Regulatory Compliance of Spintronic Devices in Pharmaceuticals
OCT 21, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Spintronics in Pharmaceuticals: Background and Objectives
Spintronics represents a revolutionary field at the intersection of quantum physics and electronics, utilizing the intrinsic spin of electrons alongside their charge to create novel electronic devices. The emergence of spintronic technology dates back to the late 1980s with the discovery of giant magnetoresistance (GMR), which earned Albert Fert and Peter Grünberg the Nobel Prize in Physics in 2007. Since then, the field has evolved significantly, moving from fundamental research to practical applications across various industries.
In the pharmaceutical context, spintronic devices offer unprecedented capabilities for drug development, manufacturing quality control, and supply chain integrity. The technology's evolution has been marked by increasing miniaturization, enhanced sensitivity, and improved energy efficiency, making it particularly suitable for integration into pharmaceutical processes that require precise measurement and monitoring capabilities.
The primary objective of implementing spintronic technology in pharmaceuticals is to enhance regulatory compliance through improved detection, measurement, and verification systems. Specifically, spintronic sensors can provide real-time monitoring of manufacturing conditions with exceptional accuracy, addressing critical regulatory requirements for process validation and quality assurance in pharmaceutical production.
Current technological trends indicate a convergence of spintronics with other emerging technologies such as artificial intelligence, blockchain, and Internet of Things (IoT), creating integrated systems capable of comprehensive compliance management. This convergence represents a significant shift from isolated quality control measures to holistic regulatory compliance ecosystems.
The pharmaceutical industry faces increasingly stringent regulatory frameworks globally, including FDA's Process Analytical Technology (PAT) initiative, EU GMP Annex 1, and various international standards for pharmaceutical manufacturing. Spintronic devices offer potential solutions to meet these evolving requirements through their unique capabilities in molecular detection, contamination identification, and process parameter monitoring.
Looking forward, the technical goals for spintronic applications in pharmaceutical compliance include developing room-temperature quantum sensors for molecular characterization, creating tamper-evident packaging with embedded spintronic elements, and establishing non-invasive quality control systems that can operate throughout the pharmaceutical supply chain without compromising product integrity.
The integration of spintronics into pharmaceutical regulatory compliance represents not merely an incremental improvement but a paradigm shift in how quality assurance and regulatory adherence are conceptualized and implemented. This technological trajectory promises to address longstanding challenges in pharmaceutical manufacturing while creating new opportunities for enhanced patient safety and product efficacy verification.
In the pharmaceutical context, spintronic devices offer unprecedented capabilities for drug development, manufacturing quality control, and supply chain integrity. The technology's evolution has been marked by increasing miniaturization, enhanced sensitivity, and improved energy efficiency, making it particularly suitable for integration into pharmaceutical processes that require precise measurement and monitoring capabilities.
The primary objective of implementing spintronic technology in pharmaceuticals is to enhance regulatory compliance through improved detection, measurement, and verification systems. Specifically, spintronic sensors can provide real-time monitoring of manufacturing conditions with exceptional accuracy, addressing critical regulatory requirements for process validation and quality assurance in pharmaceutical production.
Current technological trends indicate a convergence of spintronics with other emerging technologies such as artificial intelligence, blockchain, and Internet of Things (IoT), creating integrated systems capable of comprehensive compliance management. This convergence represents a significant shift from isolated quality control measures to holistic regulatory compliance ecosystems.
The pharmaceutical industry faces increasingly stringent regulatory frameworks globally, including FDA's Process Analytical Technology (PAT) initiative, EU GMP Annex 1, and various international standards for pharmaceutical manufacturing. Spintronic devices offer potential solutions to meet these evolving requirements through their unique capabilities in molecular detection, contamination identification, and process parameter monitoring.
Looking forward, the technical goals for spintronic applications in pharmaceutical compliance include developing room-temperature quantum sensors for molecular characterization, creating tamper-evident packaging with embedded spintronic elements, and establishing non-invasive quality control systems that can operate throughout the pharmaceutical supply chain without compromising product integrity.
The integration of spintronics into pharmaceutical regulatory compliance represents not merely an incremental improvement but a paradigm shift in how quality assurance and regulatory adherence are conceptualized and implemented. This technological trajectory promises to address longstanding challenges in pharmaceutical manufacturing while creating new opportunities for enhanced patient safety and product efficacy verification.
