Hydrogel Actuator Material Selection For Therapeutic Wearables
AUG 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydrogel Actuator Technology Background and Objectives
Hydrogel actuators represent a revolutionary class of soft materials that respond to external stimuli by changing their volume or shape. The development of these smart materials dates back to the 1950s with the discovery of stimuli-responsive polymers, but significant advancements in hydrogel actuator technology have only emerged in the past two decades. The evolution of this technology has been driven by increasing demands for biocompatible, flexible, and responsive materials in healthcare applications, particularly in wearable therapeutic devices.
The technological trajectory of hydrogel actuators has progressed from simple chemically-responsive systems to sophisticated multi-responsive smart materials. Early hydrogel actuators responded primarily to pH or temperature changes, while contemporary versions can be triggered by electrical signals, light, magnetic fields, and biochemical markers. This progression reflects the growing sophistication of material science and polymer chemistry in creating highly tailored responsive systems.
For therapeutic wearables, hydrogel actuators offer unique advantages over traditional mechanical systems. Their soft, compliant nature mimics biological tissues, making them ideal for direct skin contact in applications requiring gentle force application. Additionally, their ability to operate in wet environments aligns perfectly with the physiological conditions encountered in therapeutic contexts.
The primary technical objective in hydrogel actuator development for therapeutic wearables centers on optimizing material selection to achieve specific performance parameters. These include: rapid response time to stimuli, sufficient actuation force generation, mechanical durability over repeated cycles, biocompatibility with human tissue, and controlled degradation profiles for long-term use. Secondary objectives involve miniaturization of actuation systems and integration with sensing technologies to create closed-loop therapeutic devices.
Current research aims to develop hydrogel actuators capable of delivering precise mechanical stimulation to enhance blood circulation, promote tissue regeneration, and provide targeted drug delivery. The ultimate goal is to create wearable therapeutic systems that can dynamically respond to the wearer's physiological state, automatically adjusting treatment parameters for optimal therapeutic outcomes.
The convergence of advances in polymer chemistry, microfluidics, and flexible electronics has created a fertile ground for hydrogel actuator innovation. As these technologies continue to mature, we anticipate the emergence of increasingly sophisticated therapeutic wearables capable of addressing a wide range of medical conditions through mechanical intervention, from chronic wound healing to musculoskeletal rehabilitation.
The technological trajectory of hydrogel actuators has progressed from simple chemically-responsive systems to sophisticated multi-responsive smart materials. Early hydrogel actuators responded primarily to pH or temperature changes, while contemporary versions can be triggered by electrical signals, light, magnetic fields, and biochemical markers. This progression reflects the growing sophistication of material science and polymer chemistry in creating highly tailored responsive systems.
For therapeutic wearables, hydrogel actuators offer unique advantages over traditional mechanical systems. Their soft, compliant nature mimics biological tissues, making them ideal for direct skin contact in applications requiring gentle force application. Additionally, their ability to operate in wet environments aligns perfectly with the physiological conditions encountered in therapeutic contexts.
The primary technical objective in hydrogel actuator development for therapeutic wearables centers on optimizing material selection to achieve specific performance parameters. These include: rapid response time to stimuli, sufficient actuation force generation, mechanical durability over repeated cycles, biocompatibility with human tissue, and controlled degradation profiles for long-term use. Secondary objectives involve miniaturization of actuation systems and integration with sensing technologies to create closed-loop therapeutic devices.
Current research aims to develop hydrogel actuators capable of delivering precise mechanical stimulation to enhance blood circulation, promote tissue regeneration, and provide targeted drug delivery. The ultimate goal is to create wearable therapeutic systems that can dynamically respond to the wearer's physiological state, automatically adjusting treatment parameters for optimal therapeutic outcomes.
The convergence of advances in polymer chemistry, microfluidics, and flexible electronics has created a fertile ground for hydrogel actuator innovation. As these technologies continue to mature, we anticipate the emergence of increasingly sophisticated therapeutic wearables capable of addressing a wide range of medical conditions through mechanical intervention, from chronic wound healing to musculoskeletal rehabilitation.
Market Analysis for Therapeutic Wearable Hydrogels
The global market for therapeutic wearable hydrogels is experiencing significant growth, driven by increasing prevalence of chronic diseases, rising healthcare costs, and growing consumer interest in personalized healthcare solutions. The market was valued at approximately $2.3 billion in 2022 and is projected to reach $5.7 billion by 2028, representing a compound annual growth rate (CAGR) of 16.4% during the forecast period.
