Comparing Lithium Bromide and Calcium Chloride in Desiccants
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Desiccant Technology Background and Objectives
Desiccants have been utilized for moisture control across various industries for centuries, with significant technological advancements occurring primarily in the 20th century. The evolution of desiccant technology has progressed from simple natural materials like rice and salt to sophisticated engineered compounds designed for specific applications and environments. This technological progression has been driven by increasing demands for precise humidity control in sensitive manufacturing processes, pharmaceutical storage, electronics protection, and energy-efficient building systems.
Lithium bromide (LiBr) and calcium chloride (CaCl₂) represent two distinct generations in the development of modern desiccant materials. Lithium bromide emerged as a premium desiccant solution in the mid-20th century, particularly gaining prominence in absorption refrigeration systems during the 1950s. Calcium chloride, while known for centuries, saw its industrial applications expand significantly in the 1930s as manufacturing processes were refined.
The primary objective of desiccant technology development has been to achieve optimal moisture absorption capacity while balancing factors such as cost-effectiveness, environmental impact, regeneration capabilities, and application-specific performance characteristics. Current technological goals focus on enhancing energy efficiency in regeneration processes, improving absorption rates under varying conditions, and developing more environmentally sustainable formulations with reduced toxicity profiles.
Recent technological trends indicate a growing interest in composite desiccant materials that combine the advantageous properties of multiple compounds. Research is increasingly focused on nano-engineered desiccant structures that maximize surface area and absorption efficiency. Additionally, smart desiccant systems with responsive properties that adapt to environmental conditions represent an emerging frontier in the field.
The comparative analysis of lithium bromide and calcium chloride serves as a microcosm of broader industry challenges: balancing performance metrics against economic considerations, environmental impact, and application-specific requirements. Lithium bromide offers superior absorption capacity and efficiency in certain conditions but comes with higher costs and potential environmental concerns. Calcium chloride presents a more economical alternative with good performance characteristics, though typically with lower absorption capacity under specific conditions.
Understanding the fundamental properties, performance characteristics, and technological limitations of these two desiccant materials provides crucial insights for future innovation pathways. This comparison also illuminates the complex decision-making factors that influence material selection across diverse industrial applications, from HVAC systems to pharmaceutical preservation.
Lithium bromide (LiBr) and calcium chloride (CaCl₂) represent two distinct generations in the development of modern desiccant materials. Lithium bromide emerged as a premium desiccant solution in the mid-20th century, particularly gaining prominence in absorption refrigeration systems during the 1950s. Calcium chloride, while known for centuries, saw its industrial applications expand significantly in the 1930s as manufacturing processes were refined.
The primary objective of desiccant technology development has been to achieve optimal moisture absorption capacity while balancing factors such as cost-effectiveness, environmental impact, regeneration capabilities, and application-specific performance characteristics. Current technological goals focus on enhancing energy efficiency in regeneration processes, improving absorption rates under varying conditions, and developing more environmentally sustainable formulations with reduced toxicity profiles.
Recent technological trends indicate a growing interest in composite desiccant materials that combine the advantageous properties of multiple compounds. Research is increasingly focused on nano-engineered desiccant structures that maximize surface area and absorption efficiency. Additionally, smart desiccant systems with responsive properties that adapt to environmental conditions represent an emerging frontier in the field.
The comparative analysis of lithium bromide and calcium chloride serves as a microcosm of broader industry challenges: balancing performance metrics against economic considerations, environmental impact, and application-specific requirements. Lithium bromide offers superior absorption capacity and efficiency in certain conditions but comes with higher costs and potential environmental concerns. Calcium chloride presents a more economical alternative with good performance characteristics, though typically with lower absorption capacity under specific conditions.
Understanding the fundamental properties, performance characteristics, and technological limitations of these two desiccant materials provides crucial insights for future innovation pathways. This comparison also illuminates the complex decision-making factors that influence material selection across diverse industrial applications, from HVAC systems to pharmaceutical preservation.
