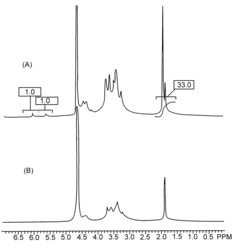
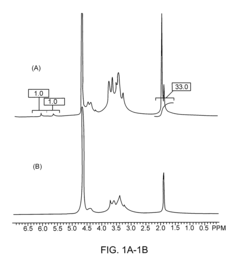
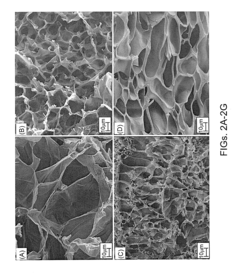
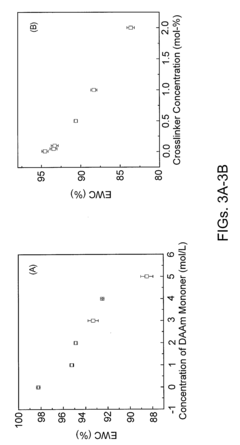
Safety And Biocompatibility Assessments For Implantable Hydrogel Actuators
AUG 29, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydrogel Actuator Technology Background and Objectives
Hydrogel actuators represent a revolutionary class of soft robotic components that have gained significant attention in the biomedical field over the past two decades. These innovative materials combine the mechanical flexibility of hydrogels with responsive properties that enable controlled movement and force generation when exposed to various stimuli such as temperature, pH, light, or electrical signals. The evolution of this technology can be traced back to the early 2000s when researchers first demonstrated the potential of hydrogels as active materials rather than passive scaffolds.
The development trajectory of hydrogel actuators has been characterized by progressive improvements in response time, force generation capacity, and biocompatibility. Early iterations suffered from slow response rates and limited mechanical strength, but recent advancements in polymer chemistry and material science have addressed many of these limitations. The incorporation of nanoparticles, carbon-based materials, and specialized polymer networks has significantly enhanced the performance characteristics of these systems.
Current technological trends in this field are moving toward multi-responsive hydrogel actuators that can react to multiple stimuli simultaneously or sequentially, allowing for more complex and precise movements. Additionally, there is growing interest in self-healing hydrogel actuators that can maintain their functionality even after mechanical damage, which is particularly valuable for long-term implantable applications.
The primary objective of research in implantable hydrogel actuators is to develop biocompatible systems that can safely interface with living tissues while performing mechanical functions. These functions may include controlled drug delivery, tissue manipulation, or assistance to compromised physiological processes. A critical goal is to ensure these materials can operate reliably within the complex and dynamic environment of the human body without triggering adverse immune responses or degrading prematurely.
Another key objective is to establish standardized protocols for safety and biocompatibility assessments specific to hydrogel actuators. While existing frameworks for evaluating implantable materials provide some guidance, the unique dynamic nature of actuators presents additional challenges that must be addressed through specialized testing methodologies.
Looking forward, the field aims to bridge the gap between laboratory demonstrations and clinical applications by focusing on long-term stability, reliability, and integration with existing medical technologies. The ultimate goal is to create implantable hydrogel actuators that can function as "smart" therapeutic devices, capable of responding to physiological changes and providing targeted interventions with minimal external control.
The development trajectory of hydrogel actuators has been characterized by progressive improvements in response time, force generation capacity, and biocompatibility. Early iterations suffered from slow response rates and limited mechanical strength, but recent advancements in polymer chemistry and material science have addressed many of these limitations. The incorporation of nanoparticles, carbon-based materials, and specialized polymer networks has significantly enhanced the performance characteristics of these systems.
Current technological trends in this field are moving toward multi-responsive hydrogel actuators that can react to multiple stimuli simultaneously or sequentially, allowing for more complex and precise movements. Additionally, there is growing interest in self-healing hydrogel actuators that can maintain their functionality even after mechanical damage, which is particularly valuable for long-term implantable applications.
