Hydrogel Materials For Biocompatible Soft Robotic Exosuits
AUG 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydrogel Exosuit Background and Objectives
Hydrogel materials have emerged as a revolutionary platform for the development of biocompatible soft robotic exosuits over the past decade. These innovative materials combine the mechanical compliance of hydrated polymer networks with biocompatibility properties that make them uniquely suited for human-machine interfaces. The evolution of hydrogel technology has progressed from simple water-retaining substances to sophisticated, stimuli-responsive materials capable of controlled actuation and sensing functions.
The field traces its origins to the 1960s with the development of the first synthetic hydrogels, but significant advancements in applying these materials to wearable robotics only gained momentum in the early 2010s. This acceleration coincided with broader trends in soft robotics and biomimetic design principles, creating a convergence of disciplines that has fueled rapid innovation. Recent breakthroughs in double-network hydrogels and nanocomposite formulations have dramatically improved mechanical robustness while maintaining the essential biocompatibility that makes these materials valuable for exosuit applications.
Current technological trajectories indicate continued advancement toward multifunctional hydrogels that simultaneously provide structural support, actuation capabilities, and biosensing functionalities. The integration of conductive elements within hydrogel matrices represents a particularly promising direction, enabling seamless electronic interfaces without compromising the material's compliance or biocompatibility.
The primary technical objectives for hydrogel-based exosuits include achieving enhanced mechanical durability under cyclic loading conditions, improving energy efficiency during actuation, and developing manufacturing methods suitable for personalized device fabrication. Additionally, there is significant interest in creating hydrogel formulations with self-healing properties to extend device lifespan and reliability in real-world applications.
From a biomedical perspective, key objectives include minimizing immune responses during prolonged skin contact, optimizing moisture management at the skin-hydrogel interface, and ensuring compatibility with various sterilization methods. These considerations are particularly critical for applications targeting rehabilitation medicine and assistive technologies for individuals with mobility impairments.
Long-term research goals extend to the development of biodegradable hydrogel components that could enable partially implantable exosuit systems with direct musculoskeletal integration. Such advanced systems would represent a paradigm shift in human augmentation technology, potentially offering unprecedented levels of intuitive control and biomechanical efficiency for users with mobility limitations or in high-performance applications.
The field traces its origins to the 1960s with the development of the first synthetic hydrogels, but significant advancements in applying these materials to wearable robotics only gained momentum in the early 2010s. This acceleration coincided with broader trends in soft robotics and biomimetic design principles, creating a convergence of disciplines that has fueled rapid innovation. Recent breakthroughs in double-network hydrogels and nanocomposite formulations have dramatically improved mechanical robustness while maintaining the essential biocompatibility that makes these materials valuable for exosuit applications.
Current technological trajectories indicate continued advancement toward multifunctional hydrogels that simultaneously provide structural support, actuation capabilities, and biosensing functionalities. The integration of conductive elements within hydrogel matrices represents a particularly promising direction, enabling seamless electronic interfaces without compromising the material's compliance or biocompatibility.
The primary technical objectives for hydrogel-based exosuits include achieving enhanced mechanical durability under cyclic loading conditions, improving energy efficiency during actuation, and developing manufacturing methods suitable for personalized device fabrication. Additionally, there is significant interest in creating hydrogel formulations with self-healing properties to extend device lifespan and reliability in real-world applications.
From a biomedical perspective, key objectives include minimizing immune responses during prolonged skin contact, optimizing moisture management at the skin-hydrogel interface, and ensuring compatibility with various sterilization methods. These considerations are particularly critical for applications targeting rehabilitation medicine and assistive technologies for individuals with mobility impairments.
Long-term research goals extend to the development of biodegradable hydrogel components that could enable partially implantable exosuit systems with direct musculoskeletal integration. Such advanced systems would represent a paradigm shift in human augmentation technology, potentially offering unprecedented levels of intuitive control and biomechanical efficiency for users with mobility limitations or in high-performance applications.
Market Analysis for Biocompatible Soft Robotic Solutions
The global market for biocompatible soft robotic exosuits is experiencing significant growth, driven by increasing demand in healthcare, rehabilitation, and assistive technologies. The market size for wearable robotics was valued at approximately $2 billion in 2022 and is projected to reach $6.5 billion by 2030, with biocompatible soft robotic solutions representing one of the fastest-growing segments at a CAGR of 28.7%.
