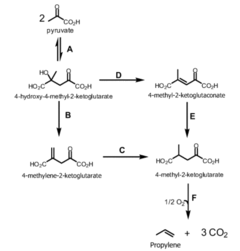
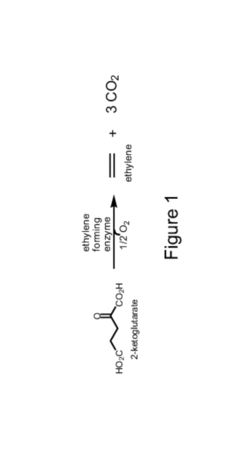
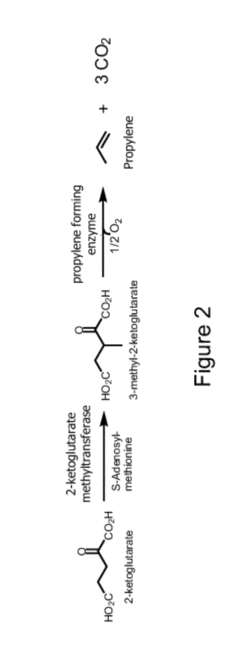
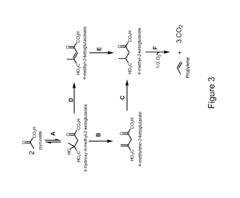
Propyne as a Precursor in Organic Synthesis of Fine Chemicals
JUL 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Propyne Chemistry Background and Objectives
Propyne, also known as methylacetylene, has emerged as a versatile and valuable precursor in the organic synthesis of fine chemicals. This three-carbon alkyne has a rich history in chemistry, dating back to its first synthesis in the late 19th century. Over the decades, propyne's unique reactivity and structural features have made it an attractive building block for a wide range of chemical transformations.
The development of propyne chemistry has been closely tied to the broader field of acetylene chemistry. As researchers explored the reactivity of triple bonds, propyne's methyl group introduced new possibilities for regioselective reactions. This characteristic has been particularly advantageous in the synthesis of complex organic molecules, where precise control over reaction outcomes is crucial.
In recent years, the importance of propyne as a precursor in fine chemical synthesis has grown significantly. This trend is driven by the increasing demand for more efficient and sustainable synthetic routes in the pharmaceutical, agrochemical, and materials science industries. Propyne's ability to participate in various reactions, including cycloadditions, hydrogenations, and C-C bond formations, makes it a versatile starting material for creating diverse molecular architectures.
The objectives of propyne chemistry in the context of fine chemical synthesis are multifaceted. Firstly, there is a strong focus on developing new methodologies that exploit propyne's unique reactivity. This includes the exploration of novel catalytic systems that can enhance selectivity and yield in propyne-based transformations. Secondly, researchers aim to expand the scope of propyne chemistry to access previously challenging or inaccessible molecular structures.
Another key objective is the integration of propyne chemistry into more sustainable synthetic processes. This aligns with the broader trend towards green chemistry, where researchers seek to minimize waste, reduce energy consumption, and utilize renewable resources. Propyne's potential as a C3 building block derived from biomass sources has garnered significant interest in this regard.
Furthermore, the field is moving towards the development of one-pot, multi-component reactions involving propyne. These approaches aim to increase synthetic efficiency by reducing the number of isolation and purification steps in complex molecule synthesis. Such advancements could lead to more economical and environmentally friendly production methods for fine chemicals.
As we look to the future, the continued exploration of propyne chemistry promises to unlock new possibilities in organic synthesis. The ongoing research efforts are expected to yield innovative synthetic strategies, expand the toolkit of organic chemists, and contribute to the development of next-generation fine chemicals with applications across various industries.
The development of propyne chemistry has been closely tied to the broader field of acetylene chemistry. As researchers explored the reactivity of triple bonds, propyne's methyl group introduced new possibilities for regioselective reactions. This characteristic has been particularly advantageous in the synthesis of complex organic molecules, where precise control over reaction outcomes is crucial.
In recent years, the importance of propyne as a precursor in fine chemical synthesis has grown significantly. This trend is driven by the increasing demand for more efficient and sustainable synthetic routes in the pharmaceutical, agrochemical, and materials science industries. Propyne's ability to participate in various reactions, including cycloadditions, hydrogenations, and C-C bond formations, makes it a versatile starting material for creating diverse molecular architectures.