The primary objective of research in implantable hydrogel actuators is to develop biocompatible systems that can safely interface with living tissues while performing mechanical functions. These functions may include controlled drug delivery, tissue manipulation, or assistance to compromised physiological processes. A critical goal is to ensure these materials can operate reliably within the complex and dynamic environment of the human body without triggering adverse immune responses or degrading prematurely.
Another key objective is to establish standardized protocols for safety and biocompatibility assessments specific to hydrogel actuators. While existing frameworks for evaluating implantable materials provide some guidance, the unique dynamic nature of actuators presents additional challenges that must be addressed through specialized testing methodologies.
Looking forward, the field aims to bridge the gap between laboratory demonstrations and clinical applications by focusing on long-term stability, reliability, and integration with existing medical technologies. The ultimate goal is to create implantable hydrogel actuators that can function as "smart" therapeutic devices, capable of responding to physiological changes and providing targeted interventions with minimal external control.
Market Analysis for Implantable Hydrogel Applications
The global market for implantable hydrogel actuators is experiencing significant growth, driven by increasing applications in medical devices, drug delivery systems, and tissue engineering. The market size for implantable hydrogels was valued at approximately $6.7 billion in 2022 and is projected to reach $12.3 billion by 2028, representing a compound annual growth rate (CAGR) of 10.7% during the forecast period.
Healthcare applications dominate the market, accounting for over 65% of the total market share. Within healthcare, drug delivery systems represent the largest segment, followed by tissue engineering and regenerative medicine. The growing prevalence of chronic diseases, coupled with an aging global population, is fueling demand for advanced implantable technologies that can provide controlled and targeted therapeutic interventions.
North America currently leads the market with a 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is expected to witness the highest growth rate during the forecast period, primarily due to increasing healthcare expenditure, improving healthcare infrastructure, and rising awareness about advanced medical technologies in countries like China, Japan, and South Korea.
Key market drivers include technological advancements in hydrogel materials, increasing research and development activities, growing demand for minimally invasive surgical procedures, and rising investments in healthcare infrastructure. The development of smart hydrogels that can respond to specific physiological stimuli has opened new avenues for applications in controlled drug release and tissue regeneration.
Market restraints include stringent regulatory requirements for implantable devices, high development costs, and concerns regarding long-term biocompatibility and safety. The complex and lengthy approval process for implantable medical devices poses a significant challenge for market entrants and can delay product commercialization.
Customer segments for implantable hydrogel actuators include hospitals and surgical centers (42%), research institutions (28%), pharmaceutical and biotechnology companies (18%), and others (12%). The demand from hospitals is primarily driven by the increasing adoption of advanced surgical techniques and the growing number of surgical procedures worldwide.
The competitive landscape is characterized by the presence of both established medical device manufacturers and emerging biotech companies. Major players are focusing on strategic collaborations, mergers and acquisitions, and significant investments in R&D to strengthen their market position and expand their product portfolios.
Future market trends indicate a shift towards personalized medicine, with customized hydrogel implants designed to meet individual patient needs. Additionally, the integration of nanotechnology with hydrogel systems is expected to enhance the functionality and efficacy of implantable actuators, further driving market growth.
Healthcare applications dominate the market, accounting for over 65% of the total market share. Within healthcare, drug delivery systems represent the largest segment, followed by tissue engineering and regenerative medicine. The growing prevalence of chronic diseases, coupled with an aging global population, is fueling demand for advanced implantable technologies that can provide controlled and targeted therapeutic interventions.
North America currently leads the market with a 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is expected to witness the highest growth rate during the forecast period, primarily due to increasing healthcare expenditure, improving healthcare infrastructure, and rising awareness about advanced medical technologies in countries like China, Japan, and South Korea.