Market Analysis for Spintronic Applications in Pharmaceutical Industry
The pharmaceutical industry is witnessing a significant transformation with the integration of advanced technologies, particularly spintronic devices. The global pharmaceutical market, valued at approximately $1.4 trillion in 2022, is projected to grow at a CAGR of 5-6% through 2030, creating substantial opportunities for innovative technologies that enhance drug development, manufacturing, and quality control processes.
Spintronic applications in pharmaceuticals represent a nascent but rapidly evolving market segment. Currently estimated at $120 million, this niche is expected to expand at a CAGR of 22% over the next five years, outpacing the growth of traditional pharmaceutical technologies. This acceleration is driven by increasing regulatory demands for precision, reliability, and data integrity in pharmaceutical processes.
Key market drivers include the growing complexity of biopharmaceuticals, which require more sophisticated analytical and monitoring tools than traditional small-molecule drugs. The rise of personalized medicine has further intensified the need for high-precision sensing technologies capable of detecting minute molecular variations, an area where spintronic sensors excel due to their exceptional sensitivity.
Geographically, North America dominates the market with approximately 45% share, followed by Europe (30%) and Asia-Pacific (20%). However, the Asia-Pacific region is experiencing the fastest growth rate, particularly in countries like China, Japan, and South Korea, which have made significant investments in both pharmaceutical manufacturing and spintronics research.
Market segmentation reveals that quality control and analytical applications currently constitute the largest application segment (40%), followed by process monitoring (30%) and research applications (25%). The remaining 5% encompasses emerging applications such as drug delivery systems incorporating spintronic components.
Demand analysis indicates that large pharmaceutical manufacturers represent the primary customer base (65%), followed by contract research organizations (20%) and academic research institutions (15%). This distribution reflects the capital-intensive nature of spintronic technology implementation and the need for specialized expertise.
Pricing trends show that while initial investment costs for spintronic systems remain high, the total cost of ownership is becoming increasingly competitive due to longer operational lifespans, reduced maintenance requirements, and significant improvements in process efficiency and error reduction.
Market barriers include high implementation costs, regulatory uncertainties regarding novel technologies, and knowledge gaps among pharmaceutical professionals about spintronic capabilities. Additionally, competition from established technologies with extensive validation histories presents a significant challenge for market penetration.
Future market projections suggest that as regulatory frameworks evolve to accommodate advanced technologies and as production scales increase, spintronic applications will likely see accelerated adoption, particularly in high-value pharmaceutical segments where precision and reliability justify premium technology investments.
Spintronic applications in pharmaceuticals represent a nascent but rapidly evolving market segment. Currently estimated at $120 million, this niche is expected to expand at a CAGR of 22% over the next five years, outpacing the growth of traditional pharmaceutical technologies. This acceleration is driven by increasing regulatory demands for precision, reliability, and data integrity in pharmaceutical processes.
Key market drivers include the growing complexity of biopharmaceuticals, which require more sophisticated analytical and monitoring tools than traditional small-molecule drugs. The rise of personalized medicine has further intensified the need for high-precision sensing technologies capable of detecting minute molecular variations, an area where spintronic sensors excel due to their exceptional sensitivity.
Geographically, North America dominates the market with approximately 45% share, followed by Europe (30%) and Asia-Pacific (20%). However, the Asia-Pacific region is experiencing the fastest growth rate, particularly in countries like China, Japan, and South Korea, which have made significant investments in both pharmaceutical manufacturing and spintronics research.
Market segmentation reveals that quality control and analytical applications currently constitute the largest application segment (40%), followed by process monitoring (30%) and research applications (25%). The remaining 5% encompasses emerging applications such as drug delivery systems incorporating spintronic components.
Demand analysis indicates that large pharmaceutical manufacturers represent the primary customer base (65%), followed by contract research organizations (20%) and academic research institutions (15%). This distribution reflects the capital-intensive nature of spintronic technology implementation and the need for specialized expertise.
Pricing trends show that while initial investment costs for spintronic systems remain high, the total cost of ownership is becoming increasingly competitive due to longer operational lifespans, reduced maintenance requirements, and significant improvements in process efficiency and error reduction.
Market barriers include high implementation costs, regulatory uncertainties regarding novel technologies, and knowledge gaps among pharmaceutical professionals about spintronic capabilities. Additionally, competition from established technologies with extensive validation histories presents a significant challenge for market penetration.
Future market projections suggest that as regulatory frameworks evolve to accommodate advanced technologies and as production scales increase, spintronic applications will likely see accelerated adoption, particularly in high-value pharmaceutical segments where precision and reliability justify premium technology investments.
