Investigation of Photon-Induced Propyne Chemistry
JUL 30, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Photon-Propyne Interaction Background and Objectives
The investigation of photon-induced propyne chemistry represents a fascinating frontier in the field of photochemistry and molecular dynamics. This research area has gained significant attention in recent years due to its potential applications in various domains, including astrochemistry, materials science, and energy conversion.
Propyne, also known as methylacetylene, is a simple hydrocarbon molecule with a unique structure that makes it particularly interesting for photochemical studies. Its triple bond and methyl group provide a model system for understanding more complex organic reactions induced by light. The interaction between photons and propyne molecules can lead to a variety of chemical transformations, including isomerization, fragmentation, and polymerization.
The historical development of this field can be traced back to early spectroscopic studies of acetylene and its derivatives in the mid-20th century. However, it was not until the advent of advanced laser technologies and sophisticated detection methods that researchers could probe the intricacies of propyne's photochemistry with high precision and time resolution.
The primary objectives of investigating photon-induced propyne chemistry are multifaceted. Firstly, researchers aim to elucidate the fundamental mechanisms underlying the interaction between light and propyne molecules. This includes understanding the electronic excitation processes, the subsequent molecular rearrangements, and the eventual chemical outcomes.
Secondly, there is a strong interest in mapping out the reaction pathways and energy landscapes involved in these photochemical processes. This knowledge is crucial for predicting and controlling the products of propyne photochemistry under various conditions. It also provides insights into the behavior of more complex hydrocarbon systems when exposed to light.
Another key objective is to explore the potential applications of propyne photochemistry. In the context of astrochemistry, understanding these reactions can help explain the formation and transformation of organic molecules in interstellar space and planetary atmospheres. In materials science, photon-induced propyne reactions could lead to novel methods for synthesizing advanced carbon-based materials with tailored properties.
Furthermore, researchers are investigating the role of propyne photochemistry in energy conversion processes. The ability of propyne to absorb and transform light energy into chemical energy could have implications for solar fuel production and other renewable energy technologies.
As the field progresses, there is an increasing focus on developing new experimental techniques and theoretical models to probe deeper into the photon-propyne interaction. This includes the use of ultrafast spectroscopy, advanced computational methods, and innovative reaction vessels that can mimic extreme conditions found in space or industrial processes.
In conclusion, the investigation of photon-induced propyne chemistry is a dynamic and evolving field with far-reaching implications. By unraveling the complexities of this seemingly simple molecule's interaction with light, researchers are paving the way for new discoveries and applications across multiple scientific disciplines.
Propyne, also known as methylacetylene, is a simple hydrocarbon molecule with a unique structure that makes it particularly interesting for photochemical studies. Its triple bond and methyl group provide a model system for understanding more complex organic reactions induced by light. The interaction between photons and propyne molecules can lead to a variety of chemical transformations, including isomerization, fragmentation, and polymerization.
The historical development of this field can be traced back to early spectroscopic studies of acetylene and its derivatives in the mid-20th century. However, it was not until the advent of advanced laser technologies and sophisticated detection methods that researchers could probe the intricacies of propyne's photochemistry with high precision and time resolution.
The primary objectives of investigating photon-induced propyne chemistry are multifaceted. Firstly, researchers aim to elucidate the fundamental mechanisms underlying the interaction between light and propyne molecules. This includes understanding the electronic excitation processes, the subsequent molecular rearrangements, and the eventual chemical outcomes.
Secondly, there is a strong interest in mapping out the reaction pathways and energy landscapes involved in these photochemical processes. This knowledge is crucial for predicting and controlling the products of propyne photochemistry under various conditions. It also provides insights into the behavior of more complex hydrocarbon systems when exposed to light.
Another key objective is to explore the potential applications of propyne photochemistry. In the context of astrochemistry, understanding these reactions can help explain the formation and transformation of organic molecules in interstellar space and planetary atmospheres. In materials science, photon-induced propyne reactions could lead to novel methods for synthesizing advanced carbon-based materials with tailored properties.
Furthermore, researchers are investigating the role of propyne photochemistry in energy conversion processes. The ability of propyne to absorb and transform light energy into chemical energy could have implications for solar fuel production and other renewable energy technologies.