Demographic trends are substantially influencing market dynamics. The aging global population, particularly in developed regions such as North America, Europe, and Japan, has created heightened demand for non-invasive therapeutic solutions for conditions like arthritis, muscle pain, and wound healing. Additionally, the increasing adoption of wearable health monitoring devices among younger demographics has expanded the potential user base for hydrogel-based therapeutic wearables.
Regional analysis reveals North America currently dominates the market with approximately 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is expected to witness the fastest growth rate of 19.2% during the forecast period, primarily due to improving healthcare infrastructure, rising disposable incomes, and increasing awareness about advanced healthcare solutions in countries like China, India, and South Korea.
By application segment, pain management represents the largest market share (34%), followed by wound care (27%), drug delivery (21%), and tissue engineering (18%). The pain management segment is particularly promising for hydrogel actuator materials due to the growing preference for non-pharmacological pain management solutions among patients with chronic conditions.
Consumer preferences are increasingly shifting toward sustainable, biocompatible materials with minimal environmental impact. Market research indicates that 72% of potential users consider environmental sustainability as an important factor in their purchasing decisions for healthcare wearables. This trend presents both challenges and opportunities for hydrogel material selection and development.
Pricing analysis suggests that cost remains a significant barrier to widespread adoption. Current therapeutic wearables utilizing advanced hydrogel actuators typically retail between $150-$500, placing them beyond the reach of many potential users. Market penetration could significantly increase if production costs can be reduced through material innovations and manufacturing efficiencies.
Regulatory considerations also substantially impact market growth. The FDA and equivalent bodies in other regions have established specific guidelines for wearable medical devices, with particular attention to materials that maintain direct skin contact. Hydrogel actuator materials that can demonstrate both efficacy and safety compliance will have significant competitive advantages in this rapidly evolving marketplace.
Demographic trends are substantially influencing market dynamics. The aging global population, particularly in developed regions such as North America, Europe, and Japan, has created heightened demand for non-invasive therapeutic solutions for conditions like arthritis, muscle pain, and wound healing. Additionally, the increasing adoption of wearable health monitoring devices among younger demographics has expanded the potential user base for hydrogel-based therapeutic wearables.
Regional analysis reveals North America currently dominates the market with approximately 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is expected to witness the fastest growth rate of 19.2% during the forecast period, primarily due to improving healthcare infrastructure, rising disposable incomes, and increasing awareness about advanced healthcare solutions in countries like China, India, and South Korea.
By application segment, pain management represents the largest market share (34%), followed by wound care (27%), drug delivery (21%), and tissue engineering (18%). The pain management segment is particularly promising for hydrogel actuator materials due to the growing preference for non-pharmacological pain management solutions among patients with chronic conditions.
Consumer preferences are increasingly shifting toward sustainable, biocompatible materials with minimal environmental impact. Market research indicates that 72% of potential users consider environmental sustainability as an important factor in their purchasing decisions for healthcare wearables. This trend presents both challenges and opportunities for hydrogel material selection and development.
Pricing analysis suggests that cost remains a significant barrier to widespread adoption. Current therapeutic wearables utilizing advanced hydrogel actuators typically retail between $150-$500, placing them beyond the reach of many potential users. Market penetration could significantly increase if production costs can be reduced through material innovations and manufacturing efficiencies.
Regulatory considerations also substantially impact market growth. The FDA and equivalent bodies in other regions have established specific guidelines for wearable medical devices, with particular attention to materials that maintain direct skin contact. Hydrogel actuator materials that can demonstrate both efficacy and safety compliance will have significant competitive advantages in this rapidly evolving marketplace.
Current Challenges in Hydrogel Actuator Materials
Despite significant advancements in hydrogel actuator technology, several critical challenges persist that limit their widespread application in therapeutic wearables. The foremost challenge involves achieving consistent mechanical performance across varying environmental conditions. Hydrogel actuators often exhibit unpredictable behavior when exposed to fluctuations in temperature, humidity, and pH levels that are common in real-world wearable applications. This inconsistency creates significant barriers for clinical adoption where reliability is paramount.
