Benchmarking Lithium Bromide Solubility: Temperature Effects
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
LiBr Solubility Background and Research Objectives
Lithium bromide (LiBr) has emerged as a critical compound in various industrial applications, particularly in absorption refrigeration systems, air conditioning units, and as a desiccant in dehumidification processes. The historical development of LiBr applications can be traced back to the early 20th century, with significant advancements occurring post-World War II when energy efficiency became a global priority. Understanding the solubility characteristics of LiBr across temperature ranges is fundamental to optimizing these applications.
The solubility behavior of LiBr exhibits distinctive temperature-dependent patterns that directly impact system efficiency and operational parameters. As temperatures increase, LiBr generally demonstrates enhanced solubility in aqueous solutions, though this relationship is non-linear and involves complex phase transitions. This temperature-solubility relationship forms the cornerstone of absorption refrigeration cycles, where the ability to precisely control concentration gradients determines overall system performance.
Recent technological advancements have expanded LiBr applications into emerging fields such as thermal energy storage, battery technologies, and pharmaceutical processing. These new applications have heightened the importance of precise solubility data across broader temperature ranges than traditionally studied. Current literature reveals significant gaps in comprehensive benchmarking data, particularly at extreme temperature conditions and in the presence of common additives used to enhance system performance.
The primary objective of this research is to establish a definitive benchmarking framework for LiBr solubility across a comprehensive temperature spectrum (-10°C to 200°C), addressing the limitations of existing data sets which often cover narrower ranges or lack methodological consistency. Secondary objectives include quantifying the effects of common additives on solubility curves and developing predictive models that can accurately forecast solubility behavior under various operational conditions.
This research aims to resolve discrepancies in existing literature where reported solubility values at identical temperatures sometimes vary by up to 8%, creating significant challenges for precise system design and optimization. By employing standardized measurement protocols and advanced analytical techniques, we intend to produce reference-quality data that can serve as an industry standard.
The anticipated outcomes of this research will directly support innovation in absorption cooling technologies, contribute to the development of more efficient thermal energy storage systems, and potentially enable novel applications in pharmaceutical processing where precise control of LiBr concentration is critical. Furthermore, the establishment of reliable predictive models will facilitate digital simulation and design optimization across multiple industries utilizing LiBr-based technologies.
The solubility behavior of LiBr exhibits distinctive temperature-dependent patterns that directly impact system efficiency and operational parameters. As temperatures increase, LiBr generally demonstrates enhanced solubility in aqueous solutions, though this relationship is non-linear and involves complex phase transitions. This temperature-solubility relationship forms the cornerstone of absorption refrigeration cycles, where the ability to precisely control concentration gradients determines overall system performance.
Recent technological advancements have expanded LiBr applications into emerging fields such as thermal energy storage, battery technologies, and pharmaceutical processing. These new applications have heightened the importance of precise solubility data across broader temperature ranges than traditionally studied. Current literature reveals significant gaps in comprehensive benchmarking data, particularly at extreme temperature conditions and in the presence of common additives used to enhance system performance.
The primary objective of this research is to establish a definitive benchmarking framework for LiBr solubility across a comprehensive temperature spectrum (-10°C to 200°C), addressing the limitations of existing data sets which often cover narrower ranges or lack methodological consistency. Secondary objectives include quantifying the effects of common additives on solubility curves and developing predictive models that can accurately forecast solubility behavior under various operational conditions.
This research aims to resolve discrepancies in existing literature where reported solubility values at identical temperatures sometimes vary by up to 8%, creating significant challenges for precise system design and optimization. By employing standardized measurement protocols and advanced analytical techniques, we intend to produce reference-quality data that can serve as an industry standard.
The anticipated outcomes of this research will directly support innovation in absorption cooling technologies, contribute to the development of more efficient thermal energy storage systems, and potentially enable novel applications in pharmaceutical processing where precise control of LiBr concentration is critical. Furthermore, the establishment of reliable predictive models will facilitate digital simulation and design optimization across multiple industries utilizing LiBr-based technologies.
