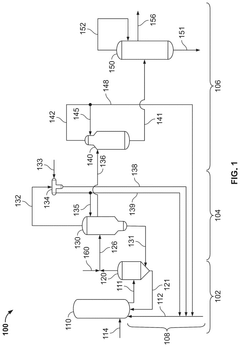
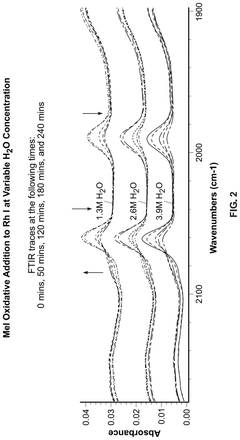
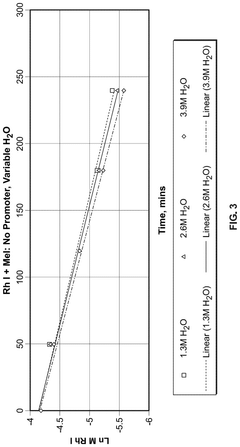
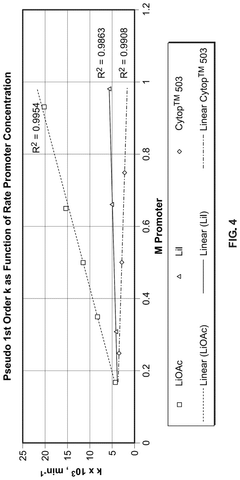
Reaction Pathways of Glacial Acetic Acid in Glass Manufacturing
AUG 5, 20258 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Acetic Acid in Glass: Background and Objectives
Glacial acetic acid plays a crucial role in the glass manufacturing process, serving as a key component in various stages of production. The use of acetic acid in glass manufacturing dates back to the early 20th century when it was first introduced as a refining agent. Since then, its applications have expanded significantly, encompassing areas such as glass etching, cleaning, and surface treatment.
The primary objective of studying the reaction pathways of glacial acetic acid in glass manufacturing is to optimize its utilization and enhance the overall efficiency of the production process. By understanding the intricate chemical interactions between acetic acid and glass components, manufacturers can improve product quality, reduce waste, and minimize environmental impact.
One of the main areas of focus is the role of acetic acid in the glass melting process. As a refining agent, it helps remove impurities and bubbles from the molten glass, resulting in a clearer and more homogeneous final product. The reaction pathways involved in this process are complex, involving multiple stages of decomposition and interaction with various glass constituents.
Another critical aspect is the use of acetic acid in glass surface treatment. The acid's ability to etch and modify glass surfaces has led to its widespread application in creating frosted or textured glass products. Understanding the reaction mechanisms at the molecular level can lead to more precise control over surface properties and the development of novel glass finishes.
The environmental implications of acetic acid usage in glass manufacturing are also a significant concern. As industries worldwide strive for sustainability, there is a growing need to investigate alternative, eco-friendly processes that can achieve similar results with reduced environmental impact. This includes exploring the potential for recycling or neutralizing acetic acid waste streams.
Recent technological advancements have opened up new avenues for research in this field. High-resolution spectroscopic techniques and computational modeling have enabled researchers to gain deeper insights into the reaction pathways of acetic acid at various stages of glass production. These tools allow for more accurate prediction of chemical behaviors and optimization of process parameters.
As we delve into the reaction pathways of glacial acetic acid in glass manufacturing, our goal is to bridge the gap between fundamental chemical understanding and practical industrial applications. By elucidating these complex processes, we aim to pave the way for innovations that will shape the future of glass production, leading to more efficient, sustainable, and versatile manufacturing techniques.
The primary objective of studying the reaction pathways of glacial acetic acid in glass manufacturing is to optimize its utilization and enhance the overall efficiency of the production process. By understanding the intricate chemical interactions between acetic acid and glass components, manufacturers can improve product quality, reduce waste, and minimize environmental impact.
One of the main areas of focus is the role of acetic acid in the glass melting process. As a refining agent, it helps remove impurities and bubbles from the molten glass, resulting in a clearer and more homogeneous final product. The reaction pathways involved in this process are complex, involving multiple stages of decomposition and interaction with various glass constituents.
