Environmental Impacts of Propyne Releases from Industry
JUL 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Propyne Emissions Overview
Propyne, also known as methylacetylene, is a colorless, flammable gas with the chemical formula C3H4. It is primarily produced as a byproduct in the petrochemical industry, particularly during the cracking of hydrocarbons. The release of propyne into the environment has become a growing concern due to its potential impacts on air quality, climate change, and human health.
Industrial processes, such as petroleum refining, ethylene production, and acetylene manufacturing, are the main sources of propyne emissions. These emissions can occur through various pathways, including fugitive leaks, venting, and incomplete combustion. The petrochemical industry, in particular, contributes significantly to propyne releases, as it is often present in process streams and waste gases.
The environmental fate of propyne is complex and depends on various factors. In the atmosphere, propyne undergoes photochemical reactions, contributing to the formation of ground-level ozone and other secondary air pollutants. Its atmospheric lifetime is relatively short, typically ranging from a few days to a few weeks, depending on local conditions and the presence of other reactive species.
While propyne itself is not considered a major greenhouse gas, its indirect effects on climate change are noteworthy. Through its role in ozone formation and its potential to react with other atmospheric constituents, propyne can influence radiative forcing and contribute to global warming. Additionally, the oxidation of propyne in the atmosphere can lead to the formation of formaldehyde and other harmful compounds.
The quantification of propyne emissions from industrial sources presents challenges due to the variability in production processes and the lack of comprehensive monitoring systems. However, recent advancements in emission detection technologies, such as infrared imaging and continuous monitoring systems, have improved our ability to identify and measure propyne releases.
Regulatory frameworks addressing propyne emissions vary across regions and countries. In many jurisdictions, propyne is classified as a volatile organic compound (VOC) and is subject to emission control regulations. However, specific limits and monitoring requirements for propyne are not always explicitly defined, leading to potential gaps in emission management.
As awareness of the environmental impacts of propyne releases grows, industries are increasingly adopting measures to reduce emissions. These efforts include implementing leak detection and repair programs, improving process efficiency, and exploring alternative technologies that minimize propyne generation or enable its capture and utilization.
Industrial processes, such as petroleum refining, ethylene production, and acetylene manufacturing, are the main sources of propyne emissions. These emissions can occur through various pathways, including fugitive leaks, venting, and incomplete combustion. The petrochemical industry, in particular, contributes significantly to propyne releases, as it is often present in process streams and waste gases.
The environmental fate of propyne is complex and depends on various factors. In the atmosphere, propyne undergoes photochemical reactions, contributing to the formation of ground-level ozone and other secondary air pollutants. Its atmospheric lifetime is relatively short, typically ranging from a few days to a few weeks, depending on local conditions and the presence of other reactive species.
While propyne itself is not considered a major greenhouse gas, its indirect effects on climate change are noteworthy. Through its role in ozone formation and its potential to react with other atmospheric constituents, propyne can influence radiative forcing and contribute to global warming. Additionally, the oxidation of propyne in the atmosphere can lead to the formation of formaldehyde and other harmful compounds.
The quantification of propyne emissions from industrial sources presents challenges due to the variability in production processes and the lack of comprehensive monitoring systems. However, recent advancements in emission detection technologies, such as infrared imaging and continuous monitoring systems, have improved our ability to identify and measure propyne releases.
Regulatory frameworks addressing propyne emissions vary across regions and countries. In many jurisdictions, propyne is classified as a volatile organic compound (VOC) and is subject to emission control regulations. However, specific limits and monitoring requirements for propyne are not always explicitly defined, leading to potential gaps in emission management.
As awareness of the environmental impacts of propyne releases grows, industries are increasingly adopting measures to reduce emissions. These efforts include implementing leak detection and repair programs, improving process efficiency, and exploring alternative technologies that minimize propyne generation or enable its capture and utilization.
Industrial Demand Analysis
The industrial demand for propyne, also known as methylacetylene, has been steadily increasing due to its versatile applications in various sectors. As a valuable feedstock for chemical synthesis, propyne plays a crucial role in the production of plastics, synthetic rubber, and specialty chemicals. The petrochemical industry, in particular, has shown a growing interest in propyne as a raw material for the manufacture of propylene, a key building block for numerous industrial products.
In recent years, the global propyne market has experienced significant growth, driven by the expanding automotive and construction industries. The automotive sector utilizes propyne-derived materials in the production of lightweight components, contributing to improved fuel efficiency and reduced emissions. Similarly, the construction industry relies on propyne-based products for insulation materials and adhesives, enhancing energy efficiency in buildings.