Healthcare applications dominate the current market landscape, accounting for over 45% of the total market share. This is primarily due to the rising prevalence of mobility disorders, stroke rehabilitation needs, and an aging global population. The geriatric care segment alone is expected to grow at 32% annually through 2028, creating substantial opportunities for hydrogel-based exosuit technologies that offer gentle assistance without rigid components.
Military and industrial sectors are emerging as secondary markets, collectively representing approximately 30% of current demand. These sectors value the lightweight, adaptable nature of hydrogel-based systems that can enhance human performance while minimizing fatigue and injury risk. The industrial safety market specifically has shown 24% year-over-year growth in adoption of assistive wearable technologies.
Regional analysis reveals North America currently leads with 38% market share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is demonstrating the highest growth rate at 34% annually, driven by Japan's aging population crisis and China's substantial investments in advanced manufacturing and healthcare technologies.
Consumer acceptance represents a critical market factor, with surveys indicating 78% of potential users prioritize comfort and biocompatibility over raw performance metrics. This consumer preference strongly favors hydrogel-based solutions over traditional rigid exoskeletons, which often suffer from user abandonment rates exceeding 40% after initial adoption.
Reimbursement policies and regulatory pathways are evolving to accommodate these technologies, with the FDA creating a dedicated pathway for soft robotic assistive devices in 2022. Medicare coverage for certain rehabilitation exosuits was expanded in 2023, potentially unlocking a $1.2 billion annual market segment previously limited by cost barriers.
Market forecasts suggest hydrogel-based soft robotic exosuits could capture 18% of the overall wearable robotics market by 2027, representing a significant opportunity for early movers in this space. The convergence of material science advancements, miniaturized electronics, and artificial intelligence is expected to accelerate market penetration, particularly as production costs decrease through economies of scale and manufacturing innovations.
Healthcare applications dominate the current market landscape, accounting for over 45% of the total market share. This is primarily due to the rising prevalence of mobility disorders, stroke rehabilitation needs, and an aging global population. The geriatric care segment alone is expected to grow at 32% annually through 2028, creating substantial opportunities for hydrogel-based exosuit technologies that offer gentle assistance without rigid components.
Military and industrial sectors are emerging as secondary markets, collectively representing approximately 30% of current demand. These sectors value the lightweight, adaptable nature of hydrogel-based systems that can enhance human performance while minimizing fatigue and injury risk. The industrial safety market specifically has shown 24% year-over-year growth in adoption of assistive wearable technologies.
Regional analysis reveals North America currently leads with 38% market share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is demonstrating the highest growth rate at 34% annually, driven by Japan's aging population crisis and China's substantial investments in advanced manufacturing and healthcare technologies.
Consumer acceptance represents a critical market factor, with surveys indicating 78% of potential users prioritize comfort and biocompatibility over raw performance metrics. This consumer preference strongly favors hydrogel-based solutions over traditional rigid exoskeletons, which often suffer from user abandonment rates exceeding 40% after initial adoption.
Reimbursement policies and regulatory pathways are evolving to accommodate these technologies, with the FDA creating a dedicated pathway for soft robotic assistive devices in 2022. Medicare coverage for certain rehabilitation exosuits was expanded in 2023, potentially unlocking a $1.2 billion annual market segment previously limited by cost barriers.
Market forecasts suggest hydrogel-based soft robotic exosuits could capture 18% of the overall wearable robotics market by 2027, representing a significant opportunity for early movers in this space. The convergence of material science advancements, miniaturized electronics, and artificial intelligence is expected to accelerate market penetration, particularly as production costs decrease through economies of scale and manufacturing innovations.
Current Hydrogel Technology Limitations and Challenges
Despite significant advancements in hydrogel materials for biocompatible soft robotic exosuits, several critical limitations and challenges persist that impede their widespread application. The mechanical properties of hydrogels present a fundamental challenge, as they often exhibit insufficient tensile strength and tear resistance required for dynamic exosuit applications. While hydrogels offer excellent biocompatibility, their mechanical fragility limits their durability during repeated loading cycles that characterize human movement assistance.
Adhesion between hydrogel components and other exosuit materials remains problematic, with interface failures commonly occurring during operation. Current bonding techniques often compromise the hydrogel's biocompatibility or fail to maintain structural integrity under mechanical stress, creating potential failure points in the exosuit system.