Another critical aspect is the use of acetic acid in glass surface treatment. The acid's ability to etch and modify glass surfaces has led to its widespread application in creating frosted or textured glass products. Understanding the reaction mechanisms at the molecular level can lead to more precise control over surface properties and the development of novel glass finishes.
The environmental implications of acetic acid usage in glass manufacturing are also a significant concern. As industries worldwide strive for sustainability, there is a growing need to investigate alternative, eco-friendly processes that can achieve similar results with reduced environmental impact. This includes exploring the potential for recycling or neutralizing acetic acid waste streams.
Recent technological advancements have opened up new avenues for research in this field. High-resolution spectroscopic techniques and computational modeling have enabled researchers to gain deeper insights into the reaction pathways of acetic acid at various stages of glass production. These tools allow for more accurate prediction of chemical behaviors and optimization of process parameters.
As we delve into the reaction pathways of glacial acetic acid in glass manufacturing, our goal is to bridge the gap between fundamental chemical understanding and practical industrial applications. By elucidating these complex processes, we aim to pave the way for innovations that will shape the future of glass production, leading to more efficient, sustainable, and versatile manufacturing techniques.
Market Analysis for Acetic Acid in Glass Production
The global market for acetic acid in glass production has been experiencing steady growth, driven by the increasing demand for high-quality glass products across various industries. Acetic acid plays a crucial role in the glass manufacturing process, particularly in the production of specialty glasses and optical fibers. The market size for acetic acid in this sector is projected to reach significant levels in the coming years, with a compound annual growth rate (CAGR) outpacing the overall chemical industry average.
The demand for acetic acid in glass production is primarily fueled by the expanding electronics and telecommunications industries, which require high-performance glass components for devices and infrastructure. Additionally, the automotive sector's shift towards advanced glass technologies for improved safety and fuel efficiency has further boosted the market. The construction industry's growing preference for energy-efficient and smart glass solutions has also contributed to the increased consumption of acetic acid in glass manufacturing.
Geographically, Asia-Pacific dominates the market, with China and India leading in terms of production and consumption. This regional dominance is attributed to the rapid industrialization, urbanization, and technological advancements in these countries. North America and Europe follow, with a focus on high-value specialty glass products that require precise chemical processes involving acetic acid.
The market landscape is characterized by a mix of large multinational chemical companies and specialized glass manufacturers. Key players are investing heavily in research and development to optimize the use of acetic acid in glass production, aiming to enhance product quality while reducing environmental impact. This has led to the development of innovative processes that improve the efficiency of acetic acid utilization in glass manufacturing.
Despite the positive outlook, the market faces challenges such as volatile raw material prices and increasing environmental regulations. The fluctuating costs of methanol and carbon monoxide, key components in acetic acid production, can impact profit margins for manufacturers. Moreover, stringent environmental policies in various countries are pushing companies to adopt greener production methods, which may require significant investments in new technologies and processes.
Looking ahead, the market for acetic acid in glass production is expected to benefit from emerging trends such as the growing demand for smart glass in building automation and the increasing use of specialty glass in the healthcare sector. The development of bio-based acetic acid production methods could also reshape the market landscape, offering more sustainable options for glass manufacturers. As the industry continues to evolve, companies that can adapt to these changing dynamics and innovate in their production processes are likely to gain a competitive edge in this growing market.
The demand for acetic acid in glass production is primarily fueled by the expanding electronics and telecommunications industries, which require high-performance glass components for devices and infrastructure. Additionally, the automotive sector's shift towards advanced glass technologies for improved safety and fuel efficiency has further boosted the market. The construction industry's growing preference for energy-efficient and smart glass solutions has also contributed to the increased consumption of acetic acid in glass manufacturing.
Geographically, Asia-Pacific dominates the market, with China and India leading in terms of production and consumption. This regional dominance is attributed to the rapid industrialization, urbanization, and technological advancements in these countries. North America and Europe follow, with a focus on high-value specialty glass products that require precise chemical processes involving acetic acid.
The market landscape is characterized by a mix of large multinational chemical companies and specialized glass manufacturers. Key players are investing heavily in research and development to optimize the use of acetic acid in glass production, aiming to enhance product quality while reducing environmental impact. This has led to the development of innovative processes that improve the efficiency of acetic acid utilization in glass manufacturing.