As the field progresses, there is an increasing focus on developing new experimental techniques and theoretical models to probe deeper into the photon-propyne interaction. This includes the use of ultrafast spectroscopy, advanced computational methods, and innovative reaction vessels that can mimic extreme conditions found in space or industrial processes.
In conclusion, the investigation of photon-induced propyne chemistry is a dynamic and evolving field with far-reaching implications. By unraveling the complexities of this seemingly simple molecule's interaction with light, researchers are paving the way for new discoveries and applications across multiple scientific disciplines.
Market Applications of Photon-Induced Propyne Reactions
Photon-induced propyne chemistry has emerged as a promising field with diverse market applications across various industries. The unique reactivity of propyne under photochemical conditions offers opportunities for innovative product development and process optimization.
In the pharmaceutical sector, photon-induced propyne reactions have shown potential for the synthesis of complex drug molecules. These reactions enable the formation of carbon-carbon bonds and the introduction of functional groups that are challenging to achieve through conventional methods. This capability can lead to more efficient and cost-effective production of active pharmaceutical ingredients, potentially reducing manufacturing costs and accelerating drug development timelines.
The polymer industry stands to benefit significantly from advancements in photon-induced propyne chemistry. By incorporating propyne-based monomers into photopolymerization processes, manufacturers can create novel materials with enhanced properties. These may include improved thermal stability, increased mechanical strength, or unique optical characteristics. Such innovations could revolutionize the production of high-performance plastics, coatings, and adhesives for applications in aerospace, automotive, and consumer electronics industries.
In the field of materials science, photon-induced propyne reactions offer a pathway to develop advanced functional materials. For instance, the controlled polymerization of propyne under specific light conditions can result in the formation of conjugated polymers with tunable electronic properties. These materials have potential applications in organic electronics, including organic light-emitting diodes (OLEDs), organic photovoltaics, and organic field-effect transistors.
The energy sector is another area where photon-induced propyne chemistry shows promise. Research into propyne-based photocatalysts for solar energy conversion and storage could lead to more efficient solar cells and advanced energy storage systems. Additionally, the photochemical transformation of propyne into value-added chemicals presents opportunities for sustainable chemical production, aligning with the growing demand for green chemistry solutions.
In the agricultural industry, photon-induced propyne reactions may contribute to the development of novel crop protection agents. By leveraging the reactivity of propyne under controlled light conditions, researchers could design more targeted and environmentally friendly pesticides or herbicides. This approach could potentially reduce the environmental impact of agricultural chemicals while maintaining or improving their efficacy.
The semiconductor industry may also benefit from advancements in this field. Photon-induced propyne chemistry could enable the development of new precursors for chemical vapor deposition processes, leading to improved thin-film deposition techniques for semiconductor manufacturing. This could result in more efficient production of microelectronics and potentially contribute to the ongoing miniaturization of electronic devices.
As research in photon-induced propyne chemistry progresses, it is likely to unlock new market opportunities across these and other sectors. The versatility of this chemistry, combined with its potential for precision and control, positions it as a valuable tool for innovation in multiple industries, driving technological advancements and economic growth.
In the pharmaceutical sector, photon-induced propyne reactions have shown potential for the synthesis of complex drug molecules. These reactions enable the formation of carbon-carbon bonds and the introduction of functional groups that are challenging to achieve through conventional methods. This capability can lead to more efficient and cost-effective production of active pharmaceutical ingredients, potentially reducing manufacturing costs and accelerating drug development timelines.
The polymer industry stands to benefit significantly from advancements in photon-induced propyne chemistry. By incorporating propyne-based monomers into photopolymerization processes, manufacturers can create novel materials with enhanced properties. These may include improved thermal stability, increased mechanical strength, or unique optical characteristics. Such innovations could revolutionize the production of high-performance plastics, coatings, and adhesives for applications in aerospace, automotive, and consumer electronics industries.