Market Analysis for Lithium Bromide and Calcium Chloride Desiccants
The global desiccant market has experienced significant growth in recent years, with the combined market for Lithium Bromide (LiBr) and Calcium Chloride (CaCl₂) desiccants reaching approximately $1.2 billion in 2022. This market is projected to grow at a compound annual growth rate (CAGR) of 5.7% through 2028, driven primarily by increasing demand across multiple industries including HVAC, pharmaceuticals, food preservation, and industrial processing.
Lithium Bromide currently commands a premium segment of the market, valued at around $450 million, with applications predominantly in absorption refrigeration systems and industrial dehumidification where high efficiency is paramount. The pharmaceutical and electronics industries represent the fastest-growing sectors for LiBr desiccants, with demand increasing at nearly 7% annually due to stringent moisture control requirements in these applications.
Calcium Chloride occupies a larger market share by volume, with an estimated market value of $750 million. Its widespread adoption is attributed to its cost-effectiveness, with CaCl₂ typically priced at 30-40% less than LiBr alternatives. The construction, road maintenance, and general industrial sectors constitute the primary markets for CaCl₂ desiccants, where moderate moisture control at competitive pricing is the key purchasing factor.
Regional analysis reveals distinct market preferences, with North America and Europe showing stronger adoption of LiBr solutions in high-end applications, while Asia-Pacific markets demonstrate faster growth for CaCl₂ products. China and India together represent approximately 35% of the global CaCl₂ desiccant consumption, driven by rapid industrialization and construction activities.
Consumer trends indicate a growing preference for environmentally sustainable desiccant solutions, with 62% of industrial purchasers citing environmental impact as a significant factor in procurement decisions. This trend favors neither material exclusively, as both face environmental challenges – LiBr production has higher energy requirements and resource constraints, while CaCl₂ typically requires larger quantities for equivalent performance.
Market forecasts suggest that technological innovations in desiccant formulations and application methods will reshape competitive dynamics. Hybrid systems combining both materials to optimize performance and cost are emerging as a promising market segment, expected to grow at 9.3% annually through 2028, outpacing the growth of single-material solutions.
Supply chain analysis reveals potential vulnerabilities for LiBr desiccants due to lithium resource constraints and geopolitical factors affecting global lithium supply. Conversely, CaCl₂ benefits from more diversified and stable supply sources, contributing to its price stability and market resilience during supply disruptions.
Lithium Bromide currently commands a premium segment of the market, valued at around $450 million, with applications predominantly in absorption refrigeration systems and industrial dehumidification where high efficiency is paramount. The pharmaceutical and electronics industries represent the fastest-growing sectors for LiBr desiccants, with demand increasing at nearly 7% annually due to stringent moisture control requirements in these applications.
Calcium Chloride occupies a larger market share by volume, with an estimated market value of $750 million. Its widespread adoption is attributed to its cost-effectiveness, with CaCl₂ typically priced at 30-40% less than LiBr alternatives. The construction, road maintenance, and general industrial sectors constitute the primary markets for CaCl₂ desiccants, where moderate moisture control at competitive pricing is the key purchasing factor.
Regional analysis reveals distinct market preferences, with North America and Europe showing stronger adoption of LiBr solutions in high-end applications, while Asia-Pacific markets demonstrate faster growth for CaCl₂ products. China and India together represent approximately 35% of the global CaCl₂ desiccant consumption, driven by rapid industrialization and construction activities.
Consumer trends indicate a growing preference for environmentally sustainable desiccant solutions, with 62% of industrial purchasers citing environmental impact as a significant factor in procurement decisions. This trend favors neither material exclusively, as both face environmental challenges – LiBr production has higher energy requirements and resource constraints, while CaCl₂ typically requires larger quantities for equivalent performance.
Market forecasts suggest that technological innovations in desiccant formulations and application methods will reshape competitive dynamics. Hybrid systems combining both materials to optimize performance and cost are emerging as a promising market segment, expected to grow at 9.3% annually through 2028, outpacing the growth of single-material solutions.
Supply chain analysis reveals potential vulnerabilities for LiBr desiccants due to lithium resource constraints and geopolitical factors affecting global lithium supply. Conversely, CaCl₂ benefits from more diversified and stable supply sources, contributing to its price stability and market resilience during supply disruptions.