Current Spintronic Technology Status and Regulatory Challenges
Spintronic technology has witnessed significant advancements over the past decade, with applications expanding beyond traditional computing into specialized fields including pharmaceutical manufacturing and quality control. Current spintronic devices leverage electron spin properties to achieve enhanced sensitivity in detection systems, reduced power consumption, and improved data processing capabilities compared to conventional electronic systems. However, the integration of these devices into pharmaceutical environments presents unique regulatory challenges that must be addressed before widespread adoption.
The global regulatory landscape for spintronic devices in pharmaceutical applications remains fragmented and underdeveloped. The FDA has not established specific guidelines for spintronic technology in drug manufacturing or quality control processes, creating uncertainty for manufacturers and potential adopters. Similarly, the European Medicines Agency (EMA) has yet to formalize requirements for these emerging technologies, though their general framework for novel technologies provides some guidance.
Technical challenges further complicate regulatory compliance. Spintronic devices often operate at the nanoscale, raising concerns about potential particle contamination in sterile pharmaceutical environments. The magnetic fields generated by these devices, while minimal, must be evaluated for their impact on biological materials and sensitive pharmaceutical compounds. Additionally, the long-term stability and calibration requirements of spintronic sensors in variable manufacturing conditions remain insufficiently documented.
Data integrity represents another critical regulatory hurdle. Pharmaceutical manufacturing requires complete, consistent, and accurate data trails for regulatory submission and compliance. Current spintronic systems lack standardized validation protocols to ensure data reliability across different operational conditions, potentially compromising their acceptability to regulatory authorities.
Material compatibility issues also present significant challenges. Many spintronic devices incorporate rare earth elements and specialized alloys that must be evaluated for biocompatibility and potential leaching when used in proximity to pharmaceutical products. The absence of industry-wide standards for these assessments creates additional regulatory uncertainty.
Geographically, spintronic technology development remains concentrated in research clusters across North America, Europe, and East Asia, with limited expertise in pharmaceutical applications specifically. This geographic distribution creates disparities in regulatory approach and technical standards, further complicating global compliance strategies.
The current regulatory gap is driving collaborative initiatives between technology developers, pharmaceutical manufacturers, and regulatory bodies to establish appropriate frameworks. Industry consortia are beginning to develop voluntary standards and best practices, which may eventually inform formal regulatory requirements as the technology matures and its pharmaceutical applications expand.
The global regulatory landscape for spintronic devices in pharmaceutical applications remains fragmented and underdeveloped. The FDA has not established specific guidelines for spintronic technology in drug manufacturing or quality control processes, creating uncertainty for manufacturers and potential adopters. Similarly, the European Medicines Agency (EMA) has yet to formalize requirements for these emerging technologies, though their general framework for novel technologies provides some guidance.
Technical challenges further complicate regulatory compliance. Spintronic devices often operate at the nanoscale, raising concerns about potential particle contamination in sterile pharmaceutical environments. The magnetic fields generated by these devices, while minimal, must be evaluated for their impact on biological materials and sensitive pharmaceutical compounds. Additionally, the long-term stability and calibration requirements of spintronic sensors in variable manufacturing conditions remain insufficiently documented.
Data integrity represents another critical regulatory hurdle. Pharmaceutical manufacturing requires complete, consistent, and accurate data trails for regulatory submission and compliance. Current spintronic systems lack standardized validation protocols to ensure data reliability across different operational conditions, potentially compromising their acceptability to regulatory authorities.
Material compatibility issues also present significant challenges. Many spintronic devices incorporate rare earth elements and specialized alloys that must be evaluated for biocompatibility and potential leaching when used in proximity to pharmaceutical products. The absence of industry-wide standards for these assessments creates additional regulatory uncertainty.
Geographically, spintronic technology development remains concentrated in research clusters across North America, Europe, and East Asia, with limited expertise in pharmaceutical applications specifically. This geographic distribution creates disparities in regulatory approach and technical standards, further complicating global compliance strategies.
The current regulatory gap is driving collaborative initiatives between technology developers, pharmaceutical manufacturers, and regulatory bodies to establish appropriate frameworks. Industry consortia are beginning to develop voluntary standards and best practices, which may eventually inform formal regulatory requirements as the technology matures and its pharmaceutical applications expand.
