Optimizing Lithium Bromide Purification in Industrial Settings
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
LiBr Purification Background and Objectives
Lithium bromide (LiBr) purification has evolved significantly since its first industrial applications in the early 20th century. Initially used primarily in absorption refrigeration systems, LiBr has expanded into various industrial applications including air conditioning systems, desiccants, pharmaceuticals, and more recently, energy storage solutions. The technical evolution of LiBr purification methods reflects broader trends in chemical engineering, moving from basic precipitation techniques to sophisticated membrane-based separation processes.
The global push for energy efficiency and sustainable industrial processes has placed renewed emphasis on optimizing LiBr purification. Impurities in LiBr solutions, even at trace levels, can significantly impact system performance through issues such as crystallization, corrosion, and reduced thermal efficiency. These challenges become particularly acute in absorption refrigeration systems where solution stability directly affects operational reliability and energy consumption.
Current purification objectives center on achieving higher purity levels while simultaneously reducing energy consumption, minimizing waste generation, and decreasing operational costs. Industry standards now typically require LiBr solutions with purity exceeding 99.5%, with particularly stringent requirements for applications in pharmaceutical manufacturing and advanced energy systems.
The technical landscape is further complicated by the presence of diverse contaminants including metal ions (particularly calcium, magnesium, and iron), organic compounds, and suspended solids. Each contaminant category presents unique separation challenges, necessitating tailored purification approaches rather than one-size-fits-all solutions.
Recent environmental regulations have added another dimension to purification goals, with increasing restrictions on discharge of bromide-containing waste streams and heightened focus on closed-loop processing. This regulatory environment has accelerated interest in recovery and recycling technologies as integral components of purification systems.
From a geographical perspective, LiBr purification technology development has historically been concentrated in industrialized nations, particularly Japan, the United States, and Germany. However, emerging economies including China and India are increasingly contributing to technological advancements in this field, driven by their expanding industrial bases and growing HVAC markets.
The ultimate technical objective in this domain is to develop economically viable purification processes that can consistently deliver ultra-high purity LiBr while minimizing environmental impact. This includes exploring novel separation mechanisms, developing advanced materials for selective ion removal, and integrating real-time monitoring systems to enable adaptive process control. Success in these endeavors would significantly enhance the performance of LiBr-based systems across multiple industries.
The global push for energy efficiency and sustainable industrial processes has placed renewed emphasis on optimizing LiBr purification. Impurities in LiBr solutions, even at trace levels, can significantly impact system performance through issues such as crystallization, corrosion, and reduced thermal efficiency. These challenges become particularly acute in absorption refrigeration systems where solution stability directly affects operational reliability and energy consumption.
Current purification objectives center on achieving higher purity levels while simultaneously reducing energy consumption, minimizing waste generation, and decreasing operational costs. Industry standards now typically require LiBr solutions with purity exceeding 99.5%, with particularly stringent requirements for applications in pharmaceutical manufacturing and advanced energy systems.
The technical landscape is further complicated by the presence of diverse contaminants including metal ions (particularly calcium, magnesium, and iron), organic compounds, and suspended solids. Each contaminant category presents unique separation challenges, necessitating tailored purification approaches rather than one-size-fits-all solutions.
Recent environmental regulations have added another dimension to purification goals, with increasing restrictions on discharge of bromide-containing waste streams and heightened focus on closed-loop processing. This regulatory environment has accelerated interest in recovery and recycling technologies as integral components of purification systems.
From a geographical perspective, LiBr purification technology development has historically been concentrated in industrialized nations, particularly Japan, the United States, and Germany. However, emerging economies including China and India are increasingly contributing to technological advancements in this field, driven by their expanding industrial bases and growing HVAC markets.
The ultimate technical objective in this domain is to develop economically viable purification processes that can consistently deliver ultra-high purity LiBr while minimizing environmental impact. This includes exploring novel separation mechanisms, developing advanced materials for selective ion removal, and integrating real-time monitoring systems to enable adaptive process control. Success in these endeavors would significantly enhance the performance of LiBr-based systems across multiple industries.
Market Analysis for High-Purity LiBr
The high-purity lithium bromide (LiBr) market has experienced significant growth in recent years, primarily driven by increasing applications in absorption refrigeration systems, air conditioning units, and pharmaceutical manufacturing. The global market value for high-purity LiBr reached approximately $320 million in 2022, with projections indicating growth to $450 million by 2027, representing a compound annual growth rate (CAGR) of 7.1%.