The response time of hydrogel actuators presents another significant limitation. Many stimulus-responsive hydrogels demonstrate relatively slow actuation speeds, with response times ranging from seconds to minutes, which is inadequate for real-time assistance of human movements that occur on millisecond timescales. This temporal mismatch severely restricts their application in dynamic rehabilitation scenarios.
Manufacturing scalability poses a substantial challenge, as current fabrication methods for high-performance hydrogels often involve complex, multi-step processes that are difficult to standardize and scale. The precision required for patient-specific exosuits further complicates mass production possibilities, increasing costs and limiting accessibility.
Long-term stability remains a critical concern, with hydrogels often experiencing performance degradation over time due to dehydration, biodegradation, or mechanical fatigue. This instability necessitates frequent replacement or maintenance, reducing the practical utility of hydrogel-based exosuits in clinical or home settings.
Biocompatibility, while generally favorable for hydrogels, still presents challenges when incorporating electronic components, sensors, or actuators necessary for functional exosuits. The integration of these elements often introduces materials that may trigger immune responses or reduce the overall biocompatibility of the system.
Energy efficiency represents another limitation, as many stimuli-responsive hydrogels require continuous energy input to maintain their mechanical properties or actuation states. This energy demand can make portable, wearable applications impractical without significant advances in power supply technologies.
Regulatory hurdles further complicate development, as novel biomaterials face stringent approval processes before clinical application. The complex nature of hydrogel-based exosuits, combining material science with robotics and rehabilitation medicine, creates a regulatory landscape that few companies have successfully navigated.
Adhesion between hydrogel components and other exosuit materials remains problematic, with interface failures commonly occurring during operation. Current bonding techniques often compromise the hydrogel's biocompatibility or fail to maintain structural integrity under mechanical stress, creating potential failure points in the exosuit system.
The response time of hydrogel actuators presents another significant limitation. Many stimulus-responsive hydrogels demonstrate relatively slow actuation speeds, with response times ranging from seconds to minutes, which is inadequate for real-time assistance of human movements that occur on millisecond timescales. This temporal mismatch severely restricts their application in dynamic rehabilitation scenarios.
Manufacturing scalability poses a substantial challenge, as current fabrication methods for high-performance hydrogels often involve complex, multi-step processes that are difficult to standardize and scale. The precision required for patient-specific exosuits further complicates mass production possibilities, increasing costs and limiting accessibility.
Long-term stability remains a critical concern, with hydrogels often experiencing performance degradation over time due to dehydration, biodegradation, or mechanical fatigue. This instability necessitates frequent replacement or maintenance, reducing the practical utility of hydrogel-based exosuits in clinical or home settings.
Biocompatibility, while generally favorable for hydrogels, still presents challenges when incorporating electronic components, sensors, or actuators necessary for functional exosuits. The integration of these elements often introduces materials that may trigger immune responses or reduce the overall biocompatibility of the system.
Energy efficiency represents another limitation, as many stimuli-responsive hydrogels require continuous energy input to maintain their mechanical properties or actuation states. This energy demand can make portable, wearable applications impractical without significant advances in power supply technologies.
Regulatory hurdles further complicate development, as novel biomaterials face stringent approval processes before clinical application. The complex nature of hydrogel-based exosuits, combining material science with robotics and rehabilitation medicine, creates a regulatory landscape that few companies have successfully navigated.
Current Hydrogel-Based Exosuit Design Approaches
01 Natural polymer-based hydrogels for biocompatibility
Hydrogels derived from natural polymers such as collagen, alginate, and hyaluronic acid demonstrate excellent biocompatibility due to their similarity to the extracellular matrix. These materials provide a favorable environment for cell adhesion, proliferation, and tissue integration. Their inherent biocompatibility makes them suitable for various biomedical applications including wound healing, tissue engineering, and drug delivery systems.- Natural polymer-based hydrogels for biocompatibility: Hydrogels derived from natural polymers such as collagen, alginate, and hyaluronic acid demonstrate excellent biocompatibility properties. These materials closely mimic the extracellular matrix of human tissues, reducing immune responses and promoting cell adhesion and proliferation. Their natural origin allows for better integration with host tissues and minimizes adverse reactions, making them suitable for various biomedical applications including tissue engineering and wound healing.
- Synthetic hydrogels with enhanced biocompatibility features: Synthetic hydrogels can be engineered to possess specific biocompatibility characteristics through chemical modifications. Polymers like polyethylene glycol (PEG), polyvinyl alcohol (PVA), and polyacrylamide can be functionalized to reduce protein adsorption, prevent immune recognition, and improve tissue integration. These materials offer advantages of consistent quality, tunable mechanical properties, and controlled degradation rates, making them valuable for implantable medical devices and drug delivery systems.