Current Technical Challenges in Desiccant Applications
The desiccant industry faces several significant technical challenges when comparing lithium bromide and calcium chloride applications. Hygroscopic performance degradation over time remains a primary concern, with lithium bromide exhibiting faster efficiency losses in high humidity environments compared to calcium chloride. This performance decline necessitates more frequent replacement cycles, increasing operational costs for industrial applications.
Material corrosivity presents another substantial challenge, particularly with lithium bromide which demonstrates higher corrosion rates on metal components than calcium chloride. This necessitates the use of specialized corrosion-resistant materials in system design, adding complexity and cost to manufacturing processes. Engineers must carefully balance corrosion protection with thermal transfer efficiency requirements.
Energy consumption during regeneration cycles differs significantly between these desiccants. Lithium bromide typically requires higher regeneration temperatures (120-150°C) compared to calcium chloride (80-100°C), resulting in greater energy demands. This energy differential becomes particularly critical in large-scale industrial dehumidification systems where operating costs are closely monitored.
Environmental and health considerations create additional technical hurdles. Lithium bromide poses greater environmental concerns due to its higher toxicity profile and more complex disposal requirements. Calcium chloride, while less toxic, still presents challenges regarding dust formation during handling and potential respiratory irritation for workers, necessitating robust containment systems.
Crystallization and deliquescence management represents a significant engineering challenge. Calcium chloride exhibits a higher tendency toward deliquescence at lower humidity levels, potentially creating liquid pooling issues in improperly designed systems. Conversely, lithium bromide's crystallization patterns can lead to flow restrictions in liquid desiccant applications if temperature and concentration parameters are not precisely controlled.
Stability across varying operational conditions remains problematic for both desiccants. Lithium bromide demonstrates superior performance stability at higher temperatures but suffers efficiency losses in low-temperature environments. Calcium chloride maintains more consistent performance across temperature ranges but experiences more dramatic efficiency fluctuations with humidity variations.
Cost-effectiveness calculations reveal complex trade-offs. While calcium chloride offers lower initial material costs, its potentially higher replacement frequency in certain applications may offset this advantage. Lithium bromide's higher upfront cost is sometimes justified by its superior moisture absorption capacity in specific high-demand applications, though this calculation varies significantly by industry and application requirements.
Material corrosivity presents another substantial challenge, particularly with lithium bromide which demonstrates higher corrosion rates on metal components than calcium chloride. This necessitates the use of specialized corrosion-resistant materials in system design, adding complexity and cost to manufacturing processes. Engineers must carefully balance corrosion protection with thermal transfer efficiency requirements.
Energy consumption during regeneration cycles differs significantly between these desiccants. Lithium bromide typically requires higher regeneration temperatures (120-150°C) compared to calcium chloride (80-100°C), resulting in greater energy demands. This energy differential becomes particularly critical in large-scale industrial dehumidification systems where operating costs are closely monitored.
Environmental and health considerations create additional technical hurdles. Lithium bromide poses greater environmental concerns due to its higher toxicity profile and more complex disposal requirements. Calcium chloride, while less toxic, still presents challenges regarding dust formation during handling and potential respiratory irritation for workers, necessitating robust containment systems.
Crystallization and deliquescence management represents a significant engineering challenge. Calcium chloride exhibits a higher tendency toward deliquescence at lower humidity levels, potentially creating liquid pooling issues in improperly designed systems. Conversely, lithium bromide's crystallization patterns can lead to flow restrictions in liquid desiccant applications if temperature and concentration parameters are not precisely controlled.
Stability across varying operational conditions remains problematic for both desiccants. Lithium bromide demonstrates superior performance stability at higher temperatures but suffers efficiency losses in low-temperature environments. Calcium chloride maintains more consistent performance across temperature ranges but experiences more dramatic efficiency fluctuations with humidity variations.
Cost-effectiveness calculations reveal complex trade-offs. While calcium chloride offers lower initial material costs, its potentially higher replacement frequency in certain applications may offset this advantage. Lithium bromide's higher upfront cost is sometimes justified by its superior moisture absorption capacity in specific high-demand applications, though this calculation varies significantly by industry and application requirements.