Despite the positive outlook, the market faces challenges such as volatile raw material prices and increasing environmental regulations. The fluctuating costs of methanol and carbon monoxide, key components in acetic acid production, can impact profit margins for manufacturers. Moreover, stringent environmental policies in various countries are pushing companies to adopt greener production methods, which may require significant investments in new technologies and processes.
Looking ahead, the market for acetic acid in glass production is expected to benefit from emerging trends such as the growing demand for smart glass in building automation and the increasing use of specialty glass in the healthcare sector. The development of bio-based acetic acid production methods could also reshape the market landscape, offering more sustainable options for glass manufacturers. As the industry continues to evolve, companies that can adapt to these changing dynamics and innovate in their production processes are likely to gain a competitive edge in this growing market.
Current Challenges in Acetic Acid Glass Manufacturing
The glass manufacturing industry faces several significant challenges in the utilization of glacial acetic acid, particularly in the reaction pathways during the production process. One of the primary issues is the corrosive nature of acetic acid, which can lead to equipment degradation and increased maintenance costs. This corrosiveness is especially problematic in high-temperature environments typical of glass manufacturing, where the acid's reactivity is amplified.
Another challenge lies in controlling the reaction rates and pathways of acetic acid during the glass formation process. The acid's behavior can be unpredictable under varying temperature and pressure conditions, leading to inconsistencies in the final product quality. This variability makes it difficult to maintain precise control over the glass properties, such as clarity, strength, and chemical resistance.
The volatility of acetic acid at elevated temperatures poses additional challenges. As the acid vaporizes, it can create pressure buildup within the manufacturing equipment, potentially leading to safety hazards and process inefficiencies. Moreover, the loss of acetic acid through vaporization can alter the stoichiometry of the glass-forming reactions, affecting the final composition and properties of the glass.
Environmental concerns also present significant challenges in acetic acid glass manufacturing. The emission of acetic acid vapors and other volatile organic compounds (VOCs) during the production process can contribute to air pollution and pose health risks to workers. Implementing effective emission control systems while maintaining production efficiency remains a complex task for manufacturers.
The interaction between acetic acid and other glass-forming components, such as silica and metal oxides, introduces further complexities. These interactions can lead to the formation of unwanted byproducts or alter the intended glass structure, necessitating careful monitoring and adjustment of reaction conditions. The presence of impurities in the acetic acid or other raw materials can exacerbate these issues, potentially catalyzing undesired side reactions or introducing defects in the final glass product.
Lastly, the energy-intensive nature of glass manufacturing processes involving acetic acid presents both economic and environmental challenges. Optimizing energy consumption while ensuring complete and controlled reactions of acetic acid remains a delicate balance. The industry continues to seek more energy-efficient methods that can maintain or improve product quality while reducing the carbon footprint associated with acetic acid glass manufacturing.
Another challenge lies in controlling the reaction rates and pathways of acetic acid during the glass formation process. The acid's behavior can be unpredictable under varying temperature and pressure conditions, leading to inconsistencies in the final product quality. This variability makes it difficult to maintain precise control over the glass properties, such as clarity, strength, and chemical resistance.
The volatility of acetic acid at elevated temperatures poses additional challenges. As the acid vaporizes, it can create pressure buildup within the manufacturing equipment, potentially leading to safety hazards and process inefficiencies. Moreover, the loss of acetic acid through vaporization can alter the stoichiometry of the glass-forming reactions, affecting the final composition and properties of the glass.
Environmental concerns also present significant challenges in acetic acid glass manufacturing. The emission of acetic acid vapors and other volatile organic compounds (VOCs) during the production process can contribute to air pollution and pose health risks to workers. Implementing effective emission control systems while maintaining production efficiency remains a complex task for manufacturers.
The interaction between acetic acid and other glass-forming components, such as silica and metal oxides, introduces further complexities. These interactions can lead to the formation of unwanted byproducts or alter the intended glass structure, necessitating careful monitoring and adjustment of reaction conditions. The presence of impurities in the acetic acid or other raw materials can exacerbate these issues, potentially catalyzing undesired side reactions or introducing defects in the final glass product.
Lastly, the energy-intensive nature of glass manufacturing processes involving acetic acid presents both economic and environmental challenges. Optimizing energy consumption while ensuring complete and controlled reactions of acetic acid remains a delicate balance. The industry continues to seek more energy-efficient methods that can maintain or improve product quality while reducing the carbon footprint associated with acetic acid glass manufacturing.