The demand for high-purity LiBr is particularly strong in regions with hot climates and growing industrial bases. Asia-Pacific currently dominates the market with 42% share, followed by North America (28%) and Europe (21%). China has emerged as both the largest producer and consumer, accounting for nearly 30% of global consumption, primarily due to its expanding HVAC industry and industrial cooling applications.
Market segmentation reveals that absorption chillers represent the largest application segment, consuming approximately 65% of high-purity LiBr production. The pharmaceutical sector accounts for 18%, while other applications including desiccants, batteries, and specialty chemicals comprise the remaining 17%. The absorption chiller segment is expected to maintain dominance due to increasing adoption of energy-efficient cooling solutions in commercial and industrial buildings.
Key market drivers include stringent environmental regulations limiting the use of conventional refrigerants, rising energy costs prompting adoption of absorption cooling technologies, and growing industrial activities in emerging economies. The pharmaceutical industry's expansion has also contributed significantly to demand growth, as high-purity LiBr is essential in certain drug manufacturing processes.
Price trends indicate volatility in recent years, with prices ranging from $8-12 per kilogram for industrial-grade LiBr to $15-25 per kilogram for pharmaceutical-grade material. This volatility stems from fluctuations in lithium raw material costs and occasional supply chain disruptions. The market has seen a 15% price increase since 2020, primarily attributed to lithium supply constraints.
Customer requirements are increasingly stringent, with specifications typically demanding 99.5% purity for industrial applications and 99.9% for pharmaceutical use. Impurity profiles, particularly regarding heavy metals and moisture content, are becoming more restrictive, creating market opportunities for advanced purification technologies.
Market challenges include raw material supply constraints, environmental concerns regarding waste streams from purification processes, and competition from alternative technologies in the cooling sector. However, opportunities exist in developing cost-effective purification methods, creating closed-loop recycling systems, and expanding applications in emerging sectors such as energy storage.
The demand for high-purity LiBr is particularly strong in regions with hot climates and growing industrial bases. Asia-Pacific currently dominates the market with 42% share, followed by North America (28%) and Europe (21%). China has emerged as both the largest producer and consumer, accounting for nearly 30% of global consumption, primarily due to its expanding HVAC industry and industrial cooling applications.
Market segmentation reveals that absorption chillers represent the largest application segment, consuming approximately 65% of high-purity LiBr production. The pharmaceutical sector accounts for 18%, while other applications including desiccants, batteries, and specialty chemicals comprise the remaining 17%. The absorption chiller segment is expected to maintain dominance due to increasing adoption of energy-efficient cooling solutions in commercial and industrial buildings.
Key market drivers include stringent environmental regulations limiting the use of conventional refrigerants, rising energy costs prompting adoption of absorption cooling technologies, and growing industrial activities in emerging economies. The pharmaceutical industry's expansion has also contributed significantly to demand growth, as high-purity LiBr is essential in certain drug manufacturing processes.
Price trends indicate volatility in recent years, with prices ranging from $8-12 per kilogram for industrial-grade LiBr to $15-25 per kilogram for pharmaceutical-grade material. This volatility stems from fluctuations in lithium raw material costs and occasional supply chain disruptions. The market has seen a 15% price increase since 2020, primarily attributed to lithium supply constraints.
Customer requirements are increasingly stringent, with specifications typically demanding 99.5% purity for industrial applications and 99.9% for pharmaceutical use. Impurity profiles, particularly regarding heavy metals and moisture content, are becoming more restrictive, creating market opportunities for advanced purification technologies.
Market challenges include raw material supply constraints, environmental concerns regarding waste streams from purification processes, and competition from alternative technologies in the cooling sector. However, opportunities exist in developing cost-effective purification methods, creating closed-loop recycling systems, and expanding applications in emerging sectors such as energy storage.
Current Purification Technologies and Challenges
The lithium bromide purification landscape in industrial settings currently employs several established technologies, each with specific advantages and limitations. Crystallization remains the most widely adopted method, utilizing temperature-controlled processes to separate LiBr from impurities based on solubility differences. This technique achieves purities of 98-99% but struggles with energy efficiency and throughput limitations when scaling to industrial volumes.
Ion exchange technologies have gained traction in recent years, employing specialized resins to selectively remove metal contaminants like calcium, magnesium, and iron from LiBr solutions. While effective for specific impurity profiles, these systems require frequent regeneration cycles and generate secondary waste streams that necessitate additional treatment, creating operational complexities in continuous production environments.
Membrane filtration represents another significant purification approach, with nanofiltration and reverse osmosis systems increasingly deployed for LiBr purification. These technologies offer advantages in continuous operation and reduced energy consumption compared to thermal methods. However, membrane fouling remains a persistent challenge, particularly when processing solutions with high concentrations of dissolved solids, leading to increased maintenance requirements and shortened membrane lifespans.