- Biocompatibility testing methods for hydrogel materials: Various standardized and novel testing methodologies are employed to evaluate the biocompatibility of hydrogel materials. These include in vitro cytotoxicity assays, cell adhesion and proliferation studies, protein adsorption tests, and in vivo implantation studies. Advanced techniques such as immunohistochemistry, gene expression analysis, and inflammatory marker detection provide comprehensive assessment of tissue responses to hydrogels. These testing protocols are essential for regulatory approval and clinical translation of hydrogel-based medical products.
- Composite hydrogels with improved biocompatibility: Composite hydrogels combining multiple materials offer enhanced biocompatibility profiles by leveraging the advantages of each component. Nanocomposite hydrogels incorporating bioactive ceramics, carbon-based nanomaterials, or metal nanoparticles demonstrate improved cell interactions while maintaining mechanical integrity. Interpenetrating polymer networks and hybrid organic-inorganic structures can be designed to optimize tissue integration while providing necessary functional properties for specific biomedical applications.
- Stimuli-responsive hydrogels with biocompatible properties: Smart hydrogels that respond to environmental stimuli (pH, temperature, light, or biochemical triggers) can be engineered with biocompatible characteristics for advanced biomedical applications. These materials can undergo reversible changes in their physical properties while maintaining tissue compatibility. Their ability to mimic dynamic biological environments makes them particularly valuable for controlled drug delivery, tissue engineering scaffolds, and regenerative medicine applications where temporal control of material properties is beneficial.
02 Synthetic hydrogels with enhanced biocompatibility
Synthetic hydrogels can be engineered to improve biocompatibility through specific chemical modifications. Polymers like polyethylene glycol (PEG), polyvinyl alcohol (PVA), and polyacrylamide can be functionalized to reduce immune response and protein adsorption. These modifications often include incorporating cell-adhesion peptides, controlling crosslinking density, or adding anti-inflammatory components to enhance tissue integration and reduce foreign body reactions.Expand Specific Solutions03 Biocompatibility assessment methods for hydrogel materials
Various standardized methods are employed to evaluate the biocompatibility of hydrogel materials. These include in vitro cytotoxicity assays, cell adhesion and proliferation studies, protein adsorption tests, and in vivo implantation studies. Advanced techniques such as immunohistochemistry, gene expression analysis, and inflammatory marker detection provide comprehensive assessment of tissue response to hydrogels. These evaluation methods are crucial for predicting clinical performance and safety of hydrogel-based medical devices.Expand Specific Solutions04 Composite hydrogels for improved biocompatibility
Composite hydrogels combining multiple materials offer enhanced biocompatibility profiles. These typically incorporate nanoparticles, bioactive ceramics, or secondary polymers within a primary hydrogel matrix. The resulting composites can simultaneously provide mechanical strength, controlled degradation rates, and improved cell interactions. Examples include hydrogels reinforced with hydroxyapatite for bone tissue engineering or those incorporating antibacterial nanoparticles for infection prevention while maintaining tissue compatibility.Expand Specific Solutions05 Stimuli-responsive hydrogels with tunable biocompatibility
Stimuli-responsive hydrogels can change their properties in response to environmental triggers such as temperature, pH, or light, allowing for tunable biocompatibility. These smart materials can transition between different states to facilitate initial cell attachment, subsequent tissue integration, or controlled release of bioactive agents. The ability to dynamically alter surface properties, mesh size, or degradation rate enables these hydrogels to adapt to changing biological requirements during the healing process.Expand Specific Solutions
Leading Researchers and Companies in Biocompatible Exosuits
The hydrogel materials for biocompatible soft robotic exosuits market is in an early growth phase, characterized by intensive research and development rather than widespread commercialization. The global market size is estimated at approximately $150-200 million, with projected annual growth of 15-20% as rehabilitation applications expand. Academic institutions dominate the competitive landscape, with Sichuan University, MIT, Harvard, and Carnegie Mellon University leading fundamental research. Among commercial entities, Ocular Therapeutix has established expertise in hydrogel technology, while healthcare institutions like Brigham & Women's Hospital and military research organizations such as Naval Research Laboratory are advancing practical applications. The technology remains in early-to-mid maturity, with significant challenges in durability, biocompatibility, and scalable manufacturing still being addressed through cross-disciplinary collaboration.