Key market drivers include technological advancements in hydrogel materials, increasing research and development activities, growing demand for minimally invasive surgical procedures, and rising investments in healthcare infrastructure. The development of smart hydrogels that can respond to specific physiological stimuli has opened new avenues for applications in controlled drug release and tissue regeneration.
Market restraints include stringent regulatory requirements for implantable devices, high development costs, and concerns regarding long-term biocompatibility and safety. The complex and lengthy approval process for implantable medical devices poses a significant challenge for market entrants and can delay product commercialization.
Customer segments for implantable hydrogel actuators include hospitals and surgical centers (42%), research institutions (28%), pharmaceutical and biotechnology companies (18%), and others (12%). The demand from hospitals is primarily driven by the increasing adoption of advanced surgical techniques and the growing number of surgical procedures worldwide.
The competitive landscape is characterized by the presence of both established medical device manufacturers and emerging biotech companies. Major players are focusing on strategic collaborations, mergers and acquisitions, and significant investments in R&D to strengthen their market position and expand their product portfolios.
Future market trends indicate a shift towards personalized medicine, with customized hydrogel implants designed to meet individual patient needs. Additionally, the integration of nanotechnology with hydrogel systems is expected to enhance the functionality and efficacy of implantable actuators, further driving market growth.
Current Safety Challenges in Implantable Hydrogel Technology
Despite significant advancements in implantable hydrogel actuator technology, several critical safety challenges persist that impede widespread clinical adoption. The primary concern involves long-term biocompatibility, as hydrogels implanted for extended periods may trigger foreign body responses, including inflammation, fibrosis, and potential rejection. These responses can compromise both device functionality and patient safety, particularly when the actuator is intended for long-term therapeutic applications.
Material degradation represents another significant challenge, as hydrogels may undergo structural changes in vivo, potentially releasing degradation products with unknown toxicity profiles. The mechanical properties of hydrogels can also change over time due to interactions with biological fluids and tissues, affecting actuator performance and potentially causing unexpected tissue damage or mechanical failure.
Sterilization compatibility poses a substantial hurdle, as conventional sterilization methods such as ethylene oxide, gamma irradiation, or autoclaving may compromise the structural integrity and functional properties of hydrogel materials. This necessitates the development of specialized sterilization protocols that maintain material properties while ensuring complete microbial elimination.
Electrical safety concerns are paramount for hydrogel actuators that incorporate electronic components or respond to electrical stimuli. The potential for electrical leakage, short circuits, or thermal damage to surrounding tissues must be thoroughly addressed, particularly in applications where the actuator interfaces with sensitive tissues such as neural or cardiac environments.
Immunogenicity remains a persistent challenge, as even hydrogels composed of biocompatible materials may contain residual chemicals, crosslinking agents, or processing additives that could elicit immune responses. The variability in patient immune reactions further complicates safety assessments and necessitates personalized approaches to biocompatibility testing.
Migration and displacement of hydrogel actuators within the body represent another safety concern, particularly for applications in dynamic tissue environments. Insufficient anchoring or material degradation may lead to device migration, potentially causing tissue damage or compromising therapeutic efficacy.
Regulatory frameworks for implantable hydrogel actuators remain underdeveloped, creating uncertainty in safety assessment protocols. Current standards often fail to address the unique characteristics of these hybrid devices that combine material, mechanical, and potentially electrical properties. This regulatory gap complicates the translation of promising laboratory technologies into clinically approved therapies.
The development of standardized, comprehensive safety testing protocols specifically designed for hydrogel actuators represents an urgent need in the field. Such protocols must address the multifaceted nature of these devices and provide clear guidelines for evaluating both short and long-term safety profiles in diverse physiological environments.
Material degradation represents another significant challenge, as hydrogels may undergo structural changes in vivo, potentially releasing degradation products with unknown toxicity profiles. The mechanical properties of hydrogels can also change over time due to interactions with biological fluids and tissues, affecting actuator performance and potentially causing unexpected tissue damage or mechanical failure.