The electronics industry has also emerged as a major consumer of propyne, particularly in the production of high-performance polymers used in electronic components and devices. As the demand for advanced electronics continues to rise, the need for propyne in this sector is expected to grow proportionally.
Geographically, Asia-Pacific has been the largest consumer of propyne, with China leading the demand due to its robust manufacturing sector and rapid industrialization. North America and Europe follow closely, driven by their well-established chemical and automotive industries. Emerging economies in Latin America and the Middle East are also showing increased interest in propyne, as they seek to diversify their industrial base and reduce dependence on imported chemicals.
Despite the growing demand, the propyne market faces challenges related to supply constraints and environmental concerns. The production of propyne is often tied to the availability of natural gas and petroleum refining processes, making it susceptible to fluctuations in energy markets. Additionally, the environmental impacts associated with propyne releases from industrial activities have raised concerns among regulators and environmental groups, potentially influencing future demand patterns.
Looking ahead, the industrial demand for propyne is projected to continue its upward trajectory, albeit with a growing emphasis on sustainable production methods and improved emission control technologies. Innovations in catalytic processes and the development of bio-based alternatives may reshape the propyne market landscape, offering new opportunities for growth while addressing environmental challenges.
In recent years, the global propyne market has experienced significant growth, driven by the expanding automotive and construction industries. The automotive sector utilizes propyne-derived materials in the production of lightweight components, contributing to improved fuel efficiency and reduced emissions. Similarly, the construction industry relies on propyne-based products for insulation materials and adhesives, enhancing energy efficiency in buildings.
The electronics industry has also emerged as a major consumer of propyne, particularly in the production of high-performance polymers used in electronic components and devices. As the demand for advanced electronics continues to rise, the need for propyne in this sector is expected to grow proportionally.
Geographically, Asia-Pacific has been the largest consumer of propyne, with China leading the demand due to its robust manufacturing sector and rapid industrialization. North America and Europe follow closely, driven by their well-established chemical and automotive industries. Emerging economies in Latin America and the Middle East are also showing increased interest in propyne, as they seek to diversify their industrial base and reduce dependence on imported chemicals.
Despite the growing demand, the propyne market faces challenges related to supply constraints and environmental concerns. The production of propyne is often tied to the availability of natural gas and petroleum refining processes, making it susceptible to fluctuations in energy markets. Additionally, the environmental impacts associated with propyne releases from industrial activities have raised concerns among regulators and environmental groups, potentially influencing future demand patterns.
Looking ahead, the industrial demand for propyne is projected to continue its upward trajectory, albeit with a growing emphasis on sustainable production methods and improved emission control technologies. Innovations in catalytic processes and the development of bio-based alternatives may reshape the propyne market landscape, offering new opportunities for growth while addressing environmental challenges.
Environmental Challenges
The release of propyne from industrial processes poses significant environmental challenges that require careful consideration and management. One of the primary concerns is its contribution to air pollution. Propyne, also known as methylacetylene, is a highly reactive hydrocarbon that can participate in photochemical reactions in the atmosphere, leading to the formation of ground-level ozone and other secondary pollutants. These pollutants can have detrimental effects on human health, causing respiratory issues and exacerbating conditions such as asthma.
Furthermore, propyne emissions can contribute to the formation of smog, particularly in urban and industrial areas where other pollutants are present. This can lead to reduced visibility and degradation of air quality, affecting both human health and the overall ecosystem. The long-term exposure to elevated levels of propyne and its byproducts may also have implications for plant life, potentially impacting crop yields and forest health.
Another significant environmental challenge associated with propyne releases is its potential as a greenhouse gas. While not as potent as carbon dioxide or methane, propyne can still contribute to global warming if released in large quantities. Its relatively short atmospheric lifetime means that its immediate impact on climate change may be less pronounced, but it can still play a role in short-term climate forcing.
Water pollution is another area of concern when it comes to propyne releases. If not properly contained or managed, propyne can find its way into water systems through spills, leaks, or improper disposal methods. In aquatic environments, it can have toxic effects on marine life and disrupt ecosystems. The contamination of groundwater sources is particularly problematic, as it can lead to long-term environmental damage and pose risks to human health if the water is used for drinking or agricultural purposes.