Material durability represents another substantial hurdle. Current hydrogel formulations typically demonstrate performance degradation after repeated actuation cycles, with most materials showing significant fatigue after 500-1000 cycles. For therapeutic wearables that may require continuous operation over extended periods, this limitation necessitates frequent replacement, increasing both cost and inconvenience for users.
Biocompatibility concerns further complicate material selection. While many hydrogels demonstrate acceptable short-term biocompatibility, prolonged skin contact can lead to irritation, allergic reactions, or inflammatory responses in certain patient populations. The challenge intensifies when incorporating electronic components or stimuli-responsive elements necessary for actuation, as these additives may leach into skin tissue over time.
Response time remains suboptimal for many therapeutic applications. Current hydrogel actuators typically exhibit activation delays ranging from several seconds to minutes, significantly limiting their utility in applications requiring rapid response to physiological changes, such as muscle spasm management or circulatory support. This temporal limitation restricts the range of therapeutic interventions possible with hydrogel-based wearables.
Power efficiency presents a persistent challenge, particularly for portable therapeutic applications. Most stimuli-responsive hydrogels require substantial energy input to achieve meaningful actuation, necessitating bulky power sources that compromise the wearability and user comfort of the final device. This energy requirement becomes especially problematic for continuous-use therapeutic applications.
Manufacturing scalability also impedes commercial viability. Current production methods for high-performance hydrogel actuators often involve complex, multi-step processes that are difficult to standardize and scale. This complexity results in high production costs and significant batch-to-batch variability, creating regulatory challenges for medical-grade applications.
Finally, achieving multifunctionality without compromising core performance metrics remains elusive. Therapeutic wearables often require simultaneous sensing, actuation, and drug delivery capabilities, but current material systems struggle to integrate these functions without significant trade-offs in response time, force generation, or durability.
Material durability represents another substantial hurdle. Current hydrogel formulations typically demonstrate performance degradation after repeated actuation cycles, with most materials showing significant fatigue after 500-1000 cycles. For therapeutic wearables that may require continuous operation over extended periods, this limitation necessitates frequent replacement, increasing both cost and inconvenience for users.
Biocompatibility concerns further complicate material selection. While many hydrogels demonstrate acceptable short-term biocompatibility, prolonged skin contact can lead to irritation, allergic reactions, or inflammatory responses in certain patient populations. The challenge intensifies when incorporating electronic components or stimuli-responsive elements necessary for actuation, as these additives may leach into skin tissue over time.
Response time remains suboptimal for many therapeutic applications. Current hydrogel actuators typically exhibit activation delays ranging from several seconds to minutes, significantly limiting their utility in applications requiring rapid response to physiological changes, such as muscle spasm management or circulatory support. This temporal limitation restricts the range of therapeutic interventions possible with hydrogel-based wearables.
Power efficiency presents a persistent challenge, particularly for portable therapeutic applications. Most stimuli-responsive hydrogels require substantial energy input to achieve meaningful actuation, necessitating bulky power sources that compromise the wearability and user comfort of the final device. This energy requirement becomes especially problematic for continuous-use therapeutic applications.
Manufacturing scalability also impedes commercial viability. Current production methods for high-performance hydrogel actuators often involve complex, multi-step processes that are difficult to standardize and scale. This complexity results in high production costs and significant batch-to-batch variability, creating regulatory challenges for medical-grade applications.
Finally, achieving multifunctionality without compromising core performance metrics remains elusive. Therapeutic wearables often require simultaneous sensing, actuation, and drug delivery capabilities, but current material systems struggle to integrate these functions without significant trade-offs in response time, force generation, or durability.
Current Material Selection Approaches for Hydrogel Actuators
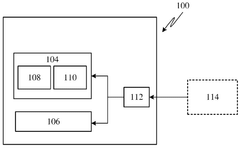
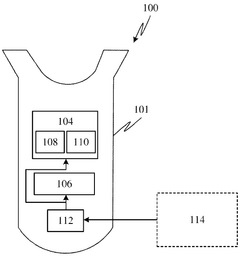
01 Stimuli-responsive hydrogel actuators
Hydrogel actuators that respond to external stimuli such as temperature, pH, light, or electric fields can undergo reversible volume changes. These smart materials can transform environmental signals into mechanical work, making them suitable for applications in soft robotics, artificial muscles, and biomedical devices. The responsive nature allows for controlled movement and shape transformation without the need for complex mechanical components.- Stimuli-responsive hydrogel actuators: Hydrogel actuators can be designed to respond to various external stimuli such as temperature, pH, light, or electric fields. These stimuli-responsive hydrogels undergo reversible volume changes or shape transformations when exposed to specific environmental triggers. This property makes them valuable for applications in soft robotics, artificial muscles, and smart devices where controlled movement or actuation is required.