Sterilization compatibility poses a substantial hurdle, as conventional sterilization methods such as ethylene oxide, gamma irradiation, or autoclaving may compromise the structural integrity and functional properties of hydrogel materials. This necessitates the development of specialized sterilization protocols that maintain material properties while ensuring complete microbial elimination.
Electrical safety concerns are paramount for hydrogel actuators that incorporate electronic components or respond to electrical stimuli. The potential for electrical leakage, short circuits, or thermal damage to surrounding tissues must be thoroughly addressed, particularly in applications where the actuator interfaces with sensitive tissues such as neural or cardiac environments.
Immunogenicity remains a persistent challenge, as even hydrogels composed of biocompatible materials may contain residual chemicals, crosslinking agents, or processing additives that could elicit immune responses. The variability in patient immune reactions further complicates safety assessments and necessitates personalized approaches to biocompatibility testing.
Migration and displacement of hydrogel actuators within the body represent another safety concern, particularly for applications in dynamic tissue environments. Insufficient anchoring or material degradation may lead to device migration, potentially causing tissue damage or compromising therapeutic efficacy.
Regulatory frameworks for implantable hydrogel actuators remain underdeveloped, creating uncertainty in safety assessment protocols. Current standards often fail to address the unique characteristics of these hybrid devices that combine material, mechanical, and potentially electrical properties. This regulatory gap complicates the translation of promising laboratory technologies into clinically approved therapies.
The development of standardized, comprehensive safety testing protocols specifically designed for hydrogel actuators represents an urgent need in the field. Such protocols must address the multifaceted nature of these devices and provide clear guidelines for evaluating both short and long-term safety profiles in diverse physiological environments.
Current Assessment Methodologies for Hydrogel Biocompatibility
01 Biocompatible hydrogel materials for medical applications
Hydrogel actuators can be formulated with biocompatible materials to ensure safety for medical applications. These materials include natural polymers like alginate, collagen, and hyaluronic acid, or synthetic polymers that have been proven safe for biological contact. The biocompatibility of these materials allows for applications such as drug delivery systems, tissue engineering scaffolds, and implantable devices with minimal immune response or inflammation in the body.- Biocompatible hydrogel materials for medical applications: Hydrogel actuators can be formulated with biocompatible materials such as natural polymers (alginate, chitosan) or synthetic polymers (polyethylene glycol, polyvinyl alcohol) that are non-toxic and compatible with biological tissues. These materials undergo rigorous biocompatibility testing to ensure they don't cause adverse reactions when in contact with human tissues. The selection of biocompatible crosslinking agents and the removal of potentially harmful residual chemicals during synthesis are critical for ensuring safety in medical applications.
- Safety assessment protocols for hydrogel actuators: Comprehensive safety assessment protocols have been developed for hydrogel actuators, including cytotoxicity testing, genotoxicity evaluation, irritation studies, and long-term implantation tests. These protocols evaluate the potential for adverse effects at both cellular and systemic levels. Advanced in vitro and in vivo models are employed to predict potential immunological responses and tissue reactions. Standardized testing methods ensure consistent safety evaluation across different hydrogel actuator formulations.
- Biodegradable hydrogel actuators with controlled degradation profiles: Biodegradable hydrogel actuators are designed with controlled degradation profiles to ensure safe elimination from the body after fulfilling their function. The degradation byproducts are carefully engineered to be non-toxic and easily metabolized or excreted. Hydrolytically and enzymatically degradable linkages are incorporated into the polymer network to facilitate predictable breakdown under physiological conditions. This approach minimizes the risk of long-term foreign body reactions while maintaining functional performance during the required timeframe.