Soil contamination is also a potential issue with propyne releases. Industrial spills or leaks can result in the accumulation of propyne and its derivatives in soil, affecting soil quality and potentially entering the food chain through uptake by plants. This can have far-reaching consequences for both terrestrial ecosystems and human food security.
The management of propyne releases also presents challenges in terms of waste disposal and treatment. Proper handling and disposal of propyne-containing waste streams are essential to prevent environmental contamination. This requires specialized equipment and processes, which can be costly and energy-intensive, potentially leading to additional environmental impacts if not managed sustainably.
Addressing these environmental challenges requires a multi-faceted approach. This includes implementing stringent emission control technologies, improving industrial processes to minimize propyne releases, and developing more effective monitoring and detection systems. Additionally, research into alternative chemicals or processes that can replace propyne in industrial applications could help mitigate its environmental impact in the long term.
Furthermore, propyne emissions can contribute to the formation of smog, particularly in urban and industrial areas where other pollutants are present. This can lead to reduced visibility and degradation of air quality, affecting both human health and the overall ecosystem. The long-term exposure to elevated levels of propyne and its byproducts may also have implications for plant life, potentially impacting crop yields and forest health.
Another significant environmental challenge associated with propyne releases is its potential as a greenhouse gas. While not as potent as carbon dioxide or methane, propyne can still contribute to global warming if released in large quantities. Its relatively short atmospheric lifetime means that its immediate impact on climate change may be less pronounced, but it can still play a role in short-term climate forcing.
Water pollution is another area of concern when it comes to propyne releases. If not properly contained or managed, propyne can find its way into water systems through spills, leaks, or improper disposal methods. In aquatic environments, it can have toxic effects on marine life and disrupt ecosystems. The contamination of groundwater sources is particularly problematic, as it can lead to long-term environmental damage and pose risks to human health if the water is used for drinking or agricultural purposes.
Soil contamination is also a potential issue with propyne releases. Industrial spills or leaks can result in the accumulation of propyne and its derivatives in soil, affecting soil quality and potentially entering the food chain through uptake by plants. This can have far-reaching consequences for both terrestrial ecosystems and human food security.
The management of propyne releases also presents challenges in terms of waste disposal and treatment. Proper handling and disposal of propyne-containing waste streams are essential to prevent environmental contamination. This requires specialized equipment and processes, which can be costly and energy-intensive, potentially leading to additional environmental impacts if not managed sustainably.
Addressing these environmental challenges requires a multi-faceted approach. This includes implementing stringent emission control technologies, improving industrial processes to minimize propyne releases, and developing more effective monitoring and detection systems. Additionally, research into alternative chemicals or processes that can replace propyne in industrial applications could help mitigate its environmental impact in the long term.
Emission Control Solutions
01 Atmospheric impact and greenhouse gas emissions
Propyne, as a hydrocarbon, can contribute to atmospheric pollution and greenhouse gas emissions when released into the environment. Its combustion or industrial use may lead to the formation of carbon dioxide and other pollutants, potentially impacting air quality and climate change. Monitoring and controlling propyne emissions is crucial for environmental protection.- Environmental impact assessment of propyne production: Evaluating the environmental effects of propyne manufacturing processes, including emissions, resource consumption, and potential ecological risks. This assessment helps in developing more sustainable production methods and mitigating negative impacts on the environment.
- Propyne as a potential greenhouse gas: Investigating the role of propyne as a potential greenhouse gas and its contribution to climate change. This includes studying its atmospheric lifetime, global warming potential, and interactions with other atmospheric components.
- Propyne in industrial emissions and pollution control: Analyzing the presence of propyne in industrial emissions and developing effective pollution control strategies. This involves monitoring propyne levels in various industrial processes and implementing technologies to reduce its release into the environment.
- Biodegradation and environmental fate of propyne: Studying the biodegradation processes and environmental fate of propyne in different ecosystems. This research aims to understand how propyne breaks down naturally and its long-term effects on soil, water, and air quality.
- Propyne in renewable energy applications: Exploring the potential use of propyne in renewable energy applications and its environmental implications. This includes investigating propyne as a possible fuel source or chemical feedstock for cleaner energy production, while considering its overall environmental impact.