The objectives of propyne chemistry in the context of fine chemical synthesis are multifaceted. Firstly, there is a strong focus on developing new methodologies that exploit propyne's unique reactivity. This includes the exploration of novel catalytic systems that can enhance selectivity and yield in propyne-based transformations. Secondly, researchers aim to expand the scope of propyne chemistry to access previously challenging or inaccessible molecular structures.
Another key objective is the integration of propyne chemistry into more sustainable synthetic processes. This aligns with the broader trend towards green chemistry, where researchers seek to minimize waste, reduce energy consumption, and utilize renewable resources. Propyne's potential as a C3 building block derived from biomass sources has garnered significant interest in this regard.
Furthermore, the field is moving towards the development of one-pot, multi-component reactions involving propyne. These approaches aim to increase synthetic efficiency by reducing the number of isolation and purification steps in complex molecule synthesis. Such advancements could lead to more economical and environmentally friendly production methods for fine chemicals.
As we look to the future, the continued exploration of propyne chemistry promises to unlock new possibilities in organic synthesis. The ongoing research efforts are expected to yield innovative synthetic strategies, expand the toolkit of organic chemists, and contribute to the development of next-generation fine chemicals with applications across various industries.
Market Analysis for Propyne-Derived Fine Chemicals
The market for propyne-derived fine chemicals has shown significant growth potential in recent years, driven by increasing demand across various industries. Propyne, also known as methylacetylene, serves as a versatile precursor in organic synthesis, enabling the production of high-value fine chemicals with diverse applications.
In the pharmaceutical sector, propyne-derived compounds play a crucial role in the synthesis of active pharmaceutical ingredients (APIs) and intermediates. The global pharmaceutical fine chemicals market is expected to expand steadily, with propyne-based products contributing to this growth. Key applications include the production of antiviral drugs, antibiotics, and other therapeutic agents.
The agrochemical industry represents another significant market for propyne-derived fine chemicals. Propyne-based intermediates are utilized in the synthesis of pesticides, herbicides, and plant growth regulators. As global agricultural productivity demands increase, the market for these specialized chemicals is projected to grow substantially.
In the field of electronic materials, propyne-derived compounds find applications in the production of advanced polymers and specialty coatings. The expanding electronics industry, particularly in emerging economies, is driving demand for these high-performance materials. Propyne-based precursors are valued for their ability to impart specific properties to electronic components and devices.
The flavors and fragrances industry also presents opportunities for propyne-derived fine chemicals. Propyne serves as a starting material for the synthesis of various aroma compounds and flavor enhancers. With growing consumer preferences for natural and complex flavor profiles, the demand for specialized propyne-based ingredients is expected to rise.
Environmental regulations and sustainability concerns are influencing market dynamics. Propyne-derived fine chemicals that offer improved environmental profiles or enable more efficient production processes are gaining traction. This trend is particularly evident in the development of green solvents and eco-friendly polymer additives.
Geographically, North America and Europe currently dominate the market for propyne-derived fine chemicals, owing to their established pharmaceutical and specialty chemical industries. However, Asia-Pacific is emerging as a rapidly growing market, driven by increasing industrialization and rising demand for advanced materials in countries like China and India.
The market landscape is characterized by a mix of large multinational chemical companies and specialized fine chemical manufacturers. Key players are investing in research and development to expand their product portfolios and improve production efficiencies. Strategic partnerships and collaborations are becoming increasingly common as companies seek to leverage complementary expertise and resources.
In the pharmaceutical sector, propyne-derived compounds play a crucial role in the synthesis of active pharmaceutical ingredients (APIs) and intermediates. The global pharmaceutical fine chemicals market is expected to expand steadily, with propyne-based products contributing to this growth. Key applications include the production of antiviral drugs, antibiotics, and other therapeutic agents.
The agrochemical industry represents another significant market for propyne-derived fine chemicals. Propyne-based intermediates are utilized in the synthesis of pesticides, herbicides, and plant growth regulators. As global agricultural productivity demands increase, the market for these specialized chemicals is projected to grow substantially.
In the field of electronic materials, propyne-derived compounds find applications in the production of advanced polymers and specialty coatings. The expanding electronics industry, particularly in emerging economies, is driving demand for these high-performance materials. Propyne-based precursors are valued for their ability to impart specific properties to electronic components and devices.
The flavors and fragrances industry also presents opportunities for propyne-derived fine chemicals. Propyne serves as a starting material for the synthesis of various aroma compounds and flavor enhancers. With growing consumer preferences for natural and complex flavor profiles, the demand for specialized propyne-based ingredients is expected to rise.