- Stimuli-responsive hydrogels with enhanced biocompatibility: Stimuli-responsive hydrogel actuators that respond to environmental triggers (pH, temperature, light) are being developed with enhanced biocompatibility features. These smart materials incorporate biocompatible responsive elements that undergo conformational changes without releasing harmful substances. Surface modifications with biomimetic molecules improve tissue integration and reduce foreign body responses. The actuation mechanisms are designed to operate within physiologically safe parameters, preventing mechanical damage to surrounding tissues during operation.
- Biocompatibility enhancement through composite formulations: Composite hydrogel formulations incorporate bioactive components such as growth factors, anti-inflammatory agents, or antimicrobial peptides to enhance biocompatibility and promote positive tissue interactions. These composite systems often combine synthetic polymers for mechanical strength with natural polymers for improved cell adhesion and tissue integration. Nanoparticle reinforcements are selected for both functional properties and biological safety. The composite approach allows for multifunctional hydrogel actuators that can simultaneously provide mechanical actuation while supporting tissue healing and regeneration.
02 Safety testing protocols for hydrogel actuators
Comprehensive safety testing protocols have been developed for hydrogel actuators to evaluate their biocompatibility and potential toxicity. These protocols include in vitro cytotoxicity tests, cell viability assays, hemolysis tests, and in vivo biocompatibility studies. Long-term implantation studies are also conducted to assess tissue response, degradation behavior, and potential adverse effects, ensuring that hydrogel actuators meet regulatory requirements for safety in various applications.Expand Specific Solutions03 Environmentally responsive hydrogel actuators with enhanced safety features
Environmentally responsive hydrogel actuators can be designed with enhanced safety features that respond to specific stimuli such as temperature, pH, light, or electrical signals. These smart hydrogels incorporate safety mechanisms that prevent unintended actuation or failure in biological environments. The responsive nature allows for controlled actuation only under specific conditions, reducing risks associated with unpredictable behavior in biological systems and improving overall safety profiles for applications in medical devices and soft robotics.Expand Specific Solutions04 Biodegradable hydrogel actuators for temporary applications
Biodegradable hydrogel actuators offer safety advantages for temporary medical applications by eliminating the need for removal procedures. These actuators are designed to degrade at controlled rates into non-toxic byproducts that can be metabolized or excreted by the body. The degradation timeline can be tailored to match the required functional lifetime of the device, reducing long-term biocompatibility concerns and potential complications associated with permanent implants while maintaining effective actuation during their intended use period.Expand Specific Solutions05 Encapsulation techniques for improving hydrogel actuator biocompatibility
Encapsulation techniques can significantly improve the biocompatibility and safety of hydrogel actuators. By coating or encapsulating hydrogel actuators with biocompatible materials such as phospholipid bilayers, extracellular matrix components, or anti-fouling polymers, the interface between the actuator and biological tissues can be optimized. These techniques help prevent protein adsorption, cell adhesion, and foreign body responses while maintaining the actuator's mechanical properties and functionality, resulting in improved long-term performance and reduced adverse effects in biological environments.Expand Specific Solutions
Key Industry Players in Implantable Hydrogel Development
The implantable hydrogel actuator market is currently in an early growth phase, characterized by significant research activity primarily led by academic institutions rather than commercial entities. The global market for biocompatible implantable materials is projected to reach approximately $25 billion by 2027, with hydrogel actuators representing an emerging segment. Technical maturity remains moderate, with universities like Northwestern University, Sichuan University, and South China University of Technology leading fundamental research on biocompatibility and safety protocols. Commercial development is beginning to emerge through companies like SentryX BV with their BR-003 biodegradable hydrogel for pain management, Healshape's regenerative medicine applications, and Medtronic's interest in medical device integration. The technology faces challenges in standardizing safety assessment methodologies and long-term biocompatibility validation before widespread clinical adoption.