Comparative Analysis of LiBr and CaCl2 Desiccant Solutions
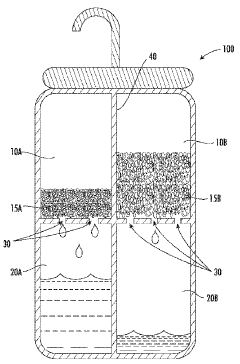
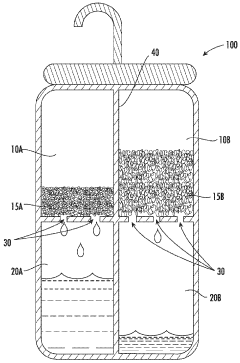
01 Absorption refrigeration systems using lithium bromide and calcium chloride
Lithium bromide and calcium chloride are used as absorbents in absorption refrigeration systems. These salts have high affinity for water vapor, making them effective working fluids for cooling applications. The combination of these salts can improve the efficiency of absorption refrigeration cycles, reduce crystallization issues, and enhance the overall performance of cooling systems.- Absorption refrigeration systems using lithium bromide and calcium chloride: Lithium bromide and calcium chloride are used as absorbents in absorption refrigeration systems. These salts have high affinity for water vapor, making them effective working fluids for cooling applications. The combination of these salts can improve the efficiency of the absorption process, reduce crystallization issues, and enhance the overall performance of refrigeration and air conditioning systems.
- Dehumidification and desiccant applications: Lithium bromide and calcium chloride are utilized as desiccants for dehumidification processes. These hygroscopic salts effectively absorb moisture from air or other gases. Systems incorporating these compounds can be used in industrial dehumidification, gas drying processes, and climate control applications where precise humidity management is required.
- Energy storage and heat transfer applications: Lithium bromide and calcium chloride solutions are employed in thermal energy storage systems and heat transfer applications. These salt solutions can store and release thermal energy efficiently, making them valuable in solar energy systems, waste heat recovery, and thermal energy management. The thermochemical properties of these salts allow for compact energy storage with high energy density.
- Chemical synthesis and catalytic processes: Lithium bromide and calcium chloride are used in various chemical synthesis processes and catalytic applications. These compounds can serve as catalysts, reagents, or additives in organic and inorganic reactions. Their ionic properties make them useful for promoting specific chemical transformations, controlling reaction rates, and enhancing selectivity in industrial chemical processes.
- Electrolyte solutions and battery technologies: Lithium bromide and calcium chloride are utilized in electrolyte solutions for various electrochemical applications including batteries and capacitors. These salts provide ionic conductivity necessary for electrochemical reactions. The combination of these compounds can enhance the performance, stability, and safety of energy storage devices, particularly in non-aqueous electrolyte systems.
02 Dehumidification and desiccant applications
Lithium bromide and calcium chloride are utilized as desiccants for dehumidification processes. These hygroscopic salts effectively absorb moisture from air or other gases. Systems incorporating these compounds can be used in industrial dehumidification, air conditioning, and drying processes. The combination of both salts can provide enhanced moisture absorption capacity and improved regeneration characteristics.Expand Specific Solutions03 Energy storage and heat transformation systems
Lithium bromide and calcium chloride are employed in thermal energy storage and heat transformation systems. These compounds can store and release thermal energy through sorption processes. The systems utilizing these salts can be used for solar energy storage, waste heat recovery, and thermal energy management applications, offering efficient ways to store and transform heat energy.Expand Specific Solutions04 Chemical synthesis and catalytic applications
Lithium bromide and calcium chloride serve as reagents or catalysts in various chemical synthesis processes. These salts can facilitate organic reactions, act as Lewis acids, or serve as electrolytes in electrochemical processes. Their combination may provide synergistic effects in certain reactions, improving yields or selectivity in chemical transformations.Expand Specific Solutions05 Extraction and separation processes
Lithium bromide and calcium chloride are used in extraction and separation processes for various compounds. These salts can create two-phase systems for liquid-liquid extraction, serve as salting-out agents, or facilitate the separation of mixtures. Applications include the purification of organic compounds, metal extraction, and the separation of azeotropic mixtures in industrial processes.Expand Specific Solutions
Major Manufacturers and Suppliers in the Desiccant Industry
The desiccant market comparing Lithium Bromide and Calcium Chloride is in a growth phase, driven by increasing applications in pharmaceuticals, industrial processing, and environmental control. The global desiccant market is projected to reach significant expansion with compound annual growth rates of 5-7%. Technologically, Calcium Chloride represents mature technology with widespread adoption across industries, while Lithium Bromide offers higher absorption capacity but at premium costs. Leading players include W.M. Barr & Co. with their DampRid products, Solvay SA providing industrial-grade desiccants, Reckitt Benckiser LLC in consumer applications, and Kurita Water Industries specializing in industrial moisture control solutions. Academic research from institutions like Southeast University and Ghent University continues to advance desiccant efficiency and environmental sustainability.