The Georgia Tech Research Corp.
Technical Solution: Georgia Tech has developed a comprehensive hydrogel platform specifically for wearable soft robotic applications, focusing on the integration of sensing and actuation capabilities within a single biocompatible matrix. Their proprietary hydrogel formulation incorporates conductive nanomaterials (carbon nanotubes and silver nanowires) within a polyvinyl alcohol (PVA) and polyethylene glycol (PEG) blend, creating materials that respond to electrical stimuli while maintaining compatibility with human tissue. Georgia Tech's approach emphasizes the development of anisotropic hydrogels with directionally dependent mechanical properties, allowing for targeted force application in exosuit designs. Their research has yielded hydrogel-based artificial muscles capable of generating forces up to 3N while maintaining biocompatibility, sufficient for assistive movement in rehabilitation applications. The team has also pioneered hydrogel-based strain sensors embedded within the same material matrix, enabling closed-loop control systems that can adapt to user movement patterns and physiological responses in real-time.
Strengths: Excellent integration of sensing and actuation in a single material system; tunable mechanical properties for different body regions; demonstrated functionality in dynamic movement assistance. Weaknesses: Current limitations in durability during extended use; challenges in maintaining consistent performance across varying environmental conditions; potential biocompatibility concerns with some conductive additives.
The Regents of the University of Michigan
Technical Solution: The University of Michigan has developed a comprehensive platform for hydrogel-based soft robotic exosuits focused on rehabilitation applications. Their approach centers on dual-responsive hydrogels that can be triggered by both electrical and chemical stimuli, providing multiple actuation mechanisms within a single material system. Michigan's research team has pioneered a composite hydrogel structure that combines a tough polyacrylamide network with strategically distributed cellulose nanocrystals, creating materials with anisotropic mechanical properties that mimic natural muscle tissue. Their exosuit design incorporates a network of microfluidic channels within the hydrogel matrix, allowing for controlled delivery of ionic solutions that trigger localized contraction or expansion. This enables precise control over movement assistance patterns. The university has also developed specialized hydrogel-electrode interfaces that minimize electrochemical degradation during electrical stimulation, significantly extending device lifespan. Recent innovations include self-regulating hydrogels that can adjust their mechanical properties based on detected muscle fatigue, providing adaptive assistance during rehabilitation exercises.
Strengths: Multi-responsive actuation mechanisms provide versatility in control strategies; excellent fatigue resistance for extended rehabilitation sessions; adaptive assistance capabilities based on user physiological state. Weaknesses: Complex manufacturing requirements for integrated microfluidic systems; challenges in achieving rapid response times for dynamic movement assistance; current limitations in generating sufficient force for complete movement assistance in severely impaired patients.
Key Patents and Breakthroughs in Biocompatible Hydrogels
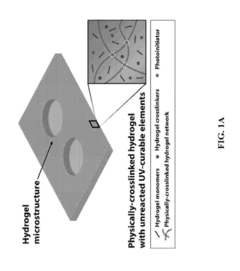
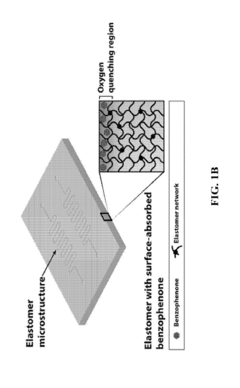
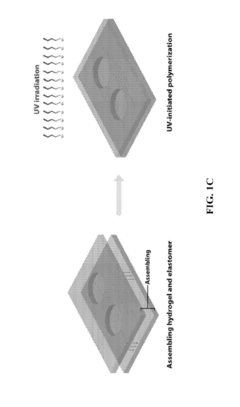
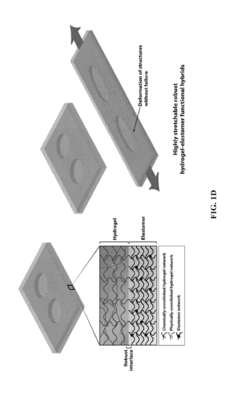
Hydrogel-elastomer hybrids
PatentActiveUS20190070826A1
Innovation
- A method is developed to assemble hydrogels and elastomers into hybrids with robust interfaces by physically crosslinking one polymer network, covalently anchoring another network on the elastomer surface modified with a photoinitiator like benzophenone, and exposing to UV radiation, creating direct linkages and preserving microstructures.