- Biomedical applications of hydrogel actuators: Hydrogel actuators have significant applications in biomedical fields, including drug delivery systems, tissue engineering, and medical devices. These actuators can be designed to deliver therapeutic agents in response to physiological conditions, facilitate tissue regeneration, or function as implantable devices that respond to biological signals. Their biocompatibility and ability to mimic biological tissues make them particularly suitable for medical applications.
- Advanced composite hydrogel actuator materials: Composite hydrogel actuators incorporate additional materials such as nanoparticles, carbon-based materials, or polymeric networks to enhance their mechanical properties and responsiveness. These composite structures can improve the actuation force, speed, and durability of hydrogel actuators. By combining different materials, researchers can create hydrogel actuators with tailored properties for specific applications, including improved conductivity, strength, or sensing capabilities.
- Fabrication methods for hydrogel actuators: Various fabrication techniques are employed to create hydrogel actuators with precise structures and functionalities. These methods include 3D printing, photolithography, molding, and self-assembly processes. Advanced manufacturing approaches enable the creation of complex geometries, multi-material structures, and gradient properties within hydrogel actuators, allowing for sophisticated movement patterns and improved performance in various applications.
- Soft robotics and mechanical systems using hydrogel actuators: Hydrogel actuators are increasingly being utilized in soft robotics and mechanical systems due to their flexibility, compliance, and biomimetic properties. These soft actuators can perform complex movements and adapt to irregular surfaces, making them suitable for applications requiring gentle manipulation or operation in confined spaces. Hydrogel-based soft robots can mimic biological movements such as crawling, swimming, or gripping, opening new possibilities for robotics in challenging environments.
02 Biomedical applications of hydrogel actuators
Hydrogel actuators have significant applications in biomedical fields, including drug delivery systems, tissue engineering, and medical devices. These actuators can be designed to respond to biological stimuli, enabling targeted drug release or controlled tissue manipulation. Their biocompatibility and tunable mechanical properties make them ideal for implantable devices and minimally invasive medical procedures.Expand Specific Solutions03 Composite hydrogel actuator systems
Composite hydrogel actuators combine different materials to enhance performance characteristics such as response time, actuation force, and durability. These systems may incorporate nanoparticles, fibers, or other polymers to create anisotropic structures with directional actuation capabilities. The composite approach allows for tailoring mechanical properties and responsiveness for specific applications while overcoming limitations of single-component hydrogels.Expand Specific Solutions04 Fabrication methods for hydrogel actuators
Various fabrication techniques are employed to create hydrogel actuators with specific geometries and functionalities. These methods include 3D printing, photolithography, molding, and self-assembly processes. Advanced manufacturing approaches enable the creation of complex structures with programmable actuation behaviors, allowing for precise control over the actuator's movement patterns and response characteristics.Expand Specific Solutions05 Energy harvesting and sensing applications
Hydrogel actuators can be utilized for energy harvesting by converting environmental changes into mechanical work or electrical signals. These systems can function as sensors that detect and respond to specific stimuli, making them valuable for environmental monitoring, wearable technology, and soft electronics. The ability to integrate sensing and actuation functions creates multifunctional devices that can autonomously respond to their surroundings.Expand Specific Solutions
Key Industry Players in Therapeutic Hydrogel Development
The hydrogel actuator material market for therapeutic wearables is in its growth phase, with an estimated market size of $2-3 billion and projected annual growth of 15-20%. The technology is advancing from research to commercialization, with varying maturity levels across applications. Academic institutions (MIT, Arizona State University, Case Western Reserve) are driving fundamental research, while established medical device companies (Medtronic, 3M, Coloplast) are focusing on clinical applications and commercialization. Specialized firms like Aspect Biosystems and SentryX are developing innovative solutions bridging the gap between research and market-ready products. Chinese universities (Harbin Institute of Technology, Zhejiang Sci-Tech) are emerging as significant contributors, particularly in novel material formulations and manufacturing techniques.