Environmental regulations and sustainability concerns are influencing market dynamics. Propyne-derived fine chemicals that offer improved environmental profiles or enable more efficient production processes are gaining traction. This trend is particularly evident in the development of green solvents and eco-friendly polymer additives.
Geographically, North America and Europe currently dominate the market for propyne-derived fine chemicals, owing to their established pharmaceutical and specialty chemical industries. However, Asia-Pacific is emerging as a rapidly growing market, driven by increasing industrialization and rising demand for advanced materials in countries like China and India.
The market landscape is characterized by a mix of large multinational chemical companies and specialized fine chemical manufacturers. Key players are investing in research and development to expand their product portfolios and improve production efficiencies. Strategic partnerships and collaborations are becoming increasingly common as companies seek to leverage complementary expertise and resources.
Current Challenges in Propyne-Based Synthesis
Despite the promising potential of propyne as a precursor in organic synthesis of fine chemicals, several significant challenges currently hinder its widespread application. One of the primary obstacles is the limited availability and high cost of propyne. As a byproduct of petroleum refining, its production is not easily scalable, leading to supply constraints and price volatility. This economic barrier often discourages researchers and industries from exploring propyne-based synthesis routes, particularly for large-scale production.
Another major challenge lies in the reactivity and handling of propyne. As a highly flammable and potentially explosive gas, propyne requires specialized equipment and safety measures for storage and use. This not only increases the complexity of experimental setups but also raises concerns about industrial-scale applications. The need for stringent safety protocols and specialized infrastructure can significantly impact the feasibility of propyne-based processes in commercial settings.
The selectivity of propyne reactions presents another hurdle in fine chemical synthesis. While propyne's triple bond offers versatile reactivity, controlling the regioselectivity and stereoselectivity of additions and coupling reactions remains challenging. Unwanted side reactions and the formation of isomeric mixtures can complicate product purification and reduce overall yield, making process optimization crucial yet difficult.
Furthermore, the development of efficient catalysts for propyne-based reactions is an ongoing challenge. Many current catalytic systems suffer from low turnover numbers, limited substrate scope, or sensitivity to reaction conditions. The search for robust, highly active, and selective catalysts that can operate under mild conditions continues to be a focus of research efforts in this field.
Environmental concerns also pose challenges to propyne-based synthesis. As sustainability becomes increasingly important in chemical manufacturing, the petroleum-derived nature of propyne raises questions about its long-term viability. Developing greener alternatives or finding renewable sources for propyne production is becoming a pressing need to ensure the future relevance of propyne-based methodologies in fine chemical synthesis.
Lastly, the scalability of propyne-based processes from laboratory to industrial scale presents significant engineering challenges. Issues such as heat management, gas-liquid mass transfer, and continuous flow processing need to be addressed to make propyne-based syntheses commercially viable for fine chemical production. Overcoming these technical hurdles requires interdisciplinary collaboration between chemists and chemical engineers.
Another major challenge lies in the reactivity and handling of propyne. As a highly flammable and potentially explosive gas, propyne requires specialized equipment and safety measures for storage and use. This not only increases the complexity of experimental setups but also raises concerns about industrial-scale applications. The need for stringent safety protocols and specialized infrastructure can significantly impact the feasibility of propyne-based processes in commercial settings.
The selectivity of propyne reactions presents another hurdle in fine chemical synthesis. While propyne's triple bond offers versatile reactivity, controlling the regioselectivity and stereoselectivity of additions and coupling reactions remains challenging. Unwanted side reactions and the formation of isomeric mixtures can complicate product purification and reduce overall yield, making process optimization crucial yet difficult.
Furthermore, the development of efficient catalysts for propyne-based reactions is an ongoing challenge. Many current catalytic systems suffer from low turnover numbers, limited substrate scope, or sensitivity to reaction conditions. The search for robust, highly active, and selective catalysts that can operate under mild conditions continues to be a focus of research efforts in this field.
Environmental concerns also pose challenges to propyne-based synthesis. As sustainability becomes increasingly important in chemical manufacturing, the petroleum-derived nature of propyne raises questions about its long-term viability. Developing greener alternatives or finding renewable sources for propyne production is becoming a pressing need to ensure the future relevance of propyne-based methodologies in fine chemical synthesis.