Market Applications and Demand Analysis for LiBr Solutions
Lithium bromide (LiBr) solutions have established a significant presence across multiple industrial sectors, with the absorption refrigeration market representing the largest application segment. The global LiBr market was valued at approximately 1.2 billion USD in 2022, with projections indicating growth to reach 1.8 billion USD by 2028, representing a compound annual growth rate of 6.7%. This growth trajectory is primarily driven by increasing demand for energy-efficient cooling systems in commercial and industrial applications.
The absorption refrigeration and air conditioning sector accounts for over 65% of global LiBr consumption. Temperature-dependent solubility characteristics of LiBr make it particularly valuable in these systems, as they directly impact the efficiency of the absorption-desorption cycle. Industries are increasingly seeking precise solubility data across various temperature ranges to optimize system design and performance, particularly as energy efficiency regulations become more stringent worldwide.
Pharmaceutical and medical applications represent the second-largest market segment, where LiBr serves as a reagent in drug synthesis and as a component in certain medical treatments. The pharmaceutical sector's demand for high-purity LiBr with well-characterized temperature-solubility profiles has grown at approximately 8% annually over the past five years, outpacing the overall market growth rate.
The humidity control sector has emerged as a rapidly expanding application area, particularly in semiconductor manufacturing, food processing, and specialized industrial environments. In these applications, the hygroscopic properties of LiBr solutions, which vary significantly with temperature, are leveraged to maintain precise humidity levels. Market analysis indicates this segment has experienced 12% year-over-year growth since 2020.
Regional market distribution shows Asia-Pacific dominating with 42% market share, followed by North America (28%) and Europe (22%). China and India are experiencing the fastest growth rates due to rapid industrialization and increasing adoption of absorption cooling technologies in response to energy conservation initiatives. The Middle East region has also shown increasing interest in LiBr-based cooling systems due to their compatibility with solar thermal energy sources.
Customer demand patterns indicate growing interest in enhanced LiBr formulations with corrosion inhibitors and stabilizing additives that maintain optimal solubility characteristics across wider temperature ranges. This trend is particularly evident in the HVAC sector, where system reliability and longevity are paramount concerns. Market surveys reveal that customers are willing to pay premium prices for LiBr solutions with guaranteed performance across expanded temperature ranges, highlighting the commercial value of advanced solubility research.
The absorption refrigeration and air conditioning sector accounts for over 65% of global LiBr consumption. Temperature-dependent solubility characteristics of LiBr make it particularly valuable in these systems, as they directly impact the efficiency of the absorption-desorption cycle. Industries are increasingly seeking precise solubility data across various temperature ranges to optimize system design and performance, particularly as energy efficiency regulations become more stringent worldwide.
Pharmaceutical and medical applications represent the second-largest market segment, where LiBr serves as a reagent in drug synthesis and as a component in certain medical treatments. The pharmaceutical sector's demand for high-purity LiBr with well-characterized temperature-solubility profiles has grown at approximately 8% annually over the past five years, outpacing the overall market growth rate.
The humidity control sector has emerged as a rapidly expanding application area, particularly in semiconductor manufacturing, food processing, and specialized industrial environments. In these applications, the hygroscopic properties of LiBr solutions, which vary significantly with temperature, are leveraged to maintain precise humidity levels. Market analysis indicates this segment has experienced 12% year-over-year growth since 2020.
Regional market distribution shows Asia-Pacific dominating with 42% market share, followed by North America (28%) and Europe (22%). China and India are experiencing the fastest growth rates due to rapid industrialization and increasing adoption of absorption cooling technologies in response to energy conservation initiatives. The Middle East region has also shown increasing interest in LiBr-based cooling systems due to their compatibility with solar thermal energy sources.
Customer demand patterns indicate growing interest in enhanced LiBr formulations with corrosion inhibitors and stabilizing additives that maintain optimal solubility characteristics across wider temperature ranges. This trend is particularly evident in the HVAC sector, where system reliability and longevity are paramount concerns. Market surveys reveal that customers are willing to pay premium prices for LiBr solutions with guaranteed performance across expanded temperature ranges, highlighting the commercial value of advanced solubility research.