Electrodialysis has emerged as a promising technology for LiBr purification, utilizing ion-selective membranes and electrical potential to separate ionic species. This approach demonstrates excellent selectivity for certain impurities but faces limitations in processing capacity and energy consumption when applied to high-concentration LiBr solutions typical in industrial absorption refrigeration systems.
The primary technical challenges across all purification methods include handling the highly corrosive nature of concentrated LiBr solutions, which accelerates equipment degradation and increases maintenance costs. Additionally, the presence of organic contaminants from process additives or system degradation products often requires multi-stage purification approaches, complicating system design and operation.
Energy efficiency represents another significant challenge, as many purification technologies require substantial thermal or electrical inputs, directly impacting operational costs and environmental footprint. This is particularly problematic for continuous industrial operations where purification must be integrated into broader production processes without creating bottlenecks.
Scale formation during purification presents ongoing difficulties, as concentrated LiBr solutions readily form deposits on heat exchange surfaces and process equipment, reducing efficiency and necessitating frequent cleaning cycles. Current mitigation strategies rely heavily on chemical additives that may introduce additional purification requirements downstream.
Ion exchange technologies have gained traction in recent years, employing specialized resins to selectively remove metal contaminants like calcium, magnesium, and iron from LiBr solutions. While effective for specific impurity profiles, these systems require frequent regeneration cycles and generate secondary waste streams that necessitate additional treatment, creating operational complexities in continuous production environments.
Membrane filtration represents another significant purification approach, with nanofiltration and reverse osmosis systems increasingly deployed for LiBr purification. These technologies offer advantages in continuous operation and reduced energy consumption compared to thermal methods. However, membrane fouling remains a persistent challenge, particularly when processing solutions with high concentrations of dissolved solids, leading to increased maintenance requirements and shortened membrane lifespans.
Electrodialysis has emerged as a promising technology for LiBr purification, utilizing ion-selective membranes and electrical potential to separate ionic species. This approach demonstrates excellent selectivity for certain impurities but faces limitations in processing capacity and energy consumption when applied to high-concentration LiBr solutions typical in industrial absorption refrigeration systems.
The primary technical challenges across all purification methods include handling the highly corrosive nature of concentrated LiBr solutions, which accelerates equipment degradation and increases maintenance costs. Additionally, the presence of organic contaminants from process additives or system degradation products often requires multi-stage purification approaches, complicating system design and operation.
Energy efficiency represents another significant challenge, as many purification technologies require substantial thermal or electrical inputs, directly impacting operational costs and environmental footprint. This is particularly problematic for continuous industrial operations where purification must be integrated into broader production processes without creating bottlenecks.
Scale formation during purification presents ongoing difficulties, as concentrated LiBr solutions readily form deposits on heat exchange surfaces and process equipment, reducing efficiency and necessitating frequent cleaning cycles. Current mitigation strategies rely heavily on chemical additives that may introduce additional purification requirements downstream.
Industrial-Scale Purification Solutions
01 Crystallization and recrystallization methods
Lithium bromide can be purified through crystallization and recrystallization processes. These methods involve dissolving the impure lithium bromide in a suitable solvent, followed by controlled crystallization to separate the pure compound from impurities. The process may include multiple crystallization steps, temperature control, and the use of specific solvents to enhance purification efficiency. This approach is effective for removing various impurities and obtaining high-purity lithium bromide suitable for industrial applications.- Crystallization and recrystallization methods: Lithium bromide can be purified through crystallization and recrystallization processes. These methods involve dissolving the impure lithium bromide in a suitable solvent, followed by controlled crystallization to separate the pure compound from impurities. The process may include multiple crystallization steps, temperature control, and the use of specific solvents to enhance purification efficiency. This approach is effective for removing various impurities and obtaining high-purity lithium bromide suitable for industrial applications.
- Ion exchange and membrane separation techniques: Ion exchange resins and membrane separation technologies are employed for lithium bromide purification. These techniques involve passing lithium bromide solutions through specialized ion exchange columns or membranes that selectively remove impurities such as metal ions, chlorides, and other contaminants. The process can be optimized by adjusting parameters like flow rate, temperature, and pH. These methods are particularly effective for removing trace impurities and can be integrated into continuous purification systems.