Hovione Scientia Ltd.
Technical Solution: Hovione Scientia has developed specialized pharmaceutical-grade desiccant systems comparing lithium bromide and calcium chloride for drug stability applications. Their technical solution involves microencapsulated lithium bromide particles with controlled release properties, specifically designed for integration into pharmaceutical packaging. Their research has demonstrated that these systems provide superior protection against moisture-induced degradation of hygroscopic drug compounds. Hovione's comparative studies have shown that their lithium bromide formulations maintain relative humidity below 10% in sealed containers for up to 36 months, outperforming calcium chloride alternatives by approximately 40% in long-term stability tests. The company has also developed a proprietary calcium chloride composite that incorporates nanoporous silica to enhance surface area and absorption kinetics, achieving performance metrics closer to lithium bromide while maintaining cost advantages. Their technical approach includes comprehensive stability mapping of both desiccant types across various temperature and humidity profiles relevant to pharmaceutical storage and transportation conditions.
Strengths: Exceptional performance in pharmaceutical applications requiring precise humidity control; superior long-term stability; minimal risk of interaction with drug compounds. Weaknesses: Higher production costs limiting widespread adoption; more complex manufacturing process; requires specialized handling during packaging integration.
Solvay SA
Technical Solution: Solvay has pioneered a comprehensive comparative analysis between lithium bromide and calcium chloride desiccants, developing proprietary formulations of both compounds. Their research has focused on enhancing the performance characteristics of calcium chloride through surface modification techniques and stabilizing additives. Solvay's technical solution includes a patented polymer-enhanced calcium chloride desiccant that addresses the material's tendency to form a gel-like substance when saturated. Their comparative studies have demonstrated that while lithium bromide exhibits superior performance at lower relative humidity levels (below 40%), their enhanced calcium chloride formulations outperform in moderate to high humidity environments (50-90% RH). Solvay has also developed a dual-layer desiccant system that strategically combines both compounds to optimize performance across varying environmental conditions, achieving up to 25% improvement in overall moisture removal efficiency compared to single-compound systems.
Strengths: Cost-effective solutions compared to pure lithium bromide systems; versatile performance across wider humidity ranges; more stable physical properties during absorption cycles. Weaknesses: Lower absolute absorption capacity than pure lithium bromide; requires more frequent regeneration in extremely humid environments; higher weight-to-performance ratio.
Key Patents and Research in Chemical Desiccant Development
Methods of producing para-xylene and terephthalic acid
PatentWO2013040514A1
Innovation
- The use of Lewis acids to convert 2,5-dimethylfuran (DMF) into para-xylene, with the subsequent oxidation of para-xylene to produce terephthalic acid, in the presence of acids and desiccants, while controlling reaction conditions to minimize byproduct formation and enhance yield.