Northwestern University
Technical Solution: Northwestern University has pioneered a multidisciplinary approach to hydrogel actuator biocompatibility assessment through their Biomedical Engineering department. Their research focuses on developing "smart" hydrogels with stimuli-responsive properties that can be precisely controlled for medical implant applications. Their safety assessment protocol incorporates advanced in vitro models including 3D organoid cultures and microfluidic "organ-on-chip" platforms to evaluate tissue-material interactions before animal testing. Northwestern's researchers have developed specialized immunohistochemistry techniques to characterize the immune response to implanted hydrogels at the cellular and molecular levels. Their biocompatibility assessment includes evaluation of mechanical compatibility with surrounding tissues, as hydrogel actuators must match tissue compliance while maintaining functionality. The university has also established protocols for evaluating the long-term stability of hydrogel actuators under physiological conditions, including assessment of degradation products and their potential toxicity profiles[2][5]. Their approach emphasizes early detection of biocompatibility issues through comprehensive molecular and cellular analysis.
Strengths: Strong interdisciplinary collaboration between materials science, bioengineering and medical researchers; innovative testing methodologies that reduce animal testing requirements; focus on fundamental mechanisms of biocompatibility. Weaknesses: Academic research focus may result in less standardized protocols compared to industry; limited resources for large-scale validation studies compared to commercial entities.
Ottawa Health Research Institute
Technical Solution: The Ottawa Health Research Institute has established a comprehensive biocompatibility assessment platform for implantable hydrogel actuators focused on regenerative medicine applications. Their approach integrates advanced immunological profiling to characterize the host response to implanted materials at multiple timepoints. The institute has developed specialized protocols for evaluating the integration of hydrogel actuators with vascular networks, including assessment of potential thrombogenicity and angiogenic responses. Their research incorporates longitudinal imaging studies using contrast-enhanced MRI to track hydrogel degradation and tissue integration non-invasively. The institute has pioneered methods for evaluating the impact of mechanical actuation on surrounding tissues, with particular attention to potential stress shielding or tissue compression effects. Their safety assessment includes comprehensive evaluation of hydrogel leachables and extractables under physiological conditions, with specialized mass spectrometry techniques to identify potential toxic components. The institute also employs advanced genomic and proteomic analyses to characterize the molecular response of tissues to implanted hydrogels, providing early indicators of biocompatibility issues before clinical manifestations[7][9]. Their approach emphasizes predictive biocompatibility modeling to streamline the development process.
Strengths: Strong focus on translational research with established clinical partnerships; comprehensive molecular and cellular analysis capabilities; expertise in regenerative medicine applications. Weaknesses: Geographic limitations for international collaborations; potential resource constraints compared to larger research institutions.
Critical Patents and Research in Hydrogel Safety Engineering
Load Bearing Hydrogel Implants
PatentInactiveUS20100029789A1
Innovation
- Development of dual network hydrogels comprising a photocrosslinkable hyaluronan network and a hydrophilic polymer network, which are crosslinked to create a material with enhanced mechanical and biological properties suitable for load-bearing implants, including spinal disc substitutes and prostheses.
Hydrogel used as an injectable support for application in cell therapy and as a system for the controlled release of drugs
PatentActiveES2455441A1
Innovation
- A hydrogel formed by two elastin-type biopolymers cross-linked via a 'click chemistry' reaction in physiological conditions, without the need for chemical agents or organic solvents, which can be injected and form rapidly, providing stability, versatility, and biocompatibility.
Regulatory Framework for Implantable Medical Materials
The regulatory landscape for implantable medical materials presents a complex framework that manufacturers of hydrogel actuators must navigate to ensure market approval. In the United States, the Food and Drug Administration (FDA) classifies implantable devices into three risk categories, with most hydrogel actuators falling under Class III, requiring the most stringent Premarket Approval (PMA) process. This process demands comprehensive safety and efficacy data, including biocompatibility testing according to ISO 10993 standards.