Lastly, the scalability of propyne-based processes from laboratory to industrial scale presents significant engineering challenges. Issues such as heat management, gas-liquid mass transfer, and continuous flow processing need to be addressed to make propyne-based syntheses commercially viable for fine chemical production. Overcoming these technical hurdles requires interdisciplinary collaboration between chemists and chemical engineers.
Existing Propyne Synthesis Methodologies
01 Synthesis and production of propyne
Various methods and processes for synthesizing and producing propyne are described. These include catalytic processes, thermal cracking, and other chemical reactions to obtain propyne from different starting materials.- Synthesis and production of propyne: Various methods and processes for synthesizing and producing propyne are described. These include catalytic processes, thermal cracking, and other chemical reactions to obtain propyne from different starting materials.
- Purification and separation of propyne: Techniques for purifying and separating propyne from other gases or mixtures are outlined. These methods may involve distillation, adsorption, or membrane separation processes to obtain high-purity propyne.
- Applications of propyne in chemical synthesis: Propyne is used as a starting material or intermediate in various chemical syntheses. It can be employed in the production of polymers, pharmaceuticals, and other organic compounds through reactions such as cycloadditions or hydrogenations.
- Storage and handling of propyne: Methods and equipment for safely storing and handling propyne are described. This includes specialized containers, pressure vessels, and safety measures to prevent accidents due to the flammable nature of propyne.
- Detection and analysis of propyne: Techniques for detecting and analyzing propyne in various environments or mixtures are presented. These may include spectroscopic methods, gas chromatography, or other analytical techniques for quantifying propyne concentrations.
02 Purification and separation of propyne
Techniques for purifying and separating propyne from mixtures or reaction products are outlined. These may involve distillation, extraction, or other separation methods to obtain high-purity propyne.Expand Specific Solutions03 Applications of propyne in chemical synthesis
Propyne is used as a reactant or intermediate in various chemical syntheses. It can be employed in the production of other chemicals, polymers, or materials with specific properties.Expand Specific Solutions04 Propyne in fuel compositions
The use of propyne in fuel compositions is explored. It may be incorporated into fuel mixtures to enhance combustion properties or as an additive for specific applications.Expand Specific Solutions05 Safety and handling of propyne
Methods and systems for the safe handling, storage, and transportation of propyne are described. This includes safety measures, equipment designs, and protocols to manage the risks associated with this flammable gas.Expand Specific Solutions
Key Players in Propyne-Based Fine Chemical Industry
The propyne-based fine chemical synthesis market is in a growth phase, driven by increasing demand for specialty chemicals across various industries. The market size is expanding, with major players like BASF Corp. and Lyondell Chemical Technology LP leading the way. Technological maturity varies, with established companies such as UBE Corp. and Braskem SA having advanced capabilities, while newer entrants like Industrial Microbes, Inc. are innovating in sustainable production methods. Academic institutions like South China University of Technology and Zhejiang University are contributing to research and development, pushing the boundaries of propyne utilization in organic synthesis.
BASF Corp.
Technical Solution: BASF has developed innovative catalytic processes for propyne utilization in fine chemical synthesis. Their approach involves a palladium-catalyzed cross-coupling reaction of propyne with aryl halides to produce substituted propargyl compounds[1]. This method allows for the efficient synthesis of complex molecules with alkyne functionalities. BASF has also explored the use of propyne in the production of methacrylate monomers, which are important precursors for various polymers and resins[2]. Their process involves the carbonylation of propyne in the presence of methanol and a palladium catalyst, resulting in high yields of methyl methacrylate[3].
Strengths: Efficient catalytic processes, high yields, and versatility in producing various fine chemicals. Weaknesses: Potential safety concerns due to the high reactivity of propyne, and the need for specialized handling equipment.
Lyondell Chemical Technology LP
Technical Solution: Lyondell has developed a proprietary process for the production of propylene oxide using propyne as a key intermediate. Their method involves the epoxidation of propyne to form propylene oxide, which is then used in the synthesis of various polyurethane precursors and other fine chemicals[4]. The company has also explored the use of propyne in the production of acrylonitrile, an important precursor for acrylic fibers and plastics. Their process involves the ammoxidation of propyne using a novel catalyst system, resulting in high selectivity and yield of acrylonitrile[5].
Strengths: Innovative use of propyne in large-scale industrial processes, high selectivity, and yield. Weaknesses: Dependence on propyne availability and potential competition from alternative feedstocks.