In the field of materials science, photon-induced propyne reactions offer a pathway to develop advanced functional materials. For instance, the controlled polymerization of propyne under specific light conditions can result in the formation of conjugated polymers with tunable electronic properties. These materials have potential applications in organic electronics, including organic light-emitting diodes (OLEDs), organic photovoltaics, and organic field-effect transistors.
The energy sector is another area where photon-induced propyne chemistry shows promise. Research into propyne-based photocatalysts for solar energy conversion and storage could lead to more efficient solar cells and advanced energy storage systems. Additionally, the photochemical transformation of propyne into value-added chemicals presents opportunities for sustainable chemical production, aligning with the growing demand for green chemistry solutions.
In the agricultural industry, photon-induced propyne reactions may contribute to the development of novel crop protection agents. By leveraging the reactivity of propyne under controlled light conditions, researchers could design more targeted and environmentally friendly pesticides or herbicides. This approach could potentially reduce the environmental impact of agricultural chemicals while maintaining or improving their efficacy.
The semiconductor industry may also benefit from advancements in this field. Photon-induced propyne chemistry could enable the development of new precursors for chemical vapor deposition processes, leading to improved thin-film deposition techniques for semiconductor manufacturing. This could result in more efficient production of microelectronics and potentially contribute to the ongoing miniaturization of electronic devices.
As research in photon-induced propyne chemistry progresses, it is likely to unlock new market opportunities across these and other sectors. The versatility of this chemistry, combined with its potential for precision and control, positions it as a valuable tool for innovation in multiple industries, driving technological advancements and economic growth.
Current Challenges in Photon-Propyne Chemistry
The field of photon-induced propyne chemistry faces several significant challenges that hinder its advancement and practical applications. One of the primary obstacles is the difficulty in achieving precise control over the photochemical reactions involving propyne molecules. The high reactivity of propyne and its tendency to undergo various side reactions under photon irradiation make it challenging to selectively produce desired products.
Another major challenge lies in the development of efficient and stable photocatalysts for propyne-based reactions. Current catalysts often suffer from rapid deactivation or limited selectivity, reducing their effectiveness in industrial applications. The search for novel, robust catalysts that can withstand prolonged exposure to light while maintaining high activity and selectivity remains an ongoing pursuit.
The complexity of reaction mechanisms in photon-induced propyne chemistry poses a significant hurdle for researchers. The intricate interplay between light absorption, energy transfer, and chemical transformations makes it difficult to elucidate the exact pathways and intermediates involved. This lack of mechanistic understanding hampers the rational design of more efficient reaction systems and limits the ability to predict and control reaction outcomes.
Scale-up and industrial implementation of photon-induced propyne reactions present additional challenges. The need for specialized photoreactors and light sources, coupled with the inherent limitations of light penetration in large-scale systems, complicates the transition from laboratory-scale experiments to industrial production. Developing cost-effective and scalable technologies for photochemical processes remains a critical area of research.
Furthermore, the energy efficiency of photon-induced propyne reactions is a concern. Many current systems require high-energy ultraviolet light, which is both costly and potentially hazardous. Shifting towards visible light-driven processes could improve energy efficiency and safety, but this transition requires the development of new photocatalysts and reaction conditions.
The stability and handling of propyne itself pose safety challenges, particularly in large-scale operations. As a highly flammable and potentially explosive gas, propyne requires careful handling and storage procedures, which can complicate its use in industrial settings. Developing safer alternatives or improved handling techniques is crucial for the widespread adoption of photon-induced propyne chemistry.
Lastly, the environmental impact of photon-induced propyne chemistry needs to be carefully considered. While photochemical processes can offer greener alternatives to traditional synthetic methods, the potential formation of harmful byproducts or the use of toxic catalysts must be addressed to ensure the sustainability of these reactions.
Another major challenge lies in the development of efficient and stable photocatalysts for propyne-based reactions. Current catalysts often suffer from rapid deactivation or limited selectivity, reducing their effectiveness in industrial applications. The search for novel, robust catalysts that can withstand prolonged exposure to light while maintaining high activity and selectivity remains an ongoing pursuit.
The complexity of reaction mechanisms in photon-induced propyne chemistry poses a significant hurdle for researchers. The intricate interplay between light absorption, energy transfer, and chemical transformations makes it difficult to elucidate the exact pathways and intermediates involved. This lack of mechanistic understanding hampers the rational design of more efficient reaction systems and limits the ability to predict and control reaction outcomes.