The European Union has implemented the Medical Device Regulation (MDR 2017/745), which replaced the previous Medical Device Directive in 2021, significantly increasing requirements for clinical evidence and post-market surveillance. Under this framework, hydrogel actuators typically qualify as Class III devices, requiring Notified Body assessment and certification before receiving CE marking.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) maintains similarly rigorous standards, while China's National Medical Products Administration (NMPA) has been harmonizing its regulatory approach with international standards, though with distinct documentation requirements and testing protocols.
Specific to hydrogel materials, regulatory bodies focus on several critical aspects: leachable compounds and degradation products, long-term stability in physiological environments, mechanical property changes over time, and potential immune responses. The FDA's guidance document on implantable polymers (2019) specifically addresses hydrogel materials, requiring additional testing for water content stability, swelling behavior, and interface interactions with surrounding tissues.
International standards play a crucial role in regulatory compliance, with ISO 10993 series serving as the cornerstone for biocompatibility assessment. For hydrogel actuators specifically, ISO 10993-13 (identification and quantification of degradation products) and ISO 10993-17 (establishment of allowable limits for leachable substances) are particularly relevant.
Recent regulatory trends indicate increasing scrutiny of materials with dynamic properties like hydrogel actuators. The FDA's 2020 guidance on "Implantable Devices with Changing Properties" specifically addresses materials that undergo intentional physical or chemical changes post-implantation, requiring additional characterization of these changes and their biological impacts.
Manufacturers must also consider regional variations in regulatory pathways, with some jurisdictions offering expedited review for innovative technologies addressing unmet medical needs, while others may require country-specific testing or documentation beyond international standards.
The European Union has implemented the Medical Device Regulation (MDR 2017/745), which replaced the previous Medical Device Directive in 2021, significantly increasing requirements for clinical evidence and post-market surveillance. Under this framework, hydrogel actuators typically qualify as Class III devices, requiring Notified Body assessment and certification before receiving CE marking.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) maintains similarly rigorous standards, while China's National Medical Products Administration (NMPA) has been harmonizing its regulatory approach with international standards, though with distinct documentation requirements and testing protocols.
Specific to hydrogel materials, regulatory bodies focus on several critical aspects: leachable compounds and degradation products, long-term stability in physiological environments, mechanical property changes over time, and potential immune responses. The FDA's guidance document on implantable polymers (2019) specifically addresses hydrogel materials, requiring additional testing for water content stability, swelling behavior, and interface interactions with surrounding tissues.
International standards play a crucial role in regulatory compliance, with ISO 10993 series serving as the cornerstone for biocompatibility assessment. For hydrogel actuators specifically, ISO 10993-13 (identification and quantification of degradation products) and ISO 10993-17 (establishment of allowable limits for leachable substances) are particularly relevant.
Recent regulatory trends indicate increasing scrutiny of materials with dynamic properties like hydrogel actuators. The FDA's 2020 guidance on "Implantable Devices with Changing Properties" specifically addresses materials that undergo intentional physical or chemical changes post-implantation, requiring additional characterization of these changes and their biological impacts.
Manufacturers must also consider regional variations in regulatory pathways, with some jurisdictions offering expedited review for innovative technologies addressing unmet medical needs, while others may require country-specific testing or documentation beyond international standards.
Long-term Performance Monitoring Strategies
Effective long-term performance monitoring of implantable hydrogel actuators represents a critical component in ensuring their continued safety and functionality within the human body. The dynamic nature of the in vivo environment necessitates comprehensive surveillance strategies that extend beyond initial implantation periods to capture potential degradation, mechanical failure, or adverse biological responses over the device's intended lifespan.
Continuous real-time monitoring systems have emerged as the gold standard for tracking hydrogel actuator performance. These systems typically incorporate embedded microsensors capable of measuring key parameters such as mechanical displacement, pressure generation, response time, and actuation frequency. Advanced monitoring platforms now integrate wireless data transmission capabilities, allowing for non-invasive assessment without requiring secondary surgical procedures for evaluation.