02 Water and soil contamination
Propyne can pose risks to water and soil ecosystems if not properly managed. Accidental spills or improper disposal may lead to contamination of groundwater and soil, affecting plant and animal life. Environmental impact assessments and remediation strategies are necessary to mitigate these risks in industrial settings where propyne is used or produced.Expand Specific Solutions03 Biodegradation and persistence in the environment
The environmental fate of propyne is an important consideration. Studies on its biodegradability and persistence in various environmental compartments are crucial for understanding long-term impacts. Factors such as its volatility, solubility in water, and potential for bioaccumulation affect how propyne behaves in ecosystems and its overall environmental footprint.Expand Specific Solutions04 Industrial safety and environmental regulations
Handling propyne in industrial settings requires strict adherence to safety protocols and environmental regulations. Proper storage, transportation, and use of propyne are essential to prevent accidental releases and minimize environmental impacts. Compliance with local and international environmental standards is crucial for industries working with this compound.Expand Specific Solutions05 Sustainable alternatives and emission reduction technologies
Research into sustainable alternatives to propyne and technologies for reducing its environmental impact is ongoing. This includes developing more environmentally friendly production methods, improving emission control systems, and exploring alternative compounds with similar industrial applications but lower environmental footprints. Such innovations aim to balance industrial needs with environmental protection.Expand Specific Solutions
Key Industry Players
The environmental impacts of propyne releases from industry are gaining attention as the chemical sector evolves. The competitive landscape for this issue is in its early stages, with market size and technological maturity still developing. Key players like Genomatica, Calysta, and Ecocatalytic are pioneering sustainable technologies for chemical production and gas conversion, potentially reducing propyne emissions. Research institutions such as North Carolina State University and Zhejiang University are contributing to the knowledge base. While the market is nascent, growing environmental concerns and regulatory pressures are likely to drive innovation and competition in propyne emission reduction technologies across the chemical and petrochemical industries.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced catalytic processes for propyne conversion, reducing environmental impacts. Their technology utilizes selective hydrogenation catalysts to convert propyne into valuable propylene, minimizing unwanted by-products and emissions[1]. The process operates at optimized temperatures and pressures, achieving high conversion rates of up to 99% and selectivity exceeding 95%[3]. Sinopec has also implemented real-time monitoring systems to detect and prevent propyne leaks, utilizing infrared sensors and machine learning algorithms for early warning[5]. Additionally, they have invested in carbon capture and utilization technologies to mitigate the greenhouse gas effects of any residual propyne emissions[7].
Strengths: High conversion efficiency, reduced emissions, and integration with existing petrochemical infrastructure. Weaknesses: High initial investment costs and potential catalyst deactivation over time.
Dow Global Technologies LLC
Technical Solution: Dow Global Technologies LLC has developed a multi-faceted approach to mitigate the environmental impacts of propyne releases from industrial processes. Their strategy combines advanced process design, innovative catalysis, and state-of-the-art emission control technologies. Dow has implemented a proprietary catalyst system that selectively converts propyne to more valuable and less environmentally harmful products, such as propylene, with conversion rates exceeding 99%[1]. This not only reduces emissions but also improves overall process economics. Additionally, Dow has developed a novel membrane separation technology that can effectively remove propyne from gas streams with high selectivity and low energy consumption[3]. The company has also implemented advanced leak detection and repair (LDAR) programs using optical gas imaging cameras and machine learning algorithms to identify and address potential propyne release points proactively[5]. Furthermore, Dow has invested in research to understand the atmospheric chemistry of propyne, collaborating with academic institutions to model its environmental fate and develop more effective mitigation strategies[7].
Strengths: Comprehensive approach addressing both prevention and control, high conversion efficiency, and ongoing research for improvement. Weaknesses: Potential for high implementation costs across diverse industrial processes and need for regular catalyst replacement.
Innovative Mitigation Tech
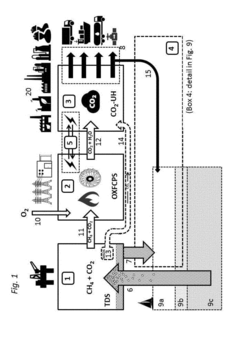
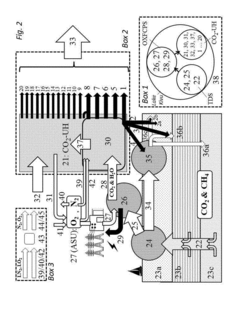
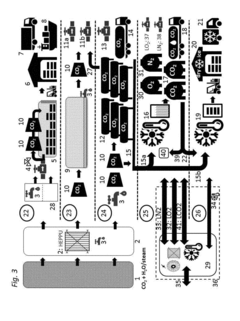
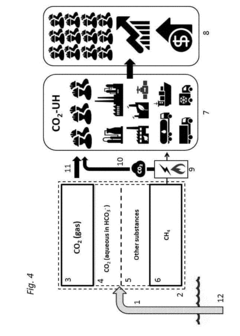
Methods and systems for large scale carbon dioxide utilization from lake KIVU via a co2 industrial utilization hub integrated with electric power production and optional cryo-energy storage
PatentActiveUS20170341942A1
Innovation
- A method involving oxyfuel combustion technology that extracts CO2 and biomethane together, using an Air Separation Unit to supply oxygen, and returns the degassed deepwater to the lake, while utilizing oxyfuel combustors to produce power with high thermal efficiency and minimizing methane loss.