Scale-up and industrial implementation of photon-induced propyne reactions present additional challenges. The need for specialized photoreactors and light sources, coupled with the inherent limitations of light penetration in large-scale systems, complicates the transition from laboratory-scale experiments to industrial production. Developing cost-effective and scalable technologies for photochemical processes remains a critical area of research.
Furthermore, the energy efficiency of photon-induced propyne reactions is a concern. Many current systems require high-energy ultraviolet light, which is both costly and potentially hazardous. Shifting towards visible light-driven processes could improve energy efficiency and safety, but this transition requires the development of new photocatalysts and reaction conditions.
The stability and handling of propyne itself pose safety challenges, particularly in large-scale operations. As a highly flammable and potentially explosive gas, propyne requires careful handling and storage procedures, which can complicate its use in industrial settings. Developing safer alternatives or improved handling techniques is crucial for the widespread adoption of photon-induced propyne chemistry.
Lastly, the environmental impact of photon-induced propyne chemistry needs to be carefully considered. While photochemical processes can offer greener alternatives to traditional synthetic methods, the potential formation of harmful byproducts or the use of toxic catalysts must be addressed to ensure the sustainability of these reactions.
Existing Methodologies for Studying Photon-Propyne Reactions
01 Synthesis and production of propyne
Various methods for synthesizing and producing propyne are described. These include catalytic processes, thermal cracking, and other chemical reactions to generate propyne from different precursors. The processes aim to improve yield, efficiency, and purity of the propyne product.- Synthesis and production of propyne: Various methods and processes for synthesizing and producing propyne are described. These include catalytic reactions, thermal decomposition, and other chemical processes to obtain propyne from different precursors.
- Propyne as a raw material in chemical processes: Propyne serves as an important raw material in various chemical processes. It is used in the production of other chemicals, polymers, and materials, showcasing its versatility in industrial applications.
- Purification and separation of propyne: Techniques for purifying and separating propyne from mixtures or reaction products are discussed. These methods aim to obtain high-purity propyne for further use in industrial or research applications.
- Propyne in fuel compositions: The use of propyne in fuel compositions is explored. It can be incorporated into various fuel blends to enhance combustion properties or as an additive to improve fuel performance.
- Propyne derivatives and their applications: Research on propyne derivatives and their potential applications in different fields is presented. These derivatives can be used in pharmaceuticals, agrochemicals, and other specialized industries.
02 Propyne as a raw material in chemical processes
Propyne serves as an important raw material in various chemical processes. It is used in the production of other chemicals, polymers, and materials. The applications include the synthesis of specialty chemicals, pharmaceuticals, and industrial products.Expand Specific Solutions03 Purification and separation of propyne
Techniques for purifying and separating propyne from mixtures are presented. These methods involve distillation, adsorption, membrane separation, and other physical or chemical processes to obtain high-purity propyne for industrial use.Expand Specific Solutions04 Propyne in fuel compositions
The use of propyne in fuel compositions is explored. It can be incorporated into various fuel blends to enhance combustion properties, improve engine performance, or reduce emissions. Research focuses on optimizing propyne content in fuel mixtures for different applications.Expand Specific Solutions05 Safety and handling of propyne
Safety measures and handling procedures for propyne are discussed. Due to its flammable and potentially explosive nature, proper storage, transportation, and use of propyne require specific precautions. Guidelines and equipment for safe handling are described to minimize risks in industrial settings.Expand Specific Solutions
Key Players in Photon-Induced Chemistry Field
The investigation of photon-induced propyne chemistry is in an early developmental stage, with a growing market potential as research progresses. The field is characterized by a diverse range of players, including academic institutions, government research organizations, and private companies. Key academic contributors include the University of Maryland, California Institute of Technology, and Brandeis University, while government entities like the French National Center for Scientific Research (CNRS) and the Spanish National Research Council (CSIC) are also actively involved. Private sector participation is evident through companies such as ExxonMobil Chemical Patents and 3M Innovative Properties, indicating increasing industrial interest. The technology's maturity is still evolving, with ongoing research focused on fundamental understanding and potential applications.