Existing Reaction Pathways of Glacial Acetic Acid
01 Esterification reactions
Glacial acetic acid is commonly used in esterification reactions, where it reacts with alcohols to form esters. This process is widely used in the production of various chemicals, fragrances, and solvents. The reaction typically requires a catalyst and often involves heating to improve yield and reaction rate.- Esterification reactions: Glacial acetic acid is commonly used in esterification reactions, where it reacts with alcohols to form esters. This process is widely applied in the production of various chemicals, fragrances, and solvents. The reaction typically requires catalysts and specific conditions to achieve high yields and selectivity.
- Acetylation processes: Glacial acetic acid serves as an acetylating agent in various chemical transformations. It can introduce acetyl groups into organic compounds, modifying their properties and creating new functional materials. This reaction pathway is crucial in pharmaceutical synthesis, polymer production, and the preparation of specialty chemicals.
- Oxidation reactions: Glacial acetic acid can participate in oxidation reactions, either as a reactant or as a solvent. These reactions are important in the synthesis of various organic compounds, including aldehydes, ketones, and carboxylic acids. The oxidation pathways often involve metal catalysts or other oxidizing agents to facilitate the transformation.
- Acid-catalyzed reactions: As a strong organic acid, glacial acetic acid can catalyze various reactions, including hydrolysis, dehydration, and rearrangements. These acid-catalyzed pathways are essential in organic synthesis, enabling the formation of new carbon-carbon bonds and the modification of functional groups in complex molecules.
- Electrochemical processes: Glacial acetic acid can be used in electrochemical reactions, serving as an electrolyte or participating directly in electrode processes. These pathways are relevant in the production of chemicals, energy storage applications, and the development of novel synthetic methodologies. Electrochemical reactions often offer more environmentally friendly alternatives to traditional chemical processes.
02 Acetylation processes
Glacial acetic acid serves as an acetylating agent in various chemical processes. It can introduce acetyl groups into organic compounds, such as in the acetylation of cellulose to produce cellulose acetate. This reaction pathway is important in the production of plastics, fibers, and pharmaceutical intermediates.Expand Specific Solutions03 Oxidation reactions
Glacial acetic acid can participate in oxidation reactions, either as a reactant or as a solvent. It can be oxidized to produce peracetic acid, a powerful oxidizing agent used in various industrial applications. Additionally, it can serve as a medium for other oxidation processes in organic synthesis.Expand Specific Solutions04 Acid-catalyzed reactions
As a strong organic acid, glacial acetic acid can catalyze various reactions, including hydrolysis, dehydration, and rearrangements. It can act as both a solvent and a catalyst in these processes, making it versatile in organic synthesis and industrial applications.Expand Specific Solutions05 Electrochemical reactions
Glacial acetic acid can be used in electrochemical processes, either as an electrolyte or as a reactant. It can undergo electrolysis to produce various compounds, including ethane and carbon dioxide. These electrochemical pathways are explored for sustainable chemical production and energy storage applications.Expand Specific Solutions
Key Players in Acetic Acid Glass Production
The competitive landscape for "Reaction Pathways of Glacial Acetic Acid in Glass Manufacturing" is characterized by a mature industry with established players and ongoing research. The market size is substantial, given the widespread use of glass in various sectors. Technologically, the field is moderately mature but still evolving, with companies like Corning, Inc., SABIC Global Technologies BV, and BASF Corp. leading innovation. These firms, along with others such as LG Chem Ltd. and Nippon Electric Glass Co., Ltd., are investing in R&D to optimize processes and improve efficiency. The involvement of academic institutions like Beijing Institute of Technology indicates ongoing scientific exploration in this area.
Corning, Inc.
Technical Solution: Corning has developed advanced glass manufacturing processes that utilize glacial acetic acid as a key component in their reaction pathways. Their proprietary method involves precise control of acetic acid concentration and temperature to optimize glass formation and properties. The company employs a multi-stage reaction process where glacial acetic acid acts as both a solvent and a reactant, facilitating the formation of high-quality glass precursors[1]. Corning's technology allows for the production of ultra-thin, flexible glass substrates with enhanced durability and optical clarity, suitable for next-generation display technologies and advanced optoelectronics[3].
Strengths: Highly controlled process resulting in superior glass quality; enables production of advanced glass forms. Weaknesses: Potentially higher production costs due to the need for high-purity glacial acetic acid; environmental concerns related to acetic acid handling and disposal.