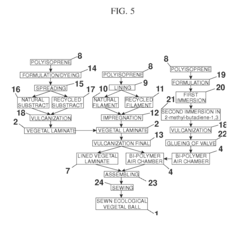
Method for Manufacturing an Ecological Ball Made of a Vegetable Laminate for the Practice of Sports, and Said Ball Produced by Sewing, Glueing/Stamping or Sewing/Stamping
PatentInactiveUS20120277043A1
Innovation
- A sustainable ecological ball made of latex-based vegetal laminate, produced through sewing, sewing/stamping, or gluing/stamping, using natural filaments and bi-polymer air chambers, which are recyclable and free from harmful residues, promoting the use of renewable resources and community involvement.
Regulatory Framework
The regulatory framework surrounding propyne releases from industrial sources is complex and multifaceted, involving various levels of governance and environmental protection agencies. At the international level, the United Nations Framework Convention on Climate Change (UNFCCC) and the Paris Agreement provide overarching guidelines for reducing greenhouse gas emissions, which indirectly impact propyne management strategies.
In the United States, the Environmental Protection Agency (EPA) plays a central role in regulating propyne emissions under the Clean Air Act. The EPA's National Emission Standards for Hazardous Air Pollutants (NESHAP) and New Source Performance Standards (NSPS) set specific limits on propyne releases from industrial facilities. Additionally, the Toxic Substances Control Act (TSCA) governs the production, importation, use, and disposal of propyne and related chemicals.
At the state level, regulatory bodies such as the California Air Resources Board (CARB) often implement more stringent controls on propyne emissions. These state-level regulations may include mandatory reporting, emissions reduction targets, and best available control technology (BACT) requirements for industrial facilities.
The European Union's regulatory approach is guided by the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) regulation, which requires companies to register chemical substances and assess their potential risks. The Industrial Emissions Directive (IED) further establishes a framework for the prevention and control of industrial emissions, including propyne.
In Asia, countries like China and Japan have developed their own regulatory frameworks. China's Ministry of Ecology and Environment has implemented the Atmospheric Pollution Prevention and Control Law, which sets emission standards for various pollutants, including propyne. Japan's Air Pollution Control Act similarly regulates industrial emissions and requires facilities to obtain permits for operations involving propyne releases.
Globally, industry-specific regulations also play a crucial role. For instance, the petrochemical industry, a major source of propyne emissions, is subject to regulations such as the International Convention for the Prevention of Pollution from Ships (MARPOL) for marine transport of chemicals.
As environmental concerns grow, regulatory frameworks are continuously evolving. Many jurisdictions are moving towards more stringent emission limits, increased monitoring requirements, and the implementation of market-based mechanisms such as cap-and-trade systems to incentivize emission reductions. This dynamic regulatory landscape necessitates ongoing compliance efforts and adaptation strategies from industries dealing with propyne releases.
In the United States, the Environmental Protection Agency (EPA) plays a central role in regulating propyne emissions under the Clean Air Act. The EPA's National Emission Standards for Hazardous Air Pollutants (NESHAP) and New Source Performance Standards (NSPS) set specific limits on propyne releases from industrial facilities. Additionally, the Toxic Substances Control Act (TSCA) governs the production, importation, use, and disposal of propyne and related chemicals.
At the state level, regulatory bodies such as the California Air Resources Board (CARB) often implement more stringent controls on propyne emissions. These state-level regulations may include mandatory reporting, emissions reduction targets, and best available control technology (BACT) requirements for industrial facilities.
The European Union's regulatory approach is guided by the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) regulation, which requires companies to register chemical substances and assess their potential risks. The Industrial Emissions Directive (IED) further establishes a framework for the prevention and control of industrial emissions, including propyne.