Centre National de la Recherche Scientifique
Technical Solution: CNRS has developed advanced spectroscopic techniques for studying photon-induced propyne chemistry. They utilize ultrafast laser spectroscopy to probe the dynamics of propyne reactions on femtosecond timescales[1]. Their approach combines pump-probe spectroscopy with mass spectrometry to track both electronic and structural changes during photochemical reactions of propyne[2]. CNRS researchers have also employed synchrotron radiation to investigate VUV-induced chemistry of propyne, providing insights into ionization and dissociation pathways[3]. Additionally, they have explored propyne reactions in astrochemical ice analogs using infrared spectroscopy to understand interstellar chemistry processes[4].
Strengths: Access to world-class spectroscopic facilities and expertise in ultrafast techniques. Weaknesses: Focus primarily on fundamental research rather than industrial applications.
ExxonMobil Chemical Patents, Inc.
Technical Solution: ExxonMobil has developed catalytic systems for propyne conversion that can be initiated or enhanced by photochemical processes. Their approach involves using transition metal complexes as photocatalysts to activate propyne for various transformations[1]. They have patented methods for selective hydrogenation of propyne using photocatalysts supported on metal oxides, which allows for precise control over reaction conditions[2]. ExxonMobil has also explored the use of plasmonic nanoparticles to enhance light absorption and catalytic activity in propyne reactions[3]. Their research extends to flow chemistry setups that integrate photochemical activation of propyne with continuous processing for improved efficiency and scalability[4].
Strengths: Strong focus on practical applications and scalable processes. Extensive resources for catalyst development and process optimization. Weaknesses: May be less focused on fundamental mechanistic studies compared to academic institutions.
Breakthrough Technologies in Photon-Propyne Interactions
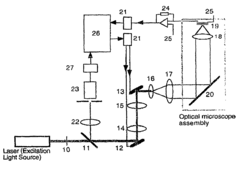
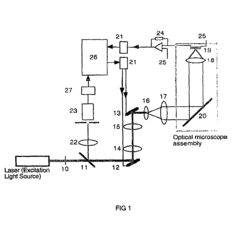
Method for generating high-contrast images of semiconductor sites via one-photon optical beam-induced current imaging and confocal reflectance microscopy
PatentInactiveUS7235988B2
Innovation
- Generating high-contrast images of semiconductor sites by taking the product of one-photon OBIC and confocal reflectance images, which are obtained from the same focused beam, leveraging the optical-sectioning capability of confocal microscopy and the exclusive OBIC signal from semiconductor materials.
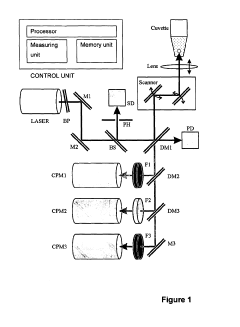
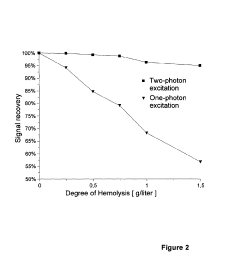
Use of two-photon excited fluorescence in assays of clinical chemistry analytes
PatentActiveUS10295465B2
Innovation
- The use of two-photon excited fluorescence (TPE) technology for clinical chemistry analyte quantification, allowing separation-free assays in microvolumes with low-cost disposable cuvettes and reduced assay volumes, while being tolerant to matrix interferences, enabling both clinical chemistry and immunoassays with a single instrument.
Safety Considerations in Photon-Propyne Experiments
Safety considerations in photon-propyne experiments are paramount due to the reactive nature of propyne and the potential hazards associated with high-energy photons. Proper risk assessment and mitigation strategies must be implemented to ensure the safety of researchers and the integrity of the experimental setup.
Propyne, also known as methylacetylene, is a highly flammable gas with a low flash point. It forms explosive mixtures with air and can undergo rapid decomposition under certain conditions. When exposed to high-energy photons, propyne may undergo various chemical reactions, including polymerization, isomerization, and fragmentation. These reactions can release significant amounts of energy and potentially lead to uncontrolled combustion or explosion if not properly managed.