Existing Regulatory Compliance Solutions for Spintronic Devices
01 Magnetic Tunnel Junction (MTJ) Structures
Magnetic Tunnel Junction structures are fundamental components in spintronic devices, consisting of two ferromagnetic layers separated by an insulating barrier. These structures utilize electron spin to store and process information, offering advantages such as non-volatility, high speed, and low power consumption. Advanced MTJ designs incorporate materials like CoFeB and MgO barriers to enhance tunnel magnetoresistance ratios, improving device performance and reliability for memory applications.- Magnetic Tunnel Junction (MTJ) Based Spintronic Devices: Magnetic Tunnel Junction (MTJ) structures are fundamental components in spintronic devices, consisting of two ferromagnetic layers separated by a thin insulating barrier. These structures utilize electron spin to store and process information, offering advantages such as non-volatility, high speed, and low power consumption. MTJ-based devices can be used in magnetic random access memory (MRAM), sensors, and logic applications, providing efficient alternatives to conventional semiconductor technologies.
- Spin-Orbit Torque (SOT) Devices: Spin-Orbit Torque (SOT) devices leverage the interaction between electron spin and orbital motion to manipulate magnetization. These devices utilize materials with strong spin-orbit coupling to generate torques that can switch magnetic states efficiently. SOT-based spintronic devices offer advantages including faster switching speeds, lower energy consumption, and improved reliability compared to conventional spin-transfer torque devices, making them promising candidates for next-generation memory and computing applications.
- Integration of Spintronic Devices with Semiconductor Technology: The integration of spintronic devices with conventional semiconductor technology enables hybrid systems that combine the advantages of both approaches. This integration involves developing compatible fabrication processes, addressing interface issues, and designing circuits that can effectively utilize spin-based components. Such hybrid systems can enhance the performance of existing semiconductor devices while introducing new functionalities based on spin properties, potentially leading to more efficient and versatile electronic systems.
- Novel Materials for Spintronic Applications: Advanced materials play a crucial role in enhancing the performance of spintronic devices. These include half-metallic ferromagnets, topological insulators, 2D materials, and various heterostructures designed to optimize spin transport properties. Novel material systems can exhibit high spin polarization, long spin coherence times, and efficient spin-charge conversion, which are essential for developing high-performance spintronic devices with improved functionality, energy efficiency, and reliability.
- Spintronic Sensors and Detection Systems: Spintronic sensors utilize the spin-dependent transport properties of electrons to detect magnetic fields, currents, or other physical quantities with high sensitivity. These sensors can be designed for various applications including biosensing, industrial monitoring, and scientific instrumentation. Compared to conventional sensors, spintronic sensors offer advantages such as higher sensitivity, smaller size, wider dynamic range, and compatibility with standard semiconductor manufacturing processes, making them valuable for advanced sensing applications.
02 Spin-Orbit Torque (SOT) Based Devices
Spin-Orbit Torque technology represents a next-generation approach in spintronics, utilizing spin-orbit coupling to manipulate magnetic moments. SOT-based devices offer advantages over conventional spin-transfer torque devices, including faster switching speeds and lower energy consumption. These devices typically employ heavy metal layers adjacent to ferromagnetic materials to generate spin currents that can efficiently switch magnetization, enabling high-performance memory and logic applications.Expand Specific Solutions03 Integration of Spintronic Devices with CMOS Technology
The integration of spintronic devices with conventional CMOS technology creates hybrid systems that combine the advantages of both technologies. This approach enables the development of energy-efficient, high-density memory-in-logic architectures. Integration techniques include back-end-of-line processing methods that allow spintronic elements to be fabricated on top of CMOS circuits, addressing interconnect challenges and thermal compatibility issues while maintaining manufacturing scalability.Expand Specific Solutions04 Novel Materials for Enhanced Spintronic Performance
Advanced materials play a crucial role in improving spintronic device performance. These include topological insulators, Heusler alloys, and two-dimensional materials that exhibit unique spin-dependent transport properties. Research focuses on materials with high spin polarization, strong spin-orbit coupling, and tunable magnetic properties. These novel materials enable higher magnetoresistance ratios, lower switching currents, and improved thermal stability, addressing key challenges in spintronic device development.Expand Specific Solutions05 Spintronic Sensors and Energy Harvesting Applications
Beyond memory applications, spintronic devices are being developed for sensing and energy harvesting purposes. Spintronic sensors utilize magnetoresistive effects to detect magnetic fields with high sensitivity and low power consumption, suitable for applications in automotive systems, biomedical devices, and industrial monitoring. Additionally, spin-based thermoelectric and mechanical energy harvesting devices convert thermal gradients or mechanical vibrations into electrical signals through spin-dependent transport mechanisms.Expand Specific Solutions
Leading Companies and Research Institutions in Pharmaceutical Spintronics
The spintronic devices regulatory compliance landscape in pharmaceuticals is currently in an emerging growth phase, with the market expected to expand significantly as technology matures. The global market remains relatively small but shows promising annual growth rates of 15-20% as pharmaceutical companies seek innovative compliance solutions. Leading players like Medtronic and Bristol Myers Squibb are investing heavily in spintronic technology integration, while specialized firms such as Broadcare Robot and Jubilee Biotech are developing niche applications. Academic-industry partnerships with MIT and University of Florida are accelerating technological advancement. The regulatory framework remains under development, with companies like Sanofi-Aventis and Novo Nordisk working closely with authorities to establish standards, indicating the technology is approaching early commercial maturity but requires further validation for widespread pharmaceutical adoption.