- Vacuum distillation and evaporation systems: Vacuum distillation and evaporation techniques are used to purify lithium bromide solutions by removing water and volatile impurities. These systems operate under reduced pressure to lower the boiling point of the solution, allowing for more efficient separation while preventing thermal decomposition. The process typically involves multiple stages of evaporation and condensation to progressively increase the concentration and purity of lithium bromide. This method is particularly useful for preparing concentrated lithium bromide solutions for absorption refrigeration applications.
- Chemical treatment and precipitation methods: Chemical treatment processes involve adding specific reagents to lithium bromide solutions to precipitate impurities or convert them into removable forms. These methods may include pH adjustment, oxidation or reduction reactions, and the addition of precipitating agents that selectively react with impurities. After the chemical treatment, filtration or other separation techniques are employed to remove the precipitated impurities. This approach is particularly effective for removing specific contaminants such as heavy metals, sulfates, and organic compounds.
- Integrated purification systems and equipment: Integrated systems combine multiple purification technologies into comprehensive equipment designed specifically for lithium bromide purification. These systems may incorporate filtration, ion exchange, crystallization, and other techniques in a sequential or parallel arrangement to achieve optimal purification results. The equipment often includes automated control systems, monitoring devices, and energy recovery components to enhance efficiency and reduce operational costs. These integrated solutions are designed for continuous operation in industrial settings where high-purity lithium bromide is required.
02 Ion exchange purification techniques
Ion exchange technology is employed for lithium bromide purification by removing impurity ions from lithium bromide solutions. The process utilizes ion exchange resins that selectively adsorb unwanted ions while allowing lithium and bromide ions to pass through. This method is particularly effective for removing metal impurities such as calcium, magnesium, and heavy metals. The purified lithium bromide solution can then be concentrated and crystallized to obtain high-purity lithium bromide salt.Expand Specific Solutions03 Membrane-based purification systems
Membrane-based technologies offer an efficient approach for lithium bromide purification. These systems utilize various membrane types such as nanofiltration, reverse osmosis, or electrodialysis to separate lithium bromide from impurities. The membranes act as selective barriers that allow lithium bromide to pass through while retaining contaminants. This method is particularly advantageous for continuous purification processes and can be integrated into absorption refrigeration systems to maintain solution quality during operation.Expand Specific Solutions04 Chemical precipitation methods
Chemical precipitation is used to purify lithium bromide by adding specific reagents that selectively react with impurities to form insoluble compounds. These precipitates can then be removed through filtration or sedimentation, leaving behind purified lithium bromide solution. Common precipitating agents include hydroxides, carbonates, or sulfides that target specific metal impurities. This method is particularly effective for removing transition metals and can be combined with other purification techniques for enhanced results.Expand Specific Solutions05 Integrated purification systems for absorption refrigeration
Specialized purification systems have been developed specifically for lithium bromide used in absorption refrigeration applications. These integrated systems continuously purify the lithium bromide solution during the refrigeration cycle, preventing accumulation of impurities that could reduce efficiency or cause corrosion. The purification components may include filtration units, degassing systems, corrosion inhibitors, and continuous monitoring equipment. These systems help maintain optimal performance of absorption chillers by ensuring the lithium bromide solution remains free of contaminants.Expand Specific Solutions
Key Industry Players and Competitors
The lithium bromide purification technology market is in a growth phase, driven by increasing demand for absorption refrigeration systems and energy-efficient cooling solutions. The global market size is expanding steadily, projected to reach significant value due to industrial applications in HVAC, refrigeration, and energy storage. From a technological maturity perspective, established players like Albemarle Corp. and Ganfeng Lithium Group have developed advanced purification methods, while research institutions such as the Institute of Process Engineering (Chinese Academy of Sciences) and Case Western Reserve University are pioneering next-generation techniques. Companies like Terralithium LLC, Aquatech International, and Veolia Water Solutions are focusing on sustainable purification processes, while Dow Global Technologies and Arkema France are advancing chemical engineering approaches. The competitive landscape shows a mix of specialized lithium producers, chemical conglomerates, and water treatment specialists competing for market share.
Institute of Process Engineering, Chinese Academy of Sciences
Technical Solution: The Institute has developed an innovative electrochemical purification process for lithium bromide that achieves exceptional purity levels while minimizing environmental impact. Their approach utilizes specially designed electrochemical cells with selective ion-permeable membranes that enable precise separation of impurities from LiBr solutions. The process operates at near-ambient temperatures, significantly reducing energy requirements compared to conventional thermal methods. Research indicates their method can reduce metal contaminants to below 10 ppm while maintaining LiBr recovery rates above 98%. The Institute's technology incorporates advanced electrode materials with enhanced durability in corrosive bromide environments, extending operational life by approximately 40% compared to standard electrodes. Their system also features an integrated regeneration cycle that recovers and reuses process chemicals, minimizing waste generation.