The use of high-energy photons in these experiments introduces additional safety concerns. Depending on the wavelength and intensity of the light source, researchers may be exposed to harmful ultraviolet or ionizing radiation. Proper shielding and personal protective equipment (PPE) are essential to minimize the risk of radiation-induced injuries or long-term health effects.
To address these safety challenges, a comprehensive safety protocol should be established. This protocol should include proper ventilation systems to prevent the accumulation of propyne gas and its potentially hazardous reaction products. Gas detection systems should be installed to monitor propyne levels and trigger alarms if concentrations approach dangerous levels. Fire suppression systems specifically designed for gas fires should be readily available in the laboratory.
Researchers must be trained in the safe handling of compressed gases and the proper use of gas regulators and delivery systems. All equipment used in the experiments should be rated for use with flammable gases and regularly inspected for leaks or damage. Experiments should be conducted in explosion-proof enclosures whenever possible to contain potential incidents.
Personal protective equipment is crucial in these experiments. Researchers should wear flame-resistant lab coats, safety goggles, and appropriate gloves. Depending on the specific experimental setup, additional PPE such as face shields or respirators may be necessary. Emergency eyewash stations and safety showers should be easily accessible in the laboratory.
Proper waste management procedures must be in place to handle any byproducts or unreacted propyne safely. This may include specialized disposal methods for potentially pyrophoric or explosive compounds formed during the experiments. Regular safety audits and risk assessments should be conducted to identify and address any emerging safety concerns as the research progresses.
By implementing these safety measures and maintaining a culture of safety awareness, researchers can minimize the risks associated with photon-induced propyne chemistry experiments and focus on advancing scientific understanding in this exciting field of study.
Propyne, also known as methylacetylene, is a highly flammable gas with a low flash point. It forms explosive mixtures with air and can undergo rapid decomposition under certain conditions. When exposed to high-energy photons, propyne may undergo various chemical reactions, including polymerization, isomerization, and fragmentation. These reactions can release significant amounts of energy and potentially lead to uncontrolled combustion or explosion if not properly managed.
The use of high-energy photons in these experiments introduces additional safety concerns. Depending on the wavelength and intensity of the light source, researchers may be exposed to harmful ultraviolet or ionizing radiation. Proper shielding and personal protective equipment (PPE) are essential to minimize the risk of radiation-induced injuries or long-term health effects.
To address these safety challenges, a comprehensive safety protocol should be established. This protocol should include proper ventilation systems to prevent the accumulation of propyne gas and its potentially hazardous reaction products. Gas detection systems should be installed to monitor propyne levels and trigger alarms if concentrations approach dangerous levels. Fire suppression systems specifically designed for gas fires should be readily available in the laboratory.
Researchers must be trained in the safe handling of compressed gases and the proper use of gas regulators and delivery systems. All equipment used in the experiments should be rated for use with flammable gases and regularly inspected for leaks or damage. Experiments should be conducted in explosion-proof enclosures whenever possible to contain potential incidents.
Personal protective equipment is crucial in these experiments. Researchers should wear flame-resistant lab coats, safety goggles, and appropriate gloves. Depending on the specific experimental setup, additional PPE such as face shields or respirators may be necessary. Emergency eyewash stations and safety showers should be easily accessible in the laboratory.
Proper waste management procedures must be in place to handle any byproducts or unreacted propyne safely. This may include specialized disposal methods for potentially pyrophoric or explosive compounds formed during the experiments. Regular safety audits and risk assessments should be conducted to identify and address any emerging safety concerns as the research progresses.
By implementing these safety measures and maintaining a culture of safety awareness, researchers can minimize the risks associated with photon-induced propyne chemistry experiments and focus on advancing scientific understanding in this exciting field of study.
Environmental Impact of Photon-Propyne Chemistry
The environmental impact of photon-propyne chemistry is a critical consideration in the investigation of this emerging field. As photon-induced propyne reactions gain traction in various applications, it is essential to assess their potential effects on ecosystems and human health.