Medtronic, Inc.
Technical Solution: Medtronic has pioneered regulatory-compliant spintronic devices for pharmaceutical delivery systems, particularly in implantable drug delivery platforms. Their technology utilizes spin-transfer torque magnetic random-access memory (STT-MRAM) to create non-volatile, radiation-resistant memory components that maintain critical dosage information even during power loss events. This addresses key regulatory concerns regarding data integrity and patient safety. Medtronic's spintronic solutions incorporate multiple redundancy systems and real-time verification protocols that meet both FDA and European Medicines Agency (EMA) requirements for medical device software. Their devices feature spintronic sensors that continuously monitor drug delivery parameters and automatically adjust to maintain compliance with prescribed therapeutic ranges, with all events logged in tamper-proof memory systems for regulatory inspection.
Strengths: Extensive experience navigating medical device regulatory frameworks; established quality management systems specifically adapted for spintronic components; strong integration capabilities with existing pharmaceutical delivery systems. Weaknesses: Solutions primarily focused on implantable devices rather than broader pharmaceutical manufacturing applications; higher cost structure compared to conventional technologies; limited compatibility with some pharmaceutical compounds.
Bristol Myers Squibb Co.
Technical Solution: Bristol Myers Squibb has developed a comprehensive spintronic monitoring system for pharmaceutical manufacturing compliance. Their platform integrates spintronic magnetic field sensors throughout the production line to create a continuous verification environment that meets cGMP requirements. The system employs tunnel magnetoresistance (TMR) sensors with sensitivity exceeding 1000% magnetoresistance ratio, allowing for detection of minute contaminants and process deviations that could affect pharmaceutical quality. BMS's regulatory compliance framework for these devices includes automated documentation generation that satisfies FDA, EMA, and ICH guidelines. Their spintronic technology also features adaptive calibration systems that maintain measurement accuracy throughout temperature fluctuations and aging effects, ensuring consistent compliance with regulatory specifications over the entire device lifecycle.
Strengths: Direct integration with pharmaceutical manufacturing expertise; comprehensive validation protocols specifically designed for regulatory inspections; extensive experience with global pharmaceutical compliance requirements. Weaknesses: Proprietary system with limited compatibility with third-party manufacturing equipment; significant initial investment required for implementation; ongoing calibration requirements to maintain regulatory compliance.
Key Patents and Technical Literature in Pharmaceutical Spintronics
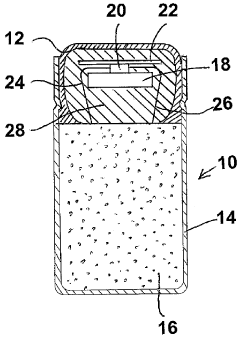
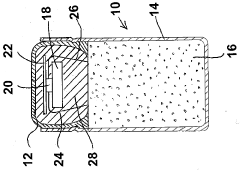
Oral drug capsule component incorporating a communication device
PatentInactiveCA2671332A1
Innovation
- Incorporating a communication device, such as an RFID tag, into an oral drug capsule that activates upon ingestion, allowing for real-time signal transmission to an external data collection device, indicating the drug has been ingested and enabling precise monitoring of compliance.
Means for monitoring compliance, facilitating automatic dispensing and childproofing strip packaged medication
PatentWO2016070272A1
Innovation
- A dispenser system that incorporates electronic compliance monitoring (ECM) and child resistance features, using reinforced strips with guide holes and sprocket wheels for automated dispensing, and pinch rollers for compact design, along with a PCB tag for tracking dosing events and wireless communication for data transmission.