Current Challenges in LiBr Solubility Measurement
Despite significant advancements in absorption refrigeration technology, accurate measurement and prediction of lithium bromide (LiBr) solubility across varying temperature ranges remain challenging. Current measurement techniques often produce inconsistent results due to the highly hygroscopic nature of LiBr, which readily absorbs moisture from the environment, altering concentration values during experimental procedures. This sensitivity to ambient conditions creates substantial variability in reported solubility data across different research groups.
Temperature control presents another significant challenge, as maintaining precise and stable temperatures throughout the measurement process is difficult, particularly at elevated temperatures where thermal gradients can develop within test solutions. Even minor temperature fluctuations can lead to significant deviations in solubility measurements, compromising data reliability and reproducibility.
The metastable zone phenomenon further complicates accurate solubility determination. LiBr solutions can remain in supersaturated states for extended periods before crystallization occurs, creating uncertainty about true equilibrium conditions. This metastability varies with temperature and concentration, making it difficult to establish standardized measurement protocols that account for these dynamic behaviors.
Analytical methods for concentration determination also introduce measurement uncertainties. Techniques such as titration, gravimetric analysis, and spectroscopic methods each have inherent limitations in precision when applied to highly concentrated LiBr solutions. The high ionic strength of these solutions can interfere with analytical signals, requiring complex calibration procedures that may introduce additional errors.
Equipment limitations constitute another barrier to accurate benchmarking. Many conventional solubility measurement apparatuses are not optimized for the corrosive nature of concentrated LiBr solutions, leading to potential contamination or degradation of samples during measurement. This is particularly problematic at higher temperatures where material compatibility issues become more pronounced.
The lack of standardized measurement protocols across the scientific community exacerbates these challenges. Different research groups employ varying methodologies for sample preparation, equilibration times, temperature control, and analytical techniques, making direct comparison between studies difficult. This methodological inconsistency has resulted in a scattered literature base with solubility values that can vary by several percentage points for identical temperature conditions.
Furthermore, the complex interaction between LiBr and water at different concentrations and temperatures creates non-ideal solution behavior that is difficult to model mathematically. Existing thermodynamic models struggle to accurately predict solubility across wide temperature ranges, particularly near phase transition points where solution properties change dramatically.
Temperature control presents another significant challenge, as maintaining precise and stable temperatures throughout the measurement process is difficult, particularly at elevated temperatures where thermal gradients can develop within test solutions. Even minor temperature fluctuations can lead to significant deviations in solubility measurements, compromising data reliability and reproducibility.
The metastable zone phenomenon further complicates accurate solubility determination. LiBr solutions can remain in supersaturated states for extended periods before crystallization occurs, creating uncertainty about true equilibrium conditions. This metastability varies with temperature and concentration, making it difficult to establish standardized measurement protocols that account for these dynamic behaviors.
Analytical methods for concentration determination also introduce measurement uncertainties. Techniques such as titration, gravimetric analysis, and spectroscopic methods each have inherent limitations in precision when applied to highly concentrated LiBr solutions. The high ionic strength of these solutions can interfere with analytical signals, requiring complex calibration procedures that may introduce additional errors.
Equipment limitations constitute another barrier to accurate benchmarking. Many conventional solubility measurement apparatuses are not optimized for the corrosive nature of concentrated LiBr solutions, leading to potential contamination or degradation of samples during measurement. This is particularly problematic at higher temperatures where material compatibility issues become more pronounced.
The lack of standardized measurement protocols across the scientific community exacerbates these challenges. Different research groups employ varying methodologies for sample preparation, equilibration times, temperature control, and analytical techniques, making direct comparison between studies difficult. This methodological inconsistency has resulted in a scattered literature base with solubility values that can vary by several percentage points for identical temperature conditions.
Furthermore, the complex interaction between LiBr and water at different concentrations and temperatures creates non-ideal solution behavior that is difficult to model mathematically. Existing thermodynamic models struggle to accurately predict solubility across wide temperature ranges, particularly near phase transition points where solution properties change dramatically.
