with a UV detector at 210 nm to improve detection accuracy, ensuring accurate analysis and reducing residual impurities in the column.
An improved reversed-phase liquid chromatographic (RP-LC) method for the determination of queitapine hemifumerate
PatentInactiveIN3136CHE2008A
Innovation
- A reversed-phase liquid chromatographic (RP-LC) method with a gradient elution using a specific mobile phase pH of 6.5 and a mixture of buffer and methanol, optimized to separate and quantify quetiapine hemifumarate and its impurities, with improved sensitivity and reproducibility, and incorporating forced degradation studies for stability indication.
Environmental Impact of LiBr Solution Impurities
The environmental implications of impurities in lithium bromide (LiBr) solutions extend far beyond operational efficiency concerns, representing significant ecological challenges that demand comprehensive assessment. When LiBr solutions containing impurities are discharged into water bodies, they can substantially alter aquatic ecosystems through increased salinity and the introduction of potentially toxic metal ions. These contaminants may include chromium, copper, and iron, which even at low concentrations can disrupt aquatic life cycles and accumulate in sediments.
The manufacturing processes for LiBr solutions themselves generate considerable environmental footprints, particularly when additional purification steps are required to remove impurities. These processes typically demand increased energy consumption and chemical usage, resulting in elevated greenhouse gas emissions and chemical waste generation. Research indicates that purification processes can increase the carbon footprint of LiBr production by 15-30% depending on the impurity levels and removal techniques employed.
Disposal of spent LiBr solutions presents another critical environmental concern. The presence of impurities often complicates recycling efforts and may necessitate specialized treatment before disposal. Without proper management, these solutions can contaminate soil and groundwater, potentially leading to long-term environmental degradation. Studies have documented cases where improper disposal has resulted in localized soil contamination with bromide levels exceeding regulatory thresholds by factors of 5-10.
Regulatory frameworks worldwide are increasingly addressing these environmental impacts. The European Union's REACH regulations and similar initiatives in North America and Asia have established stringent guidelines for the handling and disposal of LiBr solutions. Companies must now document impurity profiles and implement appropriate management strategies to maintain compliance with these evolving standards.
Encouragingly, recent technological innovations are creating pathways toward more environmentally sustainable practices. Advanced membrane filtration systems, ion-exchange technologies, and closed-loop recycling processes are reducing both the environmental impact of impurity removal and the need for raw material extraction. These technologies demonstrate potential for reducing water usage by up to 60% and waste generation by 40-50% compared to conventional methods.
The life cycle assessment of LiBr solutions with varying impurity levels reveals that environmental impacts extend throughout the entire value chain, from extraction of raw materials to end-of-life management. Quantitative analyses indicate that reducing impurities at source can decrease the overall environmental footprint by 20-35%, highlighting the importance of preventive approaches rather than end-of-pipe solutions.
The manufacturing processes for LiBr solutions themselves generate considerable environmental footprints, particularly when additional purification steps are required to remove impurities. These processes typically demand increased energy consumption and chemical usage, resulting in elevated greenhouse gas emissions and chemical waste generation. Research indicates that purification processes can increase the carbon footprint of LiBr production by 15-30% depending on the impurity levels and removal techniques employed.
Disposal of spent LiBr solutions presents another critical environmental concern. The presence of impurities often complicates recycling efforts and may necessitate specialized treatment before disposal. Without proper management, these solutions can contaminate soil and groundwater, potentially leading to long-term environmental degradation. Studies have documented cases where improper disposal has resulted in localized soil contamination with bromide levels exceeding regulatory thresholds by factors of 5-10.
Regulatory frameworks worldwide are increasingly addressing these environmental impacts. The European Union's REACH regulations and similar initiatives in North America and Asia have established stringent guidelines for the handling and disposal of LiBr solutions. Companies must now document impurity profiles and implement appropriate management strategies to maintain compliance with these evolving standards.
Encouragingly, recent technological innovations are creating pathways toward more environmentally sustainable practices. Advanced membrane filtration systems, ion-exchange technologies, and closed-loop recycling processes are reducing both the environmental impact of impurity removal and the need for raw material extraction. These technologies demonstrate potential for reducing water usage by up to 60% and waste generation by 40-50% compared to conventional methods.