Strengths: Exceptional energy efficiency with up to 60% reduction in energy consumption; precise control of impurity removal; minimal chemical consumption through regeneration cycles. Weaknesses: Higher initial technology investment; requires specialized technical expertise for operation; scale-up challenges for very high-volume applications.
Aquatech International LLC
Technical Solution: Aquatech has pioneered an advanced membrane-based purification system specifically for lithium bromide solutions used in absorption refrigeration systems. Their technology employs a combination of ultrafiltration and specialized nanofiltration membranes that selectively remove contaminants while maintaining LiBr concentration. The system incorporates proprietary anti-fouling membrane technology that extends operational life in high-concentration brine environments. Aquatech's process includes a pre-treatment stage using electrocoagulation to remove suspended solids and organic compounds, followed by multi-stage filtration. Their automated control system continuously monitors solution conductivity, pH, and turbidity, making real-time adjustments to maintain optimal purification parameters and energy efficiency.
Strengths: Continuous operation capability with minimal downtime; lower energy consumption compared to thermal purification methods; compact footprint suitable for retrofit installations. Weaknesses: Membrane replacement costs can be significant; performance may degrade with extremely high-concentration LiBr solutions; requires careful pre-treatment to prevent membrane fouling.
Critical Patents and Technical Innovations
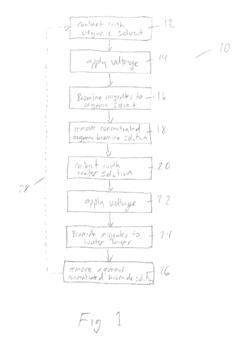
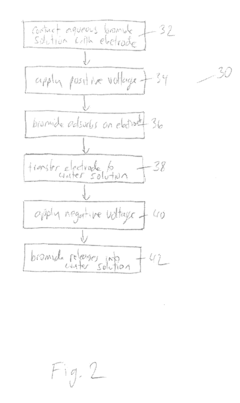
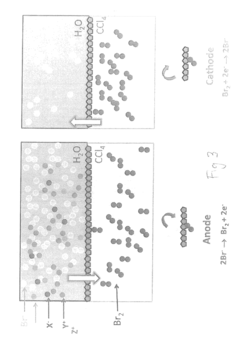
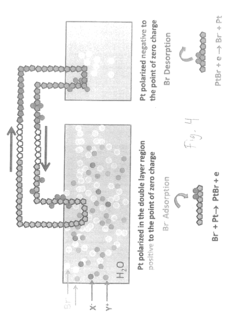
Bromide removal from aqueous solutions
PatentActiveUS20170247801A1
Innovation
- The use of an electrode, such as platinum, positioned between an aqueous bromide solution and an immiscible organic solvent like carbon tetrachloride, with controlled voltage to oxidize bromide to bromine, which then partitions into the organic phase, allowing for selective transport and purification, or employing electrochemical methods involving silver and bismuth electrodes for selective adsorption and desorption of bromide.
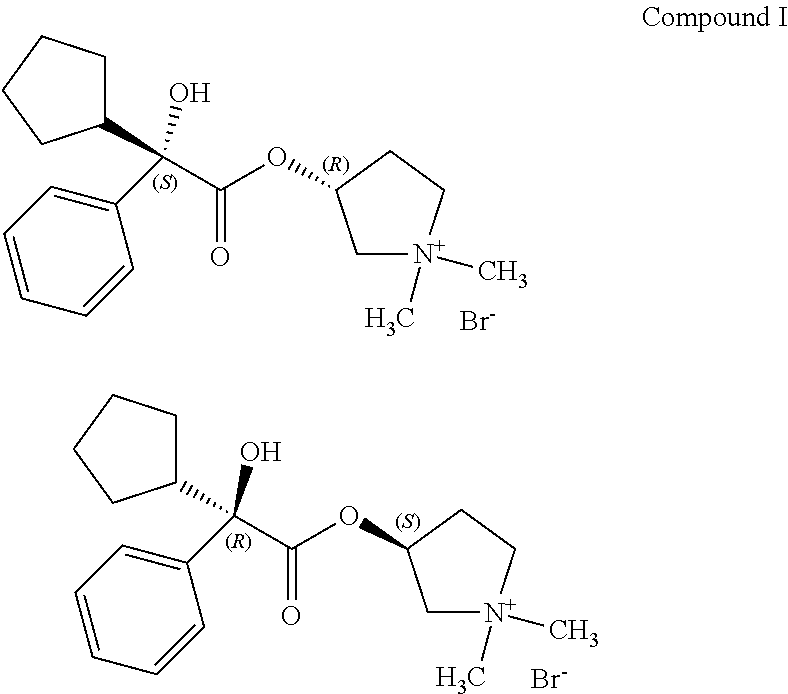
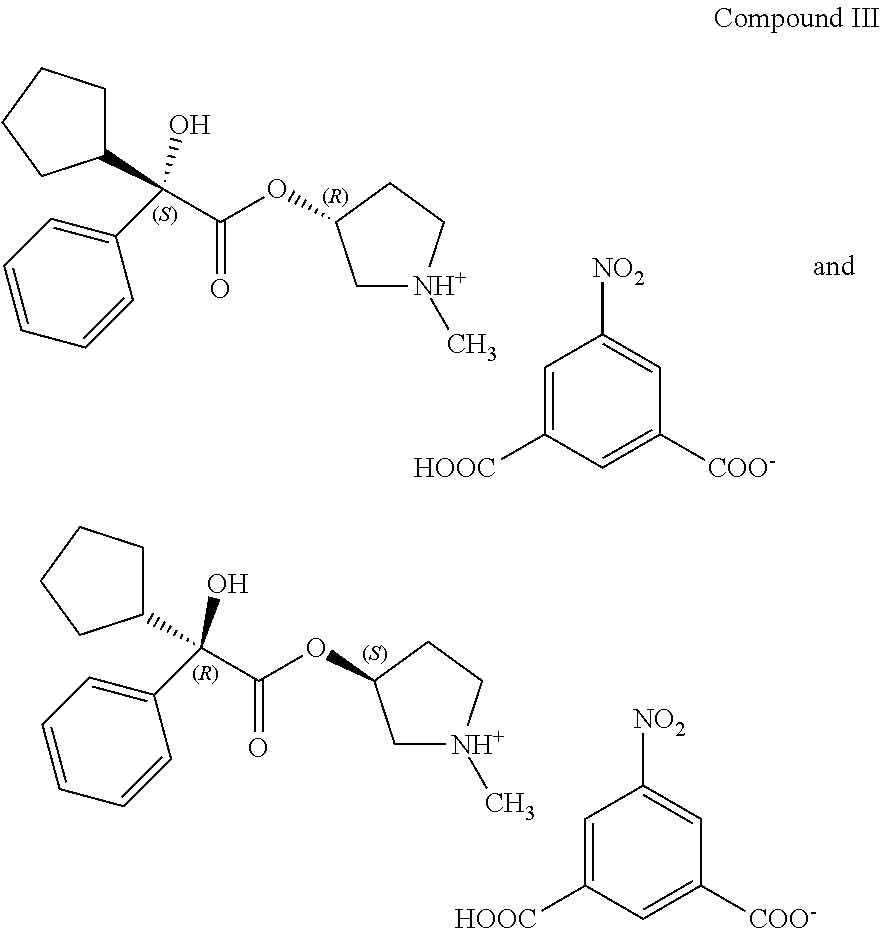
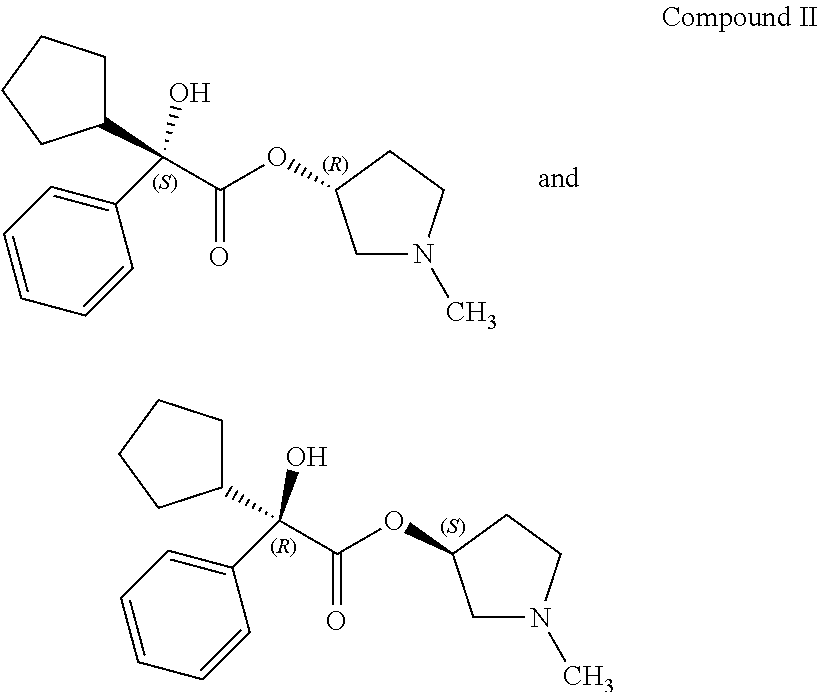
Process for preparing (3RS)-3-[(2SR)-(2-cyclopentyl-2-hydroxy-2-phenylacetyl)oxy]-1,1-dimethylp- yrrolidinium bromide
PatentActiveUS20170334848A1
Innovation
- A process involving the reaction of 1-methylpyrrolidin-3-yl-2-cyclopentyl-2-hydroxy-2-phenylacetate with 5-nitroisophthalic acid in a mixture of organic solvent and water, followed by filtration and optional purification, to produce glycopyrronium bromide with high yield and purity, reducing the need for laborious purification steps and achieving high chemical and enantiomeric purity.