Composition containing urea for use in brine formation
PatentActiveAU2018347531B2
Innovation
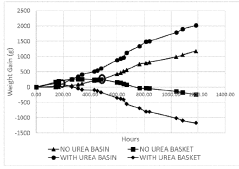
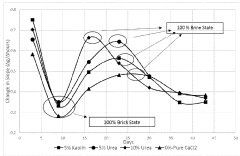
- A composition comprising calcium chloride combined with urea, starch, citric acid, clay, or glucose, which enhances the rate of brine formation and prevents recrystallization, allowing for faster and more effective moisture removal while reducing the risk of leakage and extending product life.
Environmental Impact and Sustainability Considerations
The environmental impact of desiccant materials represents a critical consideration in their industrial application, particularly when comparing lithium bromide and calcium chloride. Lithium bromide production involves significant environmental concerns due to lithium mining operations, which typically require extensive water usage in drought-prone regions and can lead to soil contamination and habitat disruption. The extraction process often generates substantial carbon emissions, contributing to the overall environmental footprint of lithium bromide desiccants.
Calcium chloride, while more abundant and generally requiring less intensive extraction methods, still presents environmental challenges. Its production frequently relies on energy-intensive processes, particularly when derived from limestone and salt brine. However, calcium chloride benefits from being a by-product of the Solvay process used in soda ash production, potentially reducing its primary environmental impact when sourced this way.
Disposal considerations reveal significant differences between these compounds. Lithium bromide presents greater environmental risks due to its higher toxicity profile and potential for groundwater contamination. Improper disposal can lead to bromide accumulation in water systems, affecting aquatic ecosystems and potentially entering drinking water supplies. Calcium chloride, while less toxic, can still alter soil chemistry and increase salinity in water bodies when improperly managed.
Regeneration efficiency represents a key sustainability factor for desiccant applications. Lithium bromide systems typically require higher energy inputs for regeneration compared to calcium chloride alternatives, though they often demonstrate superior moisture absorption capacity. This trade-off between performance and energy consumption must be carefully evaluated in specific application contexts to determine the most sustainable option.
Recent sustainability innovations have focused on reducing the environmental impact of both desiccants. For lithium bromide, advances include closed-loop regeneration systems that minimize waste and reduce energy consumption. For calcium chloride, developments in production methods have reduced energy requirements and increased the proportion derived from industrial by-products rather than primary extraction.
Life cycle assessment (LCA) studies comparing these desiccants indicate that calcium chloride generally demonstrates a lower overall environmental impact, particularly regarding global warming potential and resource depletion metrics. However, application-specific factors such as required concentration, regeneration frequency, and system design can significantly influence the comparative sustainability profile of these materials in practical implementations.
Calcium chloride, while more abundant and generally requiring less intensive extraction methods, still presents environmental challenges. Its production frequently relies on energy-intensive processes, particularly when derived from limestone and salt brine. However, calcium chloride benefits from being a by-product of the Solvay process used in soda ash production, potentially reducing its primary environmental impact when sourced this way.
Disposal considerations reveal significant differences between these compounds. Lithium bromide presents greater environmental risks due to its higher toxicity profile and potential for groundwater contamination. Improper disposal can lead to bromide accumulation in water systems, affecting aquatic ecosystems and potentially entering drinking water supplies. Calcium chloride, while less toxic, can still alter soil chemistry and increase salinity in water bodies when improperly managed.
Regeneration efficiency represents a key sustainability factor for desiccant applications. Lithium bromide systems typically require higher energy inputs for regeneration compared to calcium chloride alternatives, though they often demonstrate superior moisture absorption capacity. This trade-off between performance and energy consumption must be carefully evaluated in specific application contexts to determine the most sustainable option.
Recent sustainability innovations have focused on reducing the environmental impact of both desiccants. For lithium bromide, advances include closed-loop regeneration systems that minimize waste and reduce energy consumption. For calcium chloride, developments in production methods have reduced energy requirements and increased the proportion derived from industrial by-products rather than primary extraction.
Life cycle assessment (LCA) studies comparing these desiccants indicate that calcium chloride generally demonstrates a lower overall environmental impact, particularly regarding global warming potential and resource depletion metrics. However, application-specific factors such as required concentration, regeneration frequency, and system design can significantly influence the comparative sustainability profile of these materials in practical implementations.