The life cycle assessment of LiBr solutions with varying impurity levels reveals that environmental impacts extend throughout the entire value chain, from extraction of raw materials to end-of-life management. Quantitative analyses indicate that reducing impurities at source can decrease the overall environmental footprint by 20-35%, highlighting the importance of preventive approaches rather than end-of-pipe solutions.
Economic Analysis of Purification Technologies
The economic implications of purification technologies for lithium bromide solutions represent a critical factor in absorption refrigeration system efficiency. Initial investment costs for advanced purification systems range from $50,000 to $200,000 depending on capacity and technology sophistication, with membrane filtration systems typically requiring lower capital expenditure compared to vacuum distillation or ion exchange systems.
Operational costs must be evaluated against performance benefits, as purification technologies demonstrate varying cost-efficiency ratios. Membrane filtration technologies offer moderate initial investment with relatively low operational costs of $0.05-0.10 per cubic meter of solution treated. Ion exchange systems, while more expensive initially, provide superior impurity removal capabilities with operational costs averaging $0.15-0.25 per cubic meter, particularly effective for metallic ion removal.
Return on investment calculations indicate that high-purity lithium bromide solutions can extend equipment lifespan by 30-45%, significantly reducing maintenance costs and system downtime. Energy efficiency improvements of 8-12% have been documented in systems utilizing purified solutions, translating to substantial operational savings over system lifetime.
Market analysis reveals that facilities implementing advanced purification technologies recover their investment within 2-4 years through reduced energy consumption, maintenance requirements, and extended equipment lifespan. The economic value proposition strengthens in large-scale industrial applications where even marginal efficiency improvements yield substantial cost savings.
Comparative cost analysis between periodic solution replacement versus continuous purification demonstrates that continuous purification becomes economically advantageous for systems exceeding 500 kW cooling capacity. For smaller systems, batch purification approaches may offer more favorable economics with investment recovery periods of 18-30 months.
Environmental compliance costs must also factor into economic assessments, as regulations increasingly restrict disposal of contaminated lithium bromide solutions. Purification technologies that enable closed-loop operation can eliminate disposal costs ranging from $2-5 per gallon of contaminated solution, providing additional economic justification for implementation.
Future economic projections suggest decreasing technology costs as purification systems achieve greater market penetration, with anticipated cost reductions of 15-20% over the next five years as manufacturing scales increase and technological refinements continue.
Operational costs must be evaluated against performance benefits, as purification technologies demonstrate varying cost-efficiency ratios. Membrane filtration technologies offer moderate initial investment with relatively low operational costs of $0.05-0.10 per cubic meter of solution treated. Ion exchange systems, while more expensive initially, provide superior impurity removal capabilities with operational costs averaging $0.15-0.25 per cubic meter, particularly effective for metallic ion removal.
Return on investment calculations indicate that high-purity lithium bromide solutions can extend equipment lifespan by 30-45%, significantly reducing maintenance costs and system downtime. Energy efficiency improvements of 8-12% have been documented in systems utilizing purified solutions, translating to substantial operational savings over system lifetime.
Market analysis reveals that facilities implementing advanced purification technologies recover their investment within 2-4 years through reduced energy consumption, maintenance requirements, and extended equipment lifespan. The economic value proposition strengthens in large-scale industrial applications where even marginal efficiency improvements yield substantial cost savings.
Comparative cost analysis between periodic solution replacement versus continuous purification demonstrates that continuous purification becomes economically advantageous for systems exceeding 500 kW cooling capacity. For smaller systems, batch purification approaches may offer more favorable economics with investment recovery periods of 18-30 months.
Environmental compliance costs must also factor into economic assessments, as regulations increasingly restrict disposal of contaminated lithium bromide solutions. Purification technologies that enable closed-loop operation can eliminate disposal costs ranging from $2-5 per gallon of contaminated solution, providing additional economic justification for implementation.
Future economic projections suggest decreasing technology costs as purification systems achieve greater market penetration, with anticipated cost reductions of 15-20% over the next five years as manufacturing scales increase and technological refinements continue.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!