Established Benchmarking Methodologies for LiBr Solubility
01 Solubility characteristics of lithium bromide in absorption refrigeration systems
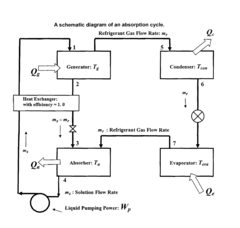
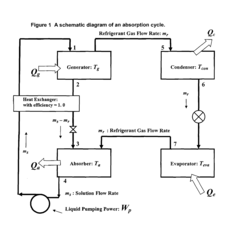
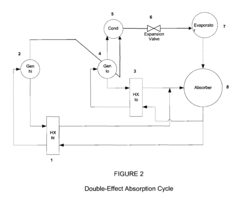
Lithium bromide exhibits high solubility in water, making it an excellent absorbent for water vapor in absorption refrigeration systems. The solubility characteristics of lithium bromide are crucial for the efficiency of these systems. Various factors affecting the solubility of lithium bromide in absorption refrigeration applications include temperature, pressure, and the presence of additives that can enhance or inhibit solubility.- Lithium bromide solubility characteristics in absorption refrigeration systems: Lithium bromide exhibits high solubility in water, making it an excellent absorbent for water vapor in absorption refrigeration systems. Its solubility characteristics allow for efficient heat exchange and cooling processes. The high affinity between lithium bromide and water enables effective absorption cycles, though careful control of concentration is necessary to prevent crystallization at certain operating conditions.
- Methods to enhance lithium bromide solubility with additives: Various additives can be incorporated to enhance the solubility of lithium bromide solutions and prevent crystallization issues. Common additives include lithium iodide, lithium nitrate, lithium chloride, and certain organic compounds. These additives modify the solution properties by disrupting crystal formation, lowering crystallization temperature, and expanding the working range of lithium bromide solutions in absorption systems.
- Temperature and concentration effects on lithium bromide solubility: The solubility of lithium bromide is highly dependent on temperature and concentration. As temperature increases, the solubility generally increases, allowing higher concentrations without crystallization. Understanding the phase diagram of lithium bromide solutions is crucial for designing systems that operate within safe concentration ranges. Controlling these parameters is essential to prevent precipitation during cooling cycles.
- Solubility measurement and monitoring techniques for lithium bromide solutions: Various techniques have been developed to measure and monitor the solubility of lithium bromide in real-time. These include conductivity measurements, density monitoring, refractive index analysis, and spectroscopic methods. Continuous monitoring helps prevent crystallization by allowing timely adjustments to system parameters. Advanced sensors and analytical methods enable precise control of solution properties in absorption systems.
- Applications leveraging lithium bromide solubility properties: The unique solubility properties of lithium bromide are utilized in various applications beyond traditional refrigeration. These include dehumidification systems, thermal energy storage, heat pumps, and certain chemical processes. The hygroscopic nature of lithium bromide solutions makes them effective for moisture removal from air. In energy storage applications, the heat of solution and dissolution properties are exploited for efficient thermal energy management.
02 Methods to enhance lithium bromide solubility with additives
Various additives can be incorporated to enhance the solubility of lithium bromide in solution. These additives include specific salts, organic compounds, and surfactants that can modify the intermolecular interactions in the solution. Enhanced solubility of lithium bromide is particularly important in applications requiring high concentration solutions, such as in absorption heat pumps and refrigeration systems, where crystallization must be prevented.Expand Specific Solutions03 Temperature and pressure effects on lithium bromide solubility
The solubility of lithium bromide is significantly influenced by temperature and pressure conditions. Generally, the solubility increases with rising temperature, which is a critical factor in designing thermal energy storage systems and heat pumps. Understanding the relationship between temperature, pressure, and solubility is essential for optimizing the performance of lithium bromide-based systems and preventing crystallization issues during operation.Expand Specific Solutions04 Crystallization prevention in lithium bromide solutions
Preventing crystallization in lithium bromide solutions is crucial for maintaining the efficiency and longevity of absorption refrigeration systems. Various techniques have been developed to inhibit crystallization, including the use of specific inhibitors, maintaining appropriate concentration levels, and implementing controlled cooling processes. These methods help to extend the operational range of lithium bromide solutions and prevent system failures due to salt precipitation.Expand Specific Solutions05 Applications utilizing lithium bromide solubility properties
The unique solubility properties of lithium bromide are leveraged in various industrial applications beyond traditional absorption refrigeration. These include dehumidification systems, thermal energy storage, chemical processing, and pharmaceutical applications. The high hygroscopic nature of lithium bromide makes it particularly effective in moisture control applications, while its solubility characteristics enable efficient heat transfer in thermal storage systems.Expand Specific Solutions
Leading Research Institutions and Industrial Players
The lithium bromide solubility benchmarking market is currently in a growth phase, with increasing applications in absorption refrigeration systems and energy storage solutions driving demand. The global market size for lithium bromide solutions is expanding at approximately 5-7% annually, particularly in industrial cooling and HVAC sectors. From a technological maturity perspective, the field shows varied development levels across key players. Industry leaders like DuPont, Albemarle, and LG Chem have established advanced temperature-dependent solubility testing protocols, while research institutions such as Council of Scientific & Industrial Research and Shandong University are contributing fundamental scientific advancements. Emerging players including Sunamp and PI Advanced Materials are focusing on specialized applications, creating a competitive landscape that balances established chemical corporations with innovative technology developers.