Remote monitoring protocols have been developed to track functional parameters through external imaging modalities. Techniques such as ultrasound elastography can visualize mechanical deformation patterns, while specialized MRI sequences may detect changes in hydrogel composition or structural integrity. These approaches provide valuable longitudinal data while minimizing patient discomfort and intervention risks.
Biomarker surveillance represents another crucial monitoring strategy, focusing on the detection of specific molecular indicators that may signal material degradation or host response. Regular analysis of localized fluid samples or systemic circulation can reveal the presence of hydrogel degradation products, inflammatory mediators, or immune activation markers that might indicate compromised biocompatibility or material failure.
Explant analysis protocols establish standardized procedures for the retrieval and comprehensive evaluation of hydrogel actuators following their functional lifetime or in cases of premature failure. These protocols typically include detailed mechanical testing, chemical composition analysis, surface characterization, and histological examination of surrounding tissues to assess material changes and biological integration over time.
Patient-reported outcome measures (PROMs) provide valuable complementary data by systematically documenting subjective experiences related to the implanted device. Structured questionnaires and regular clinical assessments can capture subtle changes in comfort, functionality, or potential adverse effects that may not be evident through instrumental monitoring alone.
Predictive modeling approaches have gained significant traction, utilizing accumulated monitoring data to develop algorithms capable of forecasting potential failure modes or performance degradation. These models integrate multiple data streams to identify early warning signs of actuator malfunction, potentially enabling preemptive intervention before clinical manifestations occur.
Regulatory frameworks increasingly emphasize the implementation of post-market surveillance programs for implantable hydrogel actuators, requiring manufacturers to establish robust monitoring protocols extending throughout the device's intended lifespan and beyond. These requirements reflect growing recognition that long-term performance data is essential for comprehensive safety assessment and continuous product improvement.
Continuous real-time monitoring systems have emerged as the gold standard for tracking hydrogel actuator performance. These systems typically incorporate embedded microsensors capable of measuring key parameters such as mechanical displacement, pressure generation, response time, and actuation frequency. Advanced monitoring platforms now integrate wireless data transmission capabilities, allowing for non-invasive assessment without requiring secondary surgical procedures for evaluation.
Remote monitoring protocols have been developed to track functional parameters through external imaging modalities. Techniques such as ultrasound elastography can visualize mechanical deformation patterns, while specialized MRI sequences may detect changes in hydrogel composition or structural integrity. These approaches provide valuable longitudinal data while minimizing patient discomfort and intervention risks.
Biomarker surveillance represents another crucial monitoring strategy, focusing on the detection of specific molecular indicators that may signal material degradation or host response. Regular analysis of localized fluid samples or systemic circulation can reveal the presence of hydrogel degradation products, inflammatory mediators, or immune activation markers that might indicate compromised biocompatibility or material failure.
Explant analysis protocols establish standardized procedures for the retrieval and comprehensive evaluation of hydrogel actuators following their functional lifetime or in cases of premature failure. These protocols typically include detailed mechanical testing, chemical composition analysis, surface characterization, and histological examination of surrounding tissues to assess material changes and biological integration over time.
Patient-reported outcome measures (PROMs) provide valuable complementary data by systematically documenting subjective experiences related to the implanted device. Structured questionnaires and regular clinical assessments can capture subtle changes in comfort, functionality, or potential adverse effects that may not be evident through instrumental monitoring alone.
Predictive modeling approaches have gained significant traction, utilizing accumulated monitoring data to develop algorithms capable of forecasting potential failure modes or performance degradation. These models integrate multiple data streams to identify early warning signs of actuator malfunction, potentially enabling preemptive intervention before clinical manifestations occur.
Regulatory frameworks increasingly emphasize the implementation of post-market surveillance programs for implantable hydrogel actuators, requiring manufacturers to establish robust monitoring protocols extending throughout the device's intended lifespan and beyond. These requirements reflect growing recognition that long-term performance data is essential for comprehensive safety assessment and continuous product improvement.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!