Environmental Impact and Sustainability Considerations
The purification of lithium bromide in industrial settings carries significant environmental implications that must be addressed to ensure sustainable practices. Traditional purification methods often involve chemical processes that generate hazardous waste streams containing heavy metals, organic contaminants, and acidic or alkaline solutions. These waste products, if improperly managed, can lead to soil contamination, water pollution, and adverse effects on aquatic ecosystems. The energy-intensive nature of conventional purification techniques also contributes substantially to carbon emissions, particularly when powered by fossil fuel-based electricity.
Recent advancements in lithium bromide purification technologies have focused on developing more environmentally friendly approaches. Membrane-based separation systems have emerged as promising alternatives, reducing chemical usage by up to 40% compared to conventional methods. These systems also demonstrate water consumption reductions of approximately 30%, addressing critical resource conservation concerns in water-stressed regions where many industrial facilities operate.
Life cycle assessment (LCA) studies indicate that implementing closed-loop recycling systems for process water and reagents can decrease the environmental footprint of lithium bromide purification operations by 25-35%. Such systems capture and treat wastewater streams, allowing for the recovery and reuse of valuable materials while minimizing discharge to the environment. This approach aligns with circular economy principles and helps facilities meet increasingly stringent environmental regulations.
The adoption of renewable energy sources to power purification processes represents another significant sustainability opportunity. Facilities transitioning to solar or wind energy for their operations have reported carbon emission reductions of 50-70% compared to those relying on conventional grid electricity. This transition not only mitigates climate impact but often delivers long-term operational cost benefits through reduced energy expenditures.
Emerging green chemistry approaches are revolutionizing lithium bromide purification by replacing toxic reagents with biodegradable alternatives. Biocatalysts and environmentally benign solvents have demonstrated comparable purification efficiencies while generating waste streams that are significantly less harmful and easier to treat. These innovations address growing regulatory pressures and consumer demands for environmentally responsible industrial practices.
Industry leaders are increasingly implementing comprehensive environmental management systems that monitor and minimize the ecological impact of their purification operations. These systems typically include real-time monitoring of emissions and effluents, regular environmental audits, and continuous improvement protocols that systematically reduce resource consumption and waste generation over time.
Recent advancements in lithium bromide purification technologies have focused on developing more environmentally friendly approaches. Membrane-based separation systems have emerged as promising alternatives, reducing chemical usage by up to 40% compared to conventional methods. These systems also demonstrate water consumption reductions of approximately 30%, addressing critical resource conservation concerns in water-stressed regions where many industrial facilities operate.
Life cycle assessment (LCA) studies indicate that implementing closed-loop recycling systems for process water and reagents can decrease the environmental footprint of lithium bromide purification operations by 25-35%. Such systems capture and treat wastewater streams, allowing for the recovery and reuse of valuable materials while minimizing discharge to the environment. This approach aligns with circular economy principles and helps facilities meet increasingly stringent environmental regulations.
The adoption of renewable energy sources to power purification processes represents another significant sustainability opportunity. Facilities transitioning to solar or wind energy for their operations have reported carbon emission reductions of 50-70% compared to those relying on conventional grid electricity. This transition not only mitigates climate impact but often delivers long-term operational cost benefits through reduced energy expenditures.
Emerging green chemistry approaches are revolutionizing lithium bromide purification by replacing toxic reagents with biodegradable alternatives. Biocatalysts and environmentally benign solvents have demonstrated comparable purification efficiencies while generating waste streams that are significantly less harmful and easier to treat. These innovations address growing regulatory pressures and consumer demands for environmentally responsible industrial practices.