Nippon Electric Glass Co., Ltd.
Technical Solution: Nippon Electric Glass has innovated in the use of glacial acetic acid for the production of ultra-thin glass for electronic devices. Their proprietary process involves using acetic acid as a key component in the glass drawing process, allowing for the creation of glass sheets with thicknesses as low as 25 micrometers[9]. This technology enables the production of flexible glass substrates that maintain excellent optical and mechanical properties. The company's approach has been particularly successful in the development of glass for foldable displays and advanced touch sensors, where the unique properties of their ultra-thin glass provide both flexibility and durability[10].
Strengths: Enables production of ultra-thin, flexible glass; suitable for next-generation electronic devices. Weaknesses: Highly specialized process that may have limited applicability outside of specific electronic applications; requires precise control over manufacturing conditions.
Key Innovations in Acetic Acid Glass Reactions
Low bromine content glacial acetic acid
PatentInactiveUS4278503A
Innovation
- A process involving thermal conversion of 3-bromo-2-butanone to 1-butene-3-one and inorganic bromides, followed by cryogenic fractional crystallization, reduces 3-bromo-2-butanone contamination by concentrating the aqueous acid mixture and rejecting the impurity, using decompression and heat treatment steps before distillative removal of organic impurities.
Aminopolycarboxylates as rate promoters for the glacial acetic acid process
PatentPendingUS20250074857A1
Innovation
- The process involves using a reaction mixture comprising a carbonylation catalyst, water, and specific rate-promoting compounds, such as Group I and Group II aminopolycarboxylate salts, to enhance the rate of acetic acid formation while reducing the amount of water required and suppressing undesirable oxidative addition reactions.
Environmental Impact of Acetic Acid in Glass Making
The use of glacial acetic acid in glass manufacturing processes has significant environmental implications that warrant careful consideration. The release of acetic acid into the environment can lead to various ecological and health concerns. When emitted into the atmosphere, acetic acid contributes to the formation of photochemical smog and acid rain, which can harm vegetation, aquatic ecosystems, and infrastructure. In aquatic environments, acetic acid can lower the pH of water bodies, potentially disrupting the delicate balance of aquatic ecosystems and affecting the survival of various species.
Furthermore, the production and transportation of glacial acetic acid for glass manufacturing purposes contribute to carbon emissions and energy consumption. The manufacturing process of acetic acid itself involves the use of fossil fuels and generates greenhouse gases, adding to the overall carbon footprint of the glass industry. Additionally, improper handling or accidental spills during transportation can result in localized environmental contamination, affecting soil quality and groundwater resources.
In terms of human health, exposure to acetic acid vapors can cause respiratory irritation, eye damage, and skin burns. Workers in glass manufacturing facilities are at particular risk and require proper safety measures and protective equipment. The potential for long-term health effects due to chronic exposure is also a concern that necessitates ongoing research and monitoring.
To mitigate these environmental impacts, the glass industry has been exploring alternative technologies and process optimizations. Some manufacturers are investigating the use of bio-based acetic acid, which can reduce the reliance on fossil fuel-derived sources. Others are implementing closed-loop systems to minimize acetic acid emissions and improve recycling efficiency. Advanced air pollution control technologies, such as scrubbers and catalytic oxidizers, are being employed to reduce atmospheric emissions of acetic acid and other volatile organic compounds.
Regulatory bodies worldwide are increasingly focusing on the environmental impact of industrial processes, including those in glass manufacturing. Stricter emission standards and waste management regulations are being implemented, pushing the industry towards more sustainable practices. This regulatory pressure, combined with growing consumer demand for environmentally friendly products, is driving innovation in cleaner glass production technologies and encouraging the adoption of circular economy principles within the industry.
Furthermore, the production and transportation of glacial acetic acid for glass manufacturing purposes contribute to carbon emissions and energy consumption. The manufacturing process of acetic acid itself involves the use of fossil fuels and generates greenhouse gases, adding to the overall carbon footprint of the glass industry. Additionally, improper handling or accidental spills during transportation can result in localized environmental contamination, affecting soil quality and groundwater resources.