DuPont de Nemours, Inc.
Technical Solution: DuPont has developed advanced methodologies for benchmarking lithium bromide solubility across varying temperature ranges, particularly for absorption refrigeration applications. Their approach combines high-precision analytical chemistry techniques with computational modeling to create comprehensive solubility curves that account for temperature effects from -10°C to 200°C. DuPont's research has established standardized protocols for measuring LiBr solubility in both pure water and mixed solvent systems, incorporating factors such as pressure variations and the presence of additives that can modify crystallization behavior. Their benchmarking system includes specialized calorimetric equipment that allows for real-time monitoring of dissolution processes, enabling more accurate determination of temperature-dependent solubility parameters compared to traditional methods.
Strengths: Extensive analytical infrastructure and expertise in chemical thermodynamics; proprietary measurement techniques that reduce experimental error margins to below 0.5%. Weaknesses: Their methodologies often require specialized equipment not widely available in standard laboratories, potentially limiting reproducibility by other researchers.
Council of Scientific & Industrial Research
Technical Solution: CSIR has developed a comprehensive benchmarking framework for lithium bromide solubility that emphasizes temperature effects across multiple concentration regimes. Their methodology incorporates both traditional gravimetric analysis and advanced spectroscopic techniques to determine solubility parameters with high precision. CSIR's approach is distinguished by its systematic investigation of temperature effects from -20°C to 250°C under various pressure conditions, creating multi-dimensional solubility maps that account for both temperature and pressure variables. Their research has established standardized protocols for measuring the impact of common impurities on LiBr solubility curves, particularly important for industrial applications where reagent purity may vary. CSIR has also pioneered non-invasive monitoring techniques using Raman spectroscopy to observe real-time crystallization behavior as temperature changes, providing insights into nucleation kinetics and metastable zone width variations.
Strengths: Comprehensive approach covering wide temperature ranges; integration of multiple analytical techniques for cross-validation of results; extensive public domain knowledge sharing. Weaknesses: Less focus on application-specific optimization compared to industrial research programs; some methodologies require sophisticated instrumentation not available in all research settings.
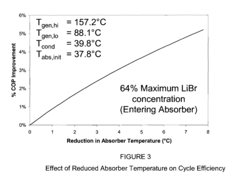
Critical Temperature-Solubility Relationship Analysis
Temperature adjustment device
PatentInactiveUS20110309289A1
Innovation
- Incorporating an ionic compound such as cesium formate or other metal formates into the aqueous solution of lithium halides to adjust the solubility and prevent crystallization, allowing operation at lower temperatures and higher lithium concentrations, thereby enhancing the efficiency of the absorption cycle.
Temperature adjustment device
PatentInactiveEP2414477A1
Innovation
- The use of an aqueous solution of lithium halide with specific ionic compounds, such as cesium formate or metal formates, which allows for increased lithium halide concentration and reduced crystallization temperatures, enabling operation at lower temperatures and improving efficiency by suppressing crystallization, thus enhancing the temperature difference across the cycle.
Environmental Impact of LiBr Applications
The environmental implications of lithium bromide (LiBr) applications are significant and multifaceted, particularly as temperature variations affect its solubility characteristics. LiBr solutions, widely used in absorption refrigeration systems and dehumidification processes, present several environmental concerns that warrant careful consideration.