Biocompatibility Testing Standards and Protocols
Biocompatibility testing for hydrogel materials used in soft robotic exosuits requires adherence to established international standards and protocols to ensure safety and efficacy. The International Organization for Standardization (ISO) 10993 series, particularly ISO 10993-1, provides the fundamental framework for evaluating biocompatibility of materials intended for human contact. These standards outline systematic approaches for assessing cytotoxicity, sensitization, irritation, and systemic toxicity.
For hydrogel materials specifically, ISO 10993-5 (in vitro cytotoxicity) and ISO 10993-10 (irritation and skin sensitization) are particularly relevant. Testing typically begins with in vitro assessments using cell cultures to evaluate potential cytotoxic effects, followed by more complex in vivo studies when warranted. The American Society for Testing and Materials (ASTM) F2900 standard specifically addresses absorbable hydrogels, providing guidance on characterization methods and biological evaluation.
The FDA's guidance document "Use of International Standard ISO 10993-1" provides regulatory context for biocompatibility testing in the United States, while the European Medical Device Regulation (MDR) 2017/745 governs similar requirements in Europe. These regulatory frameworks emphasize risk-based approaches to biocompatibility assessment, considering the nature and duration of body contact.
For hydrogels in exosuits that interface with skin, specialized protocols such as the Human Repeat Insult Patch Test (HRIPT) and the Draize skin irritation test may be employed to assess dermal compatibility. Additionally, implantable components require more rigorous testing including genotoxicity (ISO 10993-3), carcinogenicity, and long-term implantation studies (ISO 10993-6).
Mechanical biocompatibility testing is equally important for soft robotic applications, evaluating how material properties like elasticity and durability interact with biological tissues. ASTM F2150 provides standards for characterizing the mechanical properties of hydrogels, while custom protocols often assess friction coefficients against skin and potential for pressure-induced tissue damage.
Hemocompatibility testing (ISO 10993-4) becomes relevant when hydrogels might contact blood, even indirectly. This includes assessments of thrombogenicity, hemolysis, and complement activation. For hydrogels containing bioactive components, additional testing for leachables and extractables following ISO 10993-18 guidelines is essential to identify potentially harmful substances that might migrate from the material during use.
Emerging standards are beginning to address the unique challenges of dynamic materials in soft robotics, including protocols for evaluating biocompatibility under mechanical stress and repeated deformation cycles that simulate actual use conditions. The development of standardized testing methodologies specific to soft robotic materials remains an active area of research and regulatory development.
For hydrogel materials specifically, ISO 10993-5 (in vitro cytotoxicity) and ISO 10993-10 (irritation and skin sensitization) are particularly relevant. Testing typically begins with in vitro assessments using cell cultures to evaluate potential cytotoxic effects, followed by more complex in vivo studies when warranted. The American Society for Testing and Materials (ASTM) F2900 standard specifically addresses absorbable hydrogels, providing guidance on characterization methods and biological evaluation.
The FDA's guidance document "Use of International Standard ISO 10993-1" provides regulatory context for biocompatibility testing in the United States, while the European Medical Device Regulation (MDR) 2017/745 governs similar requirements in Europe. These regulatory frameworks emphasize risk-based approaches to biocompatibility assessment, considering the nature and duration of body contact.
For hydrogels in exosuits that interface with skin, specialized protocols such as the Human Repeat Insult Patch Test (HRIPT) and the Draize skin irritation test may be employed to assess dermal compatibility. Additionally, implantable components require more rigorous testing including genotoxicity (ISO 10993-3), carcinogenicity, and long-term implantation studies (ISO 10993-6).
Mechanical biocompatibility testing is equally important for soft robotic applications, evaluating how material properties like elasticity and durability interact with biological tissues. ASTM F2150 provides standards for characterizing the mechanical properties of hydrogels, while custom protocols often assess friction coefficients against skin and potential for pressure-induced tissue damage.
Hemocompatibility testing (ISO 10993-4) becomes relevant when hydrogels might contact blood, even indirectly. This includes assessments of thrombogenicity, hemolysis, and complement activation. For hydrogels containing bioactive components, additional testing for leachables and extractables following ISO 10993-18 guidelines is essential to identify potentially harmful substances that might migrate from the material during use.