One of the primary environmental concerns associated with photon-propyne chemistry is the potential release of reactive intermediates and byproducts into the atmosphere. These compounds may contribute to air pollution and participate in complex atmospheric reactions, potentially affecting air quality and climate dynamics. The formation of secondary organic aerosols (SOA) through photochemical processes involving propyne and its derivatives could have implications for particulate matter concentrations and visibility in urban areas.
Water contamination is another potential environmental risk that warrants attention. If not properly contained, propyne and its photochemical products may leach into groundwater or surface water systems. This could lead to alterations in aquatic ecosystems and potentially impact drinking water sources. The persistence and biodegradability of these compounds in aquatic environments need to be thoroughly investigated to assess long-term ecological effects.
The energy requirements for photon-induced propyne reactions must also be considered from an environmental perspective. While photochemical processes can be more energy-efficient than traditional thermal reactions, the production and disposal of light sources used in these reactions may have their own environmental footprint. Life cycle assessments of photon-propyne chemistry applications are necessary to evaluate their overall sustainability compared to conventional chemical processes.
Potential impacts on soil quality and terrestrial ecosystems should not be overlooked. Deposition of airborne photochemical products or accidental spills could affect soil chemistry and microbial communities. This may have cascading effects on plant growth and ecosystem functioning, particularly in sensitive habitats.
On a positive note, photon-propyne chemistry could offer environmental benefits in certain applications. For instance, it may enable more efficient and selective synthetic routes for pharmaceuticals and materials, potentially reducing waste and energy consumption in industrial processes. Additionally, the use of solar light as an energy source for these reactions aligns with sustainable chemistry principles and could contribute to reducing reliance on fossil fuels in chemical manufacturing.
As research in this field progresses, it is crucial to develop comprehensive environmental monitoring strategies and risk assessment protocols specific to photon-propyne chemistry. This will enable early identification of potential hazards and inform the development of mitigation measures. Collaboration between chemists, environmental scientists, and regulatory bodies will be essential in establishing guidelines for the safe and sustainable implementation of photon-induced propyne chemistry in industrial and research settings.
One of the primary environmental concerns associated with photon-propyne chemistry is the potential release of reactive intermediates and byproducts into the atmosphere. These compounds may contribute to air pollution and participate in complex atmospheric reactions, potentially affecting air quality and climate dynamics. The formation of secondary organic aerosols (SOA) through photochemical processes involving propyne and its derivatives could have implications for particulate matter concentrations and visibility in urban areas.
Water contamination is another potential environmental risk that warrants attention. If not properly contained, propyne and its photochemical products may leach into groundwater or surface water systems. This could lead to alterations in aquatic ecosystems and potentially impact drinking water sources. The persistence and biodegradability of these compounds in aquatic environments need to be thoroughly investigated to assess long-term ecological effects.
The energy requirements for photon-induced propyne reactions must also be considered from an environmental perspective. While photochemical processes can be more energy-efficient than traditional thermal reactions, the production and disposal of light sources used in these reactions may have their own environmental footprint. Life cycle assessments of photon-propyne chemistry applications are necessary to evaluate their overall sustainability compared to conventional chemical processes.
Potential impacts on soil quality and terrestrial ecosystems should not be overlooked. Deposition of airborne photochemical products or accidental spills could affect soil chemistry and microbial communities. This may have cascading effects on plant growth and ecosystem functioning, particularly in sensitive habitats.
On a positive note, photon-propyne chemistry could offer environmental benefits in certain applications. For instance, it may enable more efficient and selective synthetic routes for pharmaceuticals and materials, potentially reducing waste and energy consumption in industrial processes. Additionally, the use of solar light as an energy source for these reactions aligns with sustainable chemistry principles and could contribute to reducing reliance on fossil fuels in chemical manufacturing.
As research in this field progresses, it is crucial to develop comprehensive environmental monitoring strategies and risk assessment protocols specific to photon-propyne chemistry. This will enable early identification of potential hazards and inform the development of mitigation measures. Collaboration between chemists, environmental scientists, and regulatory bodies will be essential in establishing guidelines for the safe and sustainable implementation of photon-induced propyne chemistry in industrial and research settings.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!