In Asia, countries like China and Japan have developed their own regulatory frameworks. China's Ministry of Ecology and Environment has implemented the Atmospheric Pollution Prevention and Control Law, which sets emission standards for various pollutants, including propyne. Japan's Air Pollution Control Act similarly regulates industrial emissions and requires facilities to obtain permits for operations involving propyne releases.
Globally, industry-specific regulations also play a crucial role. For instance, the petrochemical industry, a major source of propyne emissions, is subject to regulations such as the International Convention for the Prevention of Pollution from Ships (MARPOL) for marine transport of chemicals.
As environmental concerns grow, regulatory frameworks are continuously evolving. Many jurisdictions are moving towards more stringent emission limits, increased monitoring requirements, and the implementation of market-based mechanisms such as cap-and-trade systems to incentivize emission reductions. This dynamic regulatory landscape necessitates ongoing compliance efforts and adaptation strategies from industries dealing with propyne releases.
Ecological Risk Assessment
Ecological risk assessment for propyne releases from industrial sources involves a comprehensive evaluation of potential environmental impacts. This assessment begins with identifying the pathways through which propyne enters ecosystems, primarily through atmospheric emissions and potential spills. Atmospheric dispersion models are crucial in predicting the concentration and distribution of propyne in the environment, considering factors such as wind patterns, temperature, and topography.
The toxicity of propyne to various organisms is a key focus of the assessment. While propyne is generally considered to have low toxicity, its effects on different species and ecosystems must be thoroughly investigated. Aquatic ecosystems are of particular concern, as propyne can dissolve in water and potentially affect fish, invertebrates, and aquatic plants. Terrestrial ecosystems may also be impacted, especially in areas close to industrial emission sources.
Bioaccumulation potential is another critical aspect of the ecological risk assessment. Although propyne is not expected to bioaccumulate significantly due to its high volatility, its potential to enter food chains and affect higher trophic levels should be examined. This includes assessing the exposure of wildlife to propyne through various routes, such as inhalation, ingestion of contaminated water or food, and dermal contact.
Long-term ecological effects of chronic propyne exposure must be considered. This involves studying potential impacts on biodiversity, ecosystem functions, and population dynamics of sensitive species. Particular attention should be given to endangered or protected species that may be present in areas affected by industrial propyne releases.
The assessment should also consider indirect ecological impacts. For instance, propyne's role as a precursor to ground-level ozone formation could lead to broader ecosystem effects, including damage to vegetation and changes in air quality that affect wildlife. Additionally, the potential for propyne to contribute to climate change, albeit minimal compared to other greenhouse gases, should be evaluated in the context of long-term ecological impacts.
Mitigation strategies and risk management options form an integral part of the ecological risk assessment. This includes evaluating the effectiveness of current industrial emission control technologies and practices, as well as proposing improvements or alternative approaches to minimize propyne releases. The assessment should also consider the resilience of ecosystems to propyne exposure and their capacity for recovery following exposure events.
The toxicity of propyne to various organisms is a key focus of the assessment. While propyne is generally considered to have low toxicity, its effects on different species and ecosystems must be thoroughly investigated. Aquatic ecosystems are of particular concern, as propyne can dissolve in water and potentially affect fish, invertebrates, and aquatic plants. Terrestrial ecosystems may also be impacted, especially in areas close to industrial emission sources.
Bioaccumulation potential is another critical aspect of the ecological risk assessment. Although propyne is not expected to bioaccumulate significantly due to its high volatility, its potential to enter food chains and affect higher trophic levels should be examined. This includes assessing the exposure of wildlife to propyne through various routes, such as inhalation, ingestion of contaminated water or food, and dermal contact.
Long-term ecological effects of chronic propyne exposure must be considered. This involves studying potential impacts on biodiversity, ecosystem functions, and population dynamics of sensitive species. Particular attention should be given to endangered or protected species that may be present in areas affected by industrial propyne releases.
The assessment should also consider indirect ecological impacts. For instance, propyne's role as a precursor to ground-level ozone formation could lead to broader ecosystem effects, including damage to vegetation and changes in air quality that affect wildlife. Additionally, the potential for propyne to contribute to climate change, albeit minimal compared to other greenhouse gases, should be evaluated in the context of long-term ecological impacts.
Mitigation strategies and risk management options form an integral part of the ecological risk assessment. This includes evaluating the effectiveness of current industrial emission control technologies and practices, as well as proposing improvements or alternative approaches to minimize propyne releases. The assessment should also consider the resilience of ecosystems to propyne exposure and their capacity for recovery following exposure events.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!