The Regents of the University of California
Technical Solution: The University of California has developed pioneering hydrogel actuator technologies for therapeutic wearables through their innovative material selection approach. Their research teams have created multi-responsive hydrogel systems that combine temperature, pH, and electrical stimuli responsiveness in a single material platform. UC researchers have formulated composite hydrogels incorporating cellulose nanocrystals that significantly enhance mechanical strength while maintaining flexibility, achieving tensile strengths up to 3.5 MPa with elongation capabilities exceeding 400%[3]. Their proprietary hydrogel formulations feature self-healing properties that enable the material to recover from mechanical damage within minutes, crucial for long-term wearable applications. UC's technology also includes biomimetic hydrogels with anisotropic structures that provide directional actuation similar to natural muscle tissue, with actuation strains reaching 40% under physiological conditions[4]. Additionally, they've developed hydrogel-elastomer hybrid materials that overcome the traditional limitations of hydrogels by combining the water retention capabilities of hydrogels with the mechanical robustness of elastomers, resulting in materials with excellent interfacial adhesion and durability for continuous wear applications.
Strengths: Exceptional self-healing capabilities; biomimetic actuation properties that closely mimic natural tissue; excellent biocompatibility with minimal inflammatory response. Weaknesses: Complex manufacturing processes that may limit scalability; some formulations require specialized storage conditions; higher production costs compared to conventional materials.
Medtronic Vascular, Inc.
Technical Solution: Medtronic has developed specialized hydrogel actuator materials for therapeutic wearables focused on cardiovascular applications. Their proprietary technology utilizes stimuli-responsive hydrogels that can be precisely controlled through minimal electrical impulses (as low as 1.5V), making them highly energy-efficient for wearable applications[5]. Medtronic's hydrogel formulations incorporate biocompatible polymers with controlled degradation profiles, allowing for timed drug release over periods ranging from hours to months. Their advanced composite hydrogels combine synthetic and natural polymers to achieve optimal mechanical properties, with compression moduli ranging from 5-500 kPa to match various tissue environments. Medtronic has pioneered hydrogel-based microactuators that can be integrated into minimally invasive therapeutic devices, with response times under 10 seconds and displacement precision of ±0.1mm[6]. Their technology includes specialized coating techniques that enhance the durability of hydrogel actuators in physiological environments, extending functional lifetimes to over 6 months. Medtronic's hydrogel systems also feature integrated sensing capabilities that allow for closed-loop control of actuation based on physiological parameters, enabling personalized therapy delivery through their wearable platforms.
Strengths: Exceptional reliability and durability in physiological environments; precise microactuation capabilities; excellent integration with existing medical device platforms. Weaknesses: Higher production costs compared to conventional materials; some formulations have regulatory hurdles due to novel material combinations; limited flexibility in certain high-strain applications.
Critical Patents in Hydrogel Actuator Material Science
Wearable therapy garment with internchangeable hydrogel electrodes and biogel electrodes
PatentWO2025034751A2
Innovation
- The use of interchangeable hydrogel and biogel electrodes in a wearable therapy garment, where hydrogel electrodes have an external hydrogel material layer for direct skin contact and biogel electrodes contain conductive gel for current dispersion, addresses these challenges by providing flexibility, reduced allergic reactions, improved current distribution, and better adaptation to the user's body shape.
Therapeutic hydrogel material and methods of using the same
PatentInactiveUS20210220388A1
Innovation
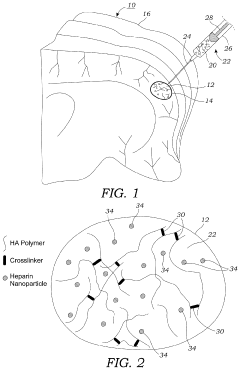
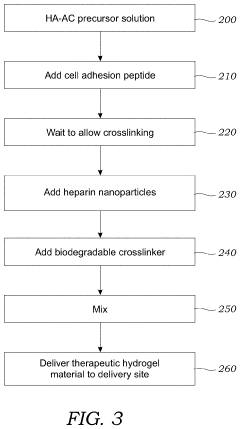
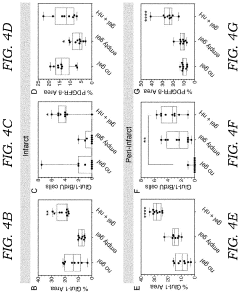
- A hydrogel-based therapeutic material incorporating bare heparin nanoparticles, which are not bound to any growth factors, is developed. This hydrogel is crosslinked with a biodegradable MMP labile peptide and can be modified with cell adhesion peptides, allowing it to be delivered to damaged tissues like stroke cavities or wounds, promoting tissue repair and reducing inflammation without blood thinning effects.