Industry leaders are increasingly implementing comprehensive environmental management systems that monitor and minimize the ecological impact of their purification operations. These systems typically include real-time monitoring of emissions and effluents, regular environmental audits, and continuous improvement protocols that systematically reduce resource consumption and waste generation over time.
Cost-Benefit Analysis of Purification Technologies
The economic viability of lithium bromide purification technologies represents a critical factor in industrial decision-making processes. When evaluating various purification methods, capital expenditure (CAPEX) must be balanced against operational expenditure (OPEX) to determine the most cost-effective approach. Conventional crystallization techniques typically require lower initial investment but incur higher ongoing costs due to energy consumption and chemical reagents. In contrast, advanced membrane filtration systems demand substantial upfront investment while offering reduced operational expenses over time.
Energy consumption patterns significantly impact the cost structure of purification processes. Thermal purification methods consume approximately 3.5-4.2 kWh per kilogram of purified lithium bromide, whereas electrochemical approaches require 2.1-2.8 kWh for equivalent output. This differential translates to substantial cost variations in energy-intensive industrial settings, particularly in regions with high electricity prices.
Recovery rates and product quality also factor prominently in cost-benefit calculations. Ion exchange technologies demonstrate recovery rates of 92-96% with 99.5% purity, while advanced crystallization methods achieve 88-93% recovery with 99.7% purity. The economic value of higher purity must be weighed against reduced recovery rates when selecting optimal purification technologies.
Maintenance requirements and system longevity represent additional cost considerations. Membrane-based systems typically require replacement components every 2-3 years, incurring costs equivalent to 15-20% of initial investment. Conversely, traditional thermal systems demand more frequent maintenance but component replacement costs are generally lower, averaging 8-12% of initial investment annually.
Environmental compliance costs increasingly influence technology selection. Advanced purification systems incorporating closed-loop water recycling and waste minimization features command premium prices but reduce environmental compliance expenses by 30-40% compared to conventional alternatives. This economic advantage becomes particularly significant in jurisdictions with stringent environmental regulations and high disposal costs.
Scalability economics reveal that membrane and electrochemical technologies demonstrate more favorable cost scaling at higher production volumes, with per-unit costs decreasing by approximately 25-30% when capacity triples. Traditional methods show more modest economies of scale, with cost reductions of 15-20% under similar expansion scenarios. This differential becomes crucial when planning facility expansions or new installations.
Return on investment (ROI) timelines vary significantly across technologies. Advanced systems with higher initial costs typically achieve ROI within 3.5-4.5 years, while conventional systems reach this milestone in 2-3 years but with lower cumulative returns over extended operational periods. These temporal considerations must align with organizational financial planning horizons and capital availability constraints.
Energy consumption patterns significantly impact the cost structure of purification processes. Thermal purification methods consume approximately 3.5-4.2 kWh per kilogram of purified lithium bromide, whereas electrochemical approaches require 2.1-2.8 kWh for equivalent output. This differential translates to substantial cost variations in energy-intensive industrial settings, particularly in regions with high electricity prices.
Recovery rates and product quality also factor prominently in cost-benefit calculations. Ion exchange technologies demonstrate recovery rates of 92-96% with 99.5% purity, while advanced crystallization methods achieve 88-93% recovery with 99.7% purity. The economic value of higher purity must be weighed against reduced recovery rates when selecting optimal purification technologies.
Maintenance requirements and system longevity represent additional cost considerations. Membrane-based systems typically require replacement components every 2-3 years, incurring costs equivalent to 15-20% of initial investment. Conversely, traditional thermal systems demand more frequent maintenance but component replacement costs are generally lower, averaging 8-12% of initial investment annually.
Environmental compliance costs increasingly influence technology selection. Advanced purification systems incorporating closed-loop water recycling and waste minimization features command premium prices but reduce environmental compliance expenses by 30-40% compared to conventional alternatives. This economic advantage becomes particularly significant in jurisdictions with stringent environmental regulations and high disposal costs.
Scalability economics reveal that membrane and electrochemical technologies demonstrate more favorable cost scaling at higher production volumes, with per-unit costs decreasing by approximately 25-30% when capacity triples. Traditional methods show more modest economies of scale, with cost reductions of 15-20% under similar expansion scenarios. This differential becomes crucial when planning facility expansions or new installations.
Return on investment (ROI) timelines vary significantly across technologies. Advanced systems with higher initial costs typically achieve ROI within 3.5-4.5 years, while conventional systems reach this milestone in 2-3 years but with lower cumulative returns over extended operational periods. These temporal considerations must align with organizational financial planning horizons and capital availability constraints.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!