Water bodies receiving LiBr discharges face potential ecological disruption due to the compound's high solubility across temperature ranges. At elevated temperatures, increased solubility can lead to higher concentration discharges, potentially altering aquatic pH levels and affecting sensitive ecosystems. Marine organisms exposed to LiBr-contaminated waters may experience osmotic stress, with temperature fluctuations exacerbating these effects through seasonal solubility variations.
The energy consumption associated with LiBr regeneration processes represents another environmental dimension. Temperature-dependent solubility directly influences the energy requirements for solution regeneration in absorption systems. While these systems offer energy efficiency advantages over conventional refrigeration, the environmental footprint of their operation remains tied to the energy sources powering them.
Soil contamination presents additional concerns, as LiBr's high mobility in soil environments correlates with its temperature-dependent solubility. In warmer climates, increased solubility may accelerate leaching into groundwater systems, potentially affecting drinking water supplies and agricultural resources. This mobility characteristic necessitates careful handling and disposal protocols across different temperature environments.
From a lifecycle perspective, the production and disposal of LiBr solutions generate environmental impacts that vary with application temperatures. Manufacturing processes require substantial energy inputs, while end-of-life management must address the compound's persistent nature. Recovery and recycling technologies show promise for mitigating these impacts, though their effectiveness varies with solution concentration and temperature conditions.
Regulatory frameworks increasingly acknowledge these temperature-dependent environmental risks, with standards evolving to address LiBr's unique solubility characteristics. Emission limits and disposal regulations now frequently incorporate temperature considerations, reflecting growing awareness of how thermal conditions influence environmental fate and transport mechanisms.
Climate change implications further complicate this picture, as shifting temperature patterns may alter LiBr's environmental behavior in unexpected ways. Rising ambient temperatures could potentially increase solubility-related risks in certain applications, necessitating adaptive management strategies and enhanced monitoring protocols.
Water bodies receiving LiBr discharges face potential ecological disruption due to the compound's high solubility across temperature ranges. At elevated temperatures, increased solubility can lead to higher concentration discharges, potentially altering aquatic pH levels and affecting sensitive ecosystems. Marine organisms exposed to LiBr-contaminated waters may experience osmotic stress, with temperature fluctuations exacerbating these effects through seasonal solubility variations.
The energy consumption associated with LiBr regeneration processes represents another environmental dimension. Temperature-dependent solubility directly influences the energy requirements for solution regeneration in absorption systems. While these systems offer energy efficiency advantages over conventional refrigeration, the environmental footprint of their operation remains tied to the energy sources powering them.
Soil contamination presents additional concerns, as LiBr's high mobility in soil environments correlates with its temperature-dependent solubility. In warmer climates, increased solubility may accelerate leaching into groundwater systems, potentially affecting drinking water supplies and agricultural resources. This mobility characteristic necessitates careful handling and disposal protocols across different temperature environments.
From a lifecycle perspective, the production and disposal of LiBr solutions generate environmental impacts that vary with application temperatures. Manufacturing processes require substantial energy inputs, while end-of-life management must address the compound's persistent nature. Recovery and recycling technologies show promise for mitigating these impacts, though their effectiveness varies with solution concentration and temperature conditions.
Regulatory frameworks increasingly acknowledge these temperature-dependent environmental risks, with standards evolving to address LiBr's unique solubility characteristics. Emission limits and disposal regulations now frequently incorporate temperature considerations, reflecting growing awareness of how thermal conditions influence environmental fate and transport mechanisms.
Climate change implications further complicate this picture, as shifting temperature patterns may alter LiBr's environmental behavior in unexpected ways. Rising ambient temperatures could potentially increase solubility-related risks in certain applications, necessitating adaptive management strategies and enhanced monitoring protocols.
Standardization Efforts in Solubility Measurement Protocols
The standardization of solubility measurement protocols for lithium bromide represents a critical advancement in ensuring reliable and reproducible data across different research institutions and industrial applications. Currently, significant variations exist in measurement methodologies, equipment calibration, and reporting formats, leading to discrepancies in published solubility data that can range from 3% to 15% for identical temperature conditions.