Innovative Propyne Reaction Mechanisms
Process for preparing alkylene oxide
PatentWO2005075444A1
Innovation
- The process involves heating only the alkene to a temperature of 60 to 120 °C, while maintaining the hydroperoxide stream at a similar temperature, to prevent fouling of the heat exchanger and allow for higher temperature epoxidation reactions without deactivating the catalyst.
Microorganisms for producing propylene and methods related thereto
PatentActiveUS20120329119A1
Innovation
- Development of non-naturally occurring microbial organisms engineered with propylene biosynthetic pathways, using nucleic acids encoding propylene pathway enzymes to produce propylene through fermentation from renewable feedstocks like sugars and syngas, eliminating the need for dehydration steps and reducing waste and emissions.
Environmental Impact of Propyne Chemistry
The use of propyne as a precursor in organic synthesis of fine chemicals has significant environmental implications that warrant careful consideration. The production and utilization of propyne involve various chemical processes that can impact the environment in multiple ways.
One of the primary environmental concerns associated with propyne chemistry is the potential for air pollution. The synthesis and handling of propyne can lead to the release of volatile organic compounds (VOCs) into the atmosphere. These emissions may contribute to the formation of ground-level ozone and smog, which can have detrimental effects on air quality and human health. Additionally, propyne itself is a flammable gas, posing risks of fire and explosion if not properly managed.
Water pollution is another critical environmental aspect to consider. The production and use of propyne in chemical synthesis often involve aqueous processes and generate wastewater. This effluent may contain organic compounds, catalysts, and other chemicals that, if not adequately treated, can contaminate water sources. Proper wastewater management and treatment systems are essential to mitigate these risks and protect aquatic ecosystems.
The energy intensity of propyne-based processes is a significant factor in their environmental footprint. The production of propyne typically requires high temperatures and pressures, consuming substantial amounts of energy. This energy demand often translates to increased greenhouse gas emissions, particularly if fossil fuels are the primary energy source. Improving energy efficiency in propyne synthesis and utilization can help reduce the overall carbon footprint of these processes.
Waste generation is an inherent challenge in chemical synthesis, including propyne-based reactions. The production of fine chemicals often involves multiple steps and purification processes, leading to the generation of by-products and waste materials. Proper disposal or recycling of these wastes is crucial to prevent soil contamination and minimize the environmental burden of propyne chemistry.
On a positive note, propyne chemistry can contribute to the development of more environmentally friendly processes in some cases. For instance, propyne-based reactions can sometimes offer higher atom economy and yield compared to alternative synthetic routes, potentially reducing overall waste generation. Additionally, propyne can serve as a building block for the synthesis of biodegradable polymers and other environmentally benign materials.
The environmental impact of propyne chemistry extends to resource consumption as well. Propyne is typically derived from fossil fuel sources, raising concerns about the sustainability of its long-term use. Exploring alternative, renewable sources for propyne or developing bio-based analogues could help address these sustainability challenges and reduce the reliance on non-renewable resources.
One of the primary environmental concerns associated with propyne chemistry is the potential for air pollution. The synthesis and handling of propyne can lead to the release of volatile organic compounds (VOCs) into the atmosphere. These emissions may contribute to the formation of ground-level ozone and smog, which can have detrimental effects on air quality and human health. Additionally, propyne itself is a flammable gas, posing risks of fire and explosion if not properly managed.
Water pollution is another critical environmental aspect to consider. The production and use of propyne in chemical synthesis often involve aqueous processes and generate wastewater. This effluent may contain organic compounds, catalysts, and other chemicals that, if not adequately treated, can contaminate water sources. Proper wastewater management and treatment systems are essential to mitigate these risks and protect aquatic ecosystems.
The energy intensity of propyne-based processes is a significant factor in their environmental footprint. The production of propyne typically requires high temperatures and pressures, consuming substantial amounts of energy. This energy demand often translates to increased greenhouse gas emissions, particularly if fossil fuels are the primary energy source. Improving energy efficiency in propyne synthesis and utilization can help reduce the overall carbon footprint of these processes.
Waste generation is an inherent challenge in chemical synthesis, including propyne-based reactions. The production of fine chemicals often involves multiple steps and purification processes, leading to the generation of by-products and waste materials. Proper disposal or recycling of these wastes is crucial to prevent soil contamination and minimize the environmental burden of propyne chemistry.