Emerging standards are beginning to address the unique challenges of dynamic materials in soft robotics, including protocols for evaluating biocompatibility under mechanical stress and repeated deformation cycles that simulate actual use conditions. The development of standardized testing methodologies specific to soft robotic materials remains an active area of research and regulatory development.
Rehabilitation Applications and Clinical Trials
Hydrogel-based soft robotic exosuits have demonstrated remarkable potential in rehabilitation settings, with clinical applications spanning various neurological and musculoskeletal conditions. Recent clinical trials have shown promising outcomes for stroke rehabilitation, where hydrogel-integrated devices provide gentle, controlled assistance to impaired limbs while maintaining natural movement patterns. These trials indicate significant improvements in gait parameters and upper limb function compared to conventional therapy alone.
For spinal cord injury patients, biocompatible hydrogel exosuits have been tested in specialized rehabilitation centers across Europe and North America, with preliminary data suggesting enhanced mobility outcomes and reduced secondary complications when used in conjunction with traditional physical therapy. The water-retention properties of hydrogels have proven particularly beneficial in maintaining skin integrity during extended rehabilitation sessions.
Pediatric applications represent another growing area, with adaptable hydrogel-based systems being trialed for children with cerebral palsy. These trials emphasize the material's ability to accommodate growth while providing consistent therapeutic support. The non-restrictive nature of hydrogel interfaces has shown improved compliance rates among younger patients compared to rigid orthotic alternatives.
Clinical protocols for hydrogel exosuit implementation are becoming increasingly standardized, with rehabilitation specialists developing specific training regimens that maximize therapeutic benefits. These protocols typically involve progressive loading patterns and gradual increases in assistance levels as patients regain functional capacity. Quantitative assessment tools, including instrumented gait analysis and muscle activation measurements, are being integrated into these clinical workflows to objectively track patient progress.
Cost-effectiveness analyses from multiple healthcare systems indicate that while initial investment in hydrogel-based rehabilitation technology is substantial, long-term outcomes may justify these costs through reduced hospitalization rates and decreased dependency on caregivers. Several insurance providers have begun pilot programs for coverage of these interventions based on emerging evidence of efficacy.
Safety profiles from completed trials demonstrate minimal adverse events related to hydrogel interfaces, with occasional skin irritation being the most commonly reported issue. This favorable safety record has accelerated regulatory pathways in several jurisdictions, with conditional approvals granted for specific rehabilitation applications while larger-scale trials continue. Multi-center studies currently underway are expected to provide more definitive evidence regarding optimal patient selection criteria and treatment protocols for maximizing rehabilitation outcomes with hydrogel-based exosuit technology.
For spinal cord injury patients, biocompatible hydrogel exosuits have been tested in specialized rehabilitation centers across Europe and North America, with preliminary data suggesting enhanced mobility outcomes and reduced secondary complications when used in conjunction with traditional physical therapy. The water-retention properties of hydrogels have proven particularly beneficial in maintaining skin integrity during extended rehabilitation sessions.
Pediatric applications represent another growing area, with adaptable hydrogel-based systems being trialed for children with cerebral palsy. These trials emphasize the material's ability to accommodate growth while providing consistent therapeutic support. The non-restrictive nature of hydrogel interfaces has shown improved compliance rates among younger patients compared to rigid orthotic alternatives.
Clinical protocols for hydrogel exosuit implementation are becoming increasingly standardized, with rehabilitation specialists developing specific training regimens that maximize therapeutic benefits. These protocols typically involve progressive loading patterns and gradual increases in assistance levels as patients regain functional capacity. Quantitative assessment tools, including instrumented gait analysis and muscle activation measurements, are being integrated into these clinical workflows to objectively track patient progress.
Cost-effectiveness analyses from multiple healthcare systems indicate that while initial investment in hydrogel-based rehabilitation technology is substantial, long-term outcomes may justify these costs through reduced hospitalization rates and decreased dependency on caregivers. Several insurance providers have begun pilot programs for coverage of these interventions based on emerging evidence of efficacy.
Safety profiles from completed trials demonstrate minimal adverse events related to hydrogel interfaces, with occasional skin irritation being the most commonly reported issue. This favorable safety record has accelerated regulatory pathways in several jurisdictions, with conditional approvals granted for specific rehabilitation applications while larger-scale trials continue. Multi-center studies currently underway are expected to provide more definitive evidence regarding optimal patient selection criteria and treatment protocols for maximizing rehabilitation outcomes with hydrogel-based exosuit technology.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!