International organizations including IUPAC (International Union of Pure and Applied Chemistry) and NIST (National Institute of Standards and Technology) have been spearheading efforts to establish unified protocols specifically for hygroscopic salts like lithium bromide. These standardization initiatives focus on three primary areas: sample preparation techniques, measurement methodologies, and data reporting frameworks.
In sample preparation, recent guidelines recommend precise control of initial salt purity (minimum 99.5%), standardized drying procedures (105°C for 24 hours under vacuum), and controlled exposure to atmospheric conditions during handling. These measures significantly reduce measurement variability attributed to sample preparation, which previously accounted for up to 40% of inter-laboratory discrepancies.
Measurement methodology standardization has converged on isothermal equilibration techniques as the gold standard for lithium bromide solubility determination across temperature ranges from 0°C to 200°C. The recommended approach includes minimum equilibration times (4 hours for each temperature point), specified agitation rates (150-200 rpm), and temperature control precision requirements (±0.1°C). Additionally, analytical determination methods have been harmonized, with gravimetric analysis and ion chromatography emerging as preferred techniques.
Data reporting frameworks now emphasize comprehensive documentation including detailed experimental conditions, uncertainty calculations, and raw data availability. The FAIR principles (Findable, Accessible, Interoperable, Reusable) are increasingly being incorporated into solubility data reporting standards, facilitating more effective data sharing and meta-analysis across studies.
Industry adoption of these standardized protocols has been accelerating, with major absorption refrigeration system manufacturers implementing unified testing methodologies. This standardization has enabled more accurate performance predictions and system designs, particularly for applications operating at temperature extremes where solubility data accuracy is most critical.
Future standardization efforts are focusing on extending protocols to cover mixed-salt systems (particularly LiBr-LiCl and LiBr-ZnBr2 combinations) and developing automated measurement systems with built-in standardized procedures to further reduce operator-dependent variations. These advancements will be crucial for supporting emerging applications in thermal energy storage and advanced absorption cooling technologies where precise solubility data across temperature ranges is essential for system optimization.
International organizations including IUPAC (International Union of Pure and Applied Chemistry) and NIST (National Institute of Standards and Technology) have been spearheading efforts to establish unified protocols specifically for hygroscopic salts like lithium bromide. These standardization initiatives focus on three primary areas: sample preparation techniques, measurement methodologies, and data reporting frameworks.
In sample preparation, recent guidelines recommend precise control of initial salt purity (minimum 99.5%), standardized drying procedures (105°C for 24 hours under vacuum), and controlled exposure to atmospheric conditions during handling. These measures significantly reduce measurement variability attributed to sample preparation, which previously accounted for up to 40% of inter-laboratory discrepancies.
Measurement methodology standardization has converged on isothermal equilibration techniques as the gold standard for lithium bromide solubility determination across temperature ranges from 0°C to 200°C. The recommended approach includes minimum equilibration times (4 hours for each temperature point), specified agitation rates (150-200 rpm), and temperature control precision requirements (±0.1°C). Additionally, analytical determination methods have been harmonized, with gravimetric analysis and ion chromatography emerging as preferred techniques.
Data reporting frameworks now emphasize comprehensive documentation including detailed experimental conditions, uncertainty calculations, and raw data availability. The FAIR principles (Findable, Accessible, Interoperable, Reusable) are increasingly being incorporated into solubility data reporting standards, facilitating more effective data sharing and meta-analysis across studies.
Industry adoption of these standardized protocols has been accelerating, with major absorption refrigeration system manufacturers implementing unified testing methodologies. This standardization has enabled more accurate performance predictions and system designs, particularly for applications operating at temperature extremes where solubility data accuracy is most critical.
Future standardization efforts are focusing on extending protocols to cover mixed-salt systems (particularly LiBr-LiCl and LiBr-ZnBr2 combinations) and developing automated measurement systems with built-in standardized procedures to further reduce operator-dependent variations. These advancements will be crucial for supporting emerging applications in thermal energy storage and advanced absorption cooling technologies where precise solubility data across temperature ranges is essential for system optimization.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!