On a positive note, propyne chemistry can contribute to the development of more environmentally friendly processes in some cases. For instance, propyne-based reactions can sometimes offer higher atom economy and yield compared to alternative synthetic routes, potentially reducing overall waste generation. Additionally, propyne can serve as a building block for the synthesis of biodegradable polymers and other environmentally benign materials.
The environmental impact of propyne chemistry extends to resource consumption as well. Propyne is typically derived from fossil fuel sources, raising concerns about the sustainability of its long-term use. Exploring alternative, renewable sources for propyne or developing bio-based analogues could help address these sustainability challenges and reduce the reliance on non-renewable resources.
Scalability of Propyne-Based Processes
The scalability of propyne-based processes is a critical factor in determining the viability of using propyne as a precursor in the organic synthesis of fine chemicals. As industrial applications often require large-scale production, it is essential to evaluate the potential for scaling up propyne-based reactions and processes.
One of the primary considerations in scaling up propyne-based processes is the availability and cost of propyne itself. Propyne is typically produced as a byproduct in ethylene production, which limits its availability and can lead to supply chain challenges for large-scale operations. However, recent advancements in on-purpose propyne production methods have shown promise in increasing the availability of this valuable precursor.
The reactivity of propyne presents both opportunities and challenges in scaling up processes. Its high reactivity allows for efficient transformations, potentially reducing reaction times and improving yields. However, this same reactivity can lead to safety concerns when handling large quantities of propyne, necessitating robust safety protocols and specialized equipment for industrial-scale operations.
Process intensification techniques have shown potential in improving the scalability of propyne-based reactions. Continuous flow chemistry, for instance, allows for better control of reaction parameters and can enhance safety by reducing the volume of reactive materials present at any given time. This approach has been successfully applied to several propyne-based syntheses, demonstrating improved scalability compared to traditional batch processes.
The selectivity of propyne-based reactions is another crucial factor in their scalability. Many fine chemicals require high levels of purity, and side reactions or byproduct formation can significantly impact the economic viability of large-scale processes. Advances in catalyst design and reaction engineering have led to improved selectivity in propyne-based transformations, making them more amenable to industrial-scale production.
Environmental considerations also play a role in the scalability of propyne-based processes. As regulations become more stringent, the ability to minimize waste and reduce the environmental impact of chemical processes becomes increasingly important. Green chemistry principles, such as atom economy and the use of renewable feedstocks, are being applied to propyne chemistry to enhance its sustainability and scalability.
In conclusion, while challenges exist, ongoing research and technological advancements are continually improving the scalability of propyne-based processes. As these developments continue, the potential for using propyne as a versatile precursor in the large-scale synthesis of fine chemicals is likely to expand, opening up new opportunities in industrial organic synthesis.
One of the primary considerations in scaling up propyne-based processes is the availability and cost of propyne itself. Propyne is typically produced as a byproduct in ethylene production, which limits its availability and can lead to supply chain challenges for large-scale operations. However, recent advancements in on-purpose propyne production methods have shown promise in increasing the availability of this valuable precursor.
The reactivity of propyne presents both opportunities and challenges in scaling up processes. Its high reactivity allows for efficient transformations, potentially reducing reaction times and improving yields. However, this same reactivity can lead to safety concerns when handling large quantities of propyne, necessitating robust safety protocols and specialized equipment for industrial-scale operations.
Process intensification techniques have shown potential in improving the scalability of propyne-based reactions. Continuous flow chemistry, for instance, allows for better control of reaction parameters and can enhance safety by reducing the volume of reactive materials present at any given time. This approach has been successfully applied to several propyne-based syntheses, demonstrating improved scalability compared to traditional batch processes.
The selectivity of propyne-based reactions is another crucial factor in their scalability. Many fine chemicals require high levels of purity, and side reactions or byproduct formation can significantly impact the economic viability of large-scale processes. Advances in catalyst design and reaction engineering have led to improved selectivity in propyne-based transformations, making them more amenable to industrial-scale production.
Environmental considerations also play a role in the scalability of propyne-based processes. As regulations become more stringent, the ability to minimize waste and reduce the environmental impact of chemical processes becomes increasingly important. Green chemistry principles, such as atom economy and the use of renewable feedstocks, are being applied to propyne chemistry to enhance its sustainability and scalability.
In conclusion, while challenges exist, ongoing research and technological advancements are continually improving the scalability of propyne-based processes. As these developments continue, the potential for using propyne as a versatile precursor in the large-scale synthesis of fine chemicals is likely to expand, opening up new opportunities in industrial organic synthesis.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!