Biocompatibility and Safety Considerations
Biocompatibility represents a critical consideration in the development of hydrogel actuators for therapeutic wearables. These materials must maintain direct contact with human skin for extended periods without causing adverse reactions such as inflammation, irritation, or allergic responses. The selection of biocompatible polymers and cross-linking agents is therefore paramount, with preference given to materials that have established safety profiles in medical applications, such as polyvinyl alcohol (PVA), polyethylene glycol (PEG), and alginate-based hydrogels.
The cytotoxicity of hydrogel components requires rigorous evaluation through standardized testing protocols including ISO 10993 series for biological evaluation of medical devices. This includes assessments for cytotoxicity, sensitization, and irritation potential. Materials that leach monomers, catalysts, or degradation products pose significant risks to users, necessitating comprehensive leachable and extractable studies during development phases.
Long-term safety considerations extend beyond initial biocompatibility to include material degradation characteristics. Hydrogels employed in therapeutic wearables must maintain structural integrity throughout their intended use period while avoiding the release of potentially harmful degradation products. The pH stability of these materials is equally important, as fluctuations can trigger skin irritation or compromise therapeutic efficacy.
Antimicrobial properties present another crucial safety dimension. Therapeutic wearables often create warm, moist environments conducive to microbial growth. Integration of inherent antimicrobial properties or compatibility with antimicrobial agents can significantly reduce infection risks without compromising the actuator's mechanical performance or biocompatibility profile.
Regulatory compliance frameworks vary globally but typically require comprehensive safety data packages. In the United States, the FDA categorizes most therapeutic wearables as medical devices, necessitating appropriate premarket submissions with supporting biocompatibility data. European markets require CE marking under the Medical Device Regulation (MDR), with specific attention to biocompatibility documentation according to harmonized standards.
The interface between hydrogel actuators and electronic components introduces additional safety considerations. Encapsulation strategies must prevent potential leakage of electronic materials into the hydrogel matrix while maintaining the actuator's performance characteristics. This becomes particularly challenging when designing systems that incorporate sensing capabilities alongside actuation functions.
Human factors engineering must account for diverse skin types and conditions. Therapeutic wearables utilizing hydrogel actuators should accommodate variations in skin sensitivity, with particular attention to vulnerable populations such as elderly users or those with compromised skin integrity. Adaptable material formulations that maintain biocompatibility across diverse user populations represent a significant advancement opportunity in this field.
The cytotoxicity of hydrogel components requires rigorous evaluation through standardized testing protocols including ISO 10993 series for biological evaluation of medical devices. This includes assessments for cytotoxicity, sensitization, and irritation potential. Materials that leach monomers, catalysts, or degradation products pose significant risks to users, necessitating comprehensive leachable and extractable studies during development phases.
Long-term safety considerations extend beyond initial biocompatibility to include material degradation characteristics. Hydrogels employed in therapeutic wearables must maintain structural integrity throughout their intended use period while avoiding the release of potentially harmful degradation products. The pH stability of these materials is equally important, as fluctuations can trigger skin irritation or compromise therapeutic efficacy.
Antimicrobial properties present another crucial safety dimension. Therapeutic wearables often create warm, moist environments conducive to microbial growth. Integration of inherent antimicrobial properties or compatibility with antimicrobial agents can significantly reduce infection risks without compromising the actuator's mechanical performance or biocompatibility profile.
Regulatory compliance frameworks vary globally but typically require comprehensive safety data packages. In the United States, the FDA categorizes most therapeutic wearables as medical devices, necessitating appropriate premarket submissions with supporting biocompatibility data. European markets require CE marking under the Medical Device Regulation (MDR), with specific attention to biocompatibility documentation according to harmonized standards.
The interface between hydrogel actuators and electronic components introduces additional safety considerations. Encapsulation strategies must prevent potential leakage of electronic materials into the hydrogel matrix while maintaining the actuator's performance characteristics. This becomes particularly challenging when designing systems that incorporate sensing capabilities alongside actuation functions.