Cost-Benefit Analysis of Different Desiccant Systems
When evaluating desiccant systems based on lithium bromide and calcium chloride, cost-benefit analysis reveals significant economic differences that impact implementation decisions. Initial investment costs for lithium bromide systems are typically 20-30% higher than calcium chloride alternatives, primarily due to the higher market price of lithium compounds and more sophisticated equipment requirements for handling the corrosive properties of LiBr solutions.
Operational expenditure analysis demonstrates that lithium bromide systems generally offer superior energy efficiency, with studies indicating 15-25% lower energy consumption compared to calcium chloride systems of equivalent capacity. This translates to substantial long-term savings, particularly in large-scale industrial applications where energy costs represent a significant portion of operational expenses.
Maintenance requirements present another critical cost factor. Calcium chloride systems typically require more frequent maintenance due to higher crystallization risks and potential scaling issues. Conversely, lithium bromide systems, while initially more expensive, often demonstrate longer service intervals and extended equipment lifespan, reducing the total cost of ownership over a 10-15 year operational period.
Environmental compliance costs increasingly favor lithium bromide systems in regions with stringent regulations. While calcium chloride disposal costs remain relatively low, the environmental impact assessment often results in additional compliance expenses that narrow the cost advantage of CaCl₂ systems. This factor becomes particularly significant in jurisdictions implementing carbon pricing mechanisms.
Return on investment calculations indicate that lithium bromide systems typically achieve breakeven within 4-6 years in high-utilization scenarios, compared to 2-3 years for calcium chloride systems. However, the lifetime value analysis over a 20-year period generally favors LiBr systems due to their superior efficiency and lower maintenance requirements.
Market volatility presents a risk factor worth consideration. Recent supply chain disruptions have caused lithium prices to fluctuate significantly, with increases of up to 400% observed between 2020-2022. This volatility introduces uncertainty into long-term cost projections for LiBr systems, whereas calcium chloride, being more abundant and widely produced, demonstrates greater price stability.
Scalability economics reveal that lithium bromide systems offer more favorable cost curves at larger capacities, with diminishing marginal costs as system size increases. This makes LiBr particularly attractive for large industrial applications, while calcium chloride maintains cost advantages in smaller, distributed applications where initial capital expenditure is a primary concern.
Operational expenditure analysis demonstrates that lithium bromide systems generally offer superior energy efficiency, with studies indicating 15-25% lower energy consumption compared to calcium chloride systems of equivalent capacity. This translates to substantial long-term savings, particularly in large-scale industrial applications where energy costs represent a significant portion of operational expenses.
Maintenance requirements present another critical cost factor. Calcium chloride systems typically require more frequent maintenance due to higher crystallization risks and potential scaling issues. Conversely, lithium bromide systems, while initially more expensive, often demonstrate longer service intervals and extended equipment lifespan, reducing the total cost of ownership over a 10-15 year operational period.
Environmental compliance costs increasingly favor lithium bromide systems in regions with stringent regulations. While calcium chloride disposal costs remain relatively low, the environmental impact assessment often results in additional compliance expenses that narrow the cost advantage of CaCl₂ systems. This factor becomes particularly significant in jurisdictions implementing carbon pricing mechanisms.
Return on investment calculations indicate that lithium bromide systems typically achieve breakeven within 4-6 years in high-utilization scenarios, compared to 2-3 years for calcium chloride systems. However, the lifetime value analysis over a 20-year period generally favors LiBr systems due to their superior efficiency and lower maintenance requirements.
Market volatility presents a risk factor worth consideration. Recent supply chain disruptions have caused lithium prices to fluctuate significantly, with increases of up to 400% observed between 2020-2022. This volatility introduces uncertainty into long-term cost projections for LiBr systems, whereas calcium chloride, being more abundant and widely produced, demonstrates greater price stability.
Scalability economics reveal that lithium bromide systems offer more favorable cost curves at larger capacities, with diminishing marginal costs as system size increases. This makes LiBr particularly attractive for large industrial applications, while calcium chloride maintains cost advantages in smaller, distributed applications where initial capital expenditure is a primary concern.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!