In terms of human health, exposure to acetic acid vapors can cause respiratory irritation, eye damage, and skin burns. Workers in glass manufacturing facilities are at particular risk and require proper safety measures and protective equipment. The potential for long-term health effects due to chronic exposure is also a concern that necessitates ongoing research and monitoring.
To mitigate these environmental impacts, the glass industry has been exploring alternative technologies and process optimizations. Some manufacturers are investigating the use of bio-based acetic acid, which can reduce the reliance on fossil fuel-derived sources. Others are implementing closed-loop systems to minimize acetic acid emissions and improve recycling efficiency. Advanced air pollution control technologies, such as scrubbers and catalytic oxidizers, are being employed to reduce atmospheric emissions of acetic acid and other volatile organic compounds.
Regulatory bodies worldwide are increasingly focusing on the environmental impact of industrial processes, including those in glass manufacturing. Stricter emission standards and waste management regulations are being implemented, pushing the industry towards more sustainable practices. This regulatory pressure, combined with growing consumer demand for environmentally friendly products, is driving innovation in cleaner glass production technologies and encouraging the adoption of circular economy principles within the industry.
Safety Protocols for Handling Glacial Acetic Acid
Handling glacial acetic acid in glass manufacturing requires strict adherence to comprehensive safety protocols due to its corrosive and flammable nature. Personal protective equipment (PPE) is essential, including chemical-resistant gloves, goggles, face shields, and appropriate clothing to prevent skin contact. Proper respiratory protection, such as a full-face respirator with organic vapor cartridges, is necessary when working with glacial acetic acid vapors.
Storage and transportation of glacial acetic acid demand careful consideration. It should be stored in a cool, dry, well-ventilated area away from sources of ignition and incompatible materials. Containers must be properly labeled and kept tightly closed when not in use. Secondary containment systems are recommended to prevent spills from spreading.
Workplace engineering controls play a crucial role in minimizing exposure risks. These include the use of closed systems, local exhaust ventilation, and fume hoods to capture and remove vapors. Regular maintenance and inspection of equipment and storage facilities are essential to prevent leaks and ensure proper functioning of safety systems.
Emergency response procedures must be established and regularly practiced. This includes having readily available eyewash stations, safety showers, and spill control materials. Employees should be trained in proper spill cleanup techniques and the use of neutralizing agents such as sodium bicarbonate or lime.
Proper waste disposal is critical to prevent environmental contamination. Glacial acetic acid waste should be collected in appropriate containers and disposed of through authorized chemical waste disposal services. Dilution with water before disposal is not recommended due to the heat generated during the process.
Regular safety training and education programs are vital to ensure all personnel are aware of the hazards associated with glacial acetic acid and are proficient in following safety protocols. This includes training on proper handling techniques, emergency procedures, and the use of safety equipment.
Monitoring and record-keeping are essential components of a comprehensive safety program. This includes regular air quality monitoring, medical surveillance for exposed workers, and maintaining detailed logs of all incidents and near-misses to identify areas for improvement in safety practices.
Storage and transportation of glacial acetic acid demand careful consideration. It should be stored in a cool, dry, well-ventilated area away from sources of ignition and incompatible materials. Containers must be properly labeled and kept tightly closed when not in use. Secondary containment systems are recommended to prevent spills from spreading.
Workplace engineering controls play a crucial role in minimizing exposure risks. These include the use of closed systems, local exhaust ventilation, and fume hoods to capture and remove vapors. Regular maintenance and inspection of equipment and storage facilities are essential to prevent leaks and ensure proper functioning of safety systems.
Emergency response procedures must be established and regularly practiced. This includes having readily available eyewash stations, safety showers, and spill control materials. Employees should be trained in proper spill cleanup techniques and the use of neutralizing agents such as sodium bicarbonate or lime.
Proper waste disposal is critical to prevent environmental contamination. Glacial acetic acid waste should be collected in appropriate containers and disposed of through authorized chemical waste disposal services. Dilution with water before disposal is not recommended due to the heat generated during the process.
Regular safety training and education programs are vital to ensure all personnel are aware of the hazards associated with glacial acetic acid and are proficient in following safety protocols. This includes training on proper handling techniques, emergency procedures, and the use of safety equipment.
Monitoring and record-keeping are essential components of a comprehensive safety program. This includes regular air quality monitoring, medical surveillance for exposed workers, and maintaining detailed logs of all incidents and near-misses to identify areas for improvement in safety practices.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!