Human factors engineering must account for diverse skin types and conditions. Therapeutic wearables utilizing hydrogel actuators should accommodate variations in skin sensitivity, with particular attention to vulnerable populations such as elderly users or those with compromised skin integrity. Adaptable material formulations that maintain biocompatibility across diverse user populations represent a significant advancement opportunity in this field.
Manufacturing Scalability and Cost Analysis
The manufacturing scalability of hydrogel actuator materials represents a critical consideration for therapeutic wearable devices transitioning from laboratory prototypes to commercial products. Current production methods for high-performance hydrogel actuators often involve complex synthesis procedures requiring precise control of polymerization conditions, which presents significant challenges for large-scale manufacturing. Batch-to-batch consistency remains a persistent issue, with variations in crosslinking density and network structure potentially affecting the mechanical and responsive properties of the final actuators.
Cost analysis reveals that raw material expenses constitute approximately 30-40% of total production costs for hydrogel-based wearables. Specialty monomers and stimuli-responsive components command premium prices, particularly for advanced formulations incorporating temperature-sensitive polymers like poly(N-isopropylacrylamide) or biocompatible variants. Equipment investment for precision manufacturing represents another substantial cost factor, with specialized molding and patterning technologies requiring significant capital expenditure.
Energy consumption during production presents both economic and sustainability challenges. Conventional thermal polymerization methods demand considerable energy input, while alternative approaches like photopolymerization require specialized UV equipment but offer improved energy efficiency. Water purification systems represent an often-overlooked infrastructure requirement, as contaminants can significantly impact hydrogel performance characteristics.
Scaling considerations must address the transition from laboratory-scale synthesis (typically producing grams of material) to industrial production (requiring kilograms or tons). Continuous flow reactors show promise for increasing throughput while maintaining quality control, though implementation requires substantial process engineering expertise. Recent innovations in microfluidic manufacturing techniques demonstrate potential for precise control over hydrogel architecture at increased production volumes.
Cost reduction pathways include the development of standardized formulations that balance performance requirements with material accessibility. Several research groups have successfully substituted expensive specialty components with more affordable alternatives while maintaining key functional properties. Process optimization through automation and quality control systems can significantly reduce labor costs, which currently account for 25-35% of production expenses in semi-automated facilities.
Market analysis indicates that economies of scale could potentially reduce unit costs by 40-60% when production volumes exceed 100,000 units annually. However, this threshold remains challenging for specialized therapeutic applications with smaller target populations. Strategic partnerships between material developers and established medical device manufacturers offer promising pathways to leverage existing production infrastructure and distribution channels, potentially accelerating market entry while managing capital requirements.
Cost analysis reveals that raw material expenses constitute approximately 30-40% of total production costs for hydrogel-based wearables. Specialty monomers and stimuli-responsive components command premium prices, particularly for advanced formulations incorporating temperature-sensitive polymers like poly(N-isopropylacrylamide) or biocompatible variants. Equipment investment for precision manufacturing represents another substantial cost factor, with specialized molding and patterning technologies requiring significant capital expenditure.
Energy consumption during production presents both economic and sustainability challenges. Conventional thermal polymerization methods demand considerable energy input, while alternative approaches like photopolymerization require specialized UV equipment but offer improved energy efficiency. Water purification systems represent an often-overlooked infrastructure requirement, as contaminants can significantly impact hydrogel performance characteristics.
Scaling considerations must address the transition from laboratory-scale synthesis (typically producing grams of material) to industrial production (requiring kilograms or tons). Continuous flow reactors show promise for increasing throughput while maintaining quality control, though implementation requires substantial process engineering expertise. Recent innovations in microfluidic manufacturing techniques demonstrate potential for precise control over hydrogel architecture at increased production volumes.
Cost reduction pathways include the development of standardized formulations that balance performance requirements with material accessibility. Several research groups have successfully substituted expensive specialty components with more affordable alternatives while maintaining key functional properties. Process optimization through automation and quality control systems can significantly reduce labor costs, which currently account for 25-35% of production expenses in semi-automated facilities.
Market analysis indicates that economies of scale could potentially reduce unit costs by 40-60% when production volumes exceed 100,000 units annually. However, this threshold remains challenging for specialized therapeutic applications with smaller target populations. Strategic partnerships between material developers and established medical device manufacturers offer promising pathways to leverage existing production infrastructure and distribution channels, potentially accelerating market entry while managing capital requirements.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!