Global Regulatory Framework for Pharmaceutical Manufacturing Technologies
The pharmaceutical manufacturing sector operates within a complex web of international and national regulatory frameworks that govern the adoption of new technologies. For spintronic devices, which represent an emerging technology in pharmaceutical manufacturing processes, navigating this regulatory landscape is particularly challenging due to the novelty of the technology and varying regional approaches to regulation.
At the international level, the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) provides guidelines that influence regulatory frameworks across major pharmaceutical markets. The ICH Q8, Q9, Q10, and Q11 guidelines, which address pharmaceutical development, quality risk management, pharmaceutical quality systems, and development and manufacture of drug substances respectively, are particularly relevant for spintronic device implementation in pharmaceutical manufacturing.
The U.S. Food and Drug Administration (FDA) has established the framework for Process Analytical Technology (PAT) and Quality by Design (QbD) approaches, which encourage innovation in manufacturing technologies while maintaining strict quality standards. Under this framework, spintronic devices must demonstrate compliance with 21 CFR Part 11 for electronic records and signatures, as well as adherence to Good Manufacturing Practice (GMP) regulations.
In the European Union, the European Medicines Agency (EMA) oversees compliance through the EU GMP guidelines and the Medical Device Regulation (MDR). Spintronic devices used in pharmaceutical manufacturing must meet the requirements of Annex 11 of the EU GMP, which addresses computerized systems, and potentially the MDR if the device directly interfaces with medicinal products.
The Pharmaceutical Inspection Co-operation Scheme (PIC/S) provides another layer of international standards that influence national regulatory frameworks across its 54 member countries. PIC/S guidelines emphasize the validation of computerized systems and the integrity of data generated by such systems, which directly impacts the implementation of spintronic technologies.
In Asia, regulatory bodies such as Japan's Pharmaceuticals and Medical Devices Agency (PMDA) and China's National Medical Products Administration (NMPA) have established their own frameworks that manufacturers must navigate. These frameworks often incorporate elements of international standards but may include country-specific requirements that add complexity to global compliance strategies.
Emerging markets present additional regulatory challenges, with varying levels of sophistication in their regulatory frameworks and different approaches to the adoption of new technologies. Manufacturers implementing spintronic devices must develop comprehensive regulatory strategies that account for these regional variations while maintaining compliance with core quality and safety principles.
At the international level, the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) provides guidelines that influence regulatory frameworks across major pharmaceutical markets. The ICH Q8, Q9, Q10, and Q11 guidelines, which address pharmaceutical development, quality risk management, pharmaceutical quality systems, and development and manufacture of drug substances respectively, are particularly relevant for spintronic device implementation in pharmaceutical manufacturing.
The U.S. Food and Drug Administration (FDA) has established the framework for Process Analytical Technology (PAT) and Quality by Design (QbD) approaches, which encourage innovation in manufacturing technologies while maintaining strict quality standards. Under this framework, spintronic devices must demonstrate compliance with 21 CFR Part 11 for electronic records and signatures, as well as adherence to Good Manufacturing Practice (GMP) regulations.
In the European Union, the European Medicines Agency (EMA) oversees compliance through the EU GMP guidelines and the Medical Device Regulation (MDR). Spintronic devices used in pharmaceutical manufacturing must meet the requirements of Annex 11 of the EU GMP, which addresses computerized systems, and potentially the MDR if the device directly interfaces with medicinal products.
The Pharmaceutical Inspection Co-operation Scheme (PIC/S) provides another layer of international standards that influence national regulatory frameworks across its 54 member countries. PIC/S guidelines emphasize the validation of computerized systems and the integrity of data generated by such systems, which directly impacts the implementation of spintronic technologies.
In Asia, regulatory bodies such as Japan's Pharmaceuticals and Medical Devices Agency (PMDA) and China's National Medical Products Administration (NMPA) have established their own frameworks that manufacturers must navigate. These frameworks often incorporate elements of international standards but may include country-specific requirements that add complexity to global compliance strategies.
Emerging markets present additional regulatory challenges, with varying levels of sophistication in their regulatory frameworks and different approaches to the adoption of new technologies. Manufacturers implementing spintronic devices must develop comprehensive regulatory strategies that account for these regional variations while maintaining compliance with core quality and safety principles.
Risk Assessment and Validation Protocols for Spintronic Implementations
The implementation of spintronic devices in pharmaceutical environments necessitates comprehensive risk assessment frameworks and validation protocols to ensure regulatory compliance. Current methodologies for evaluating spintronic technologies in pharmaceutical applications remain underdeveloped, creating significant regulatory challenges.
Risk assessment for spintronic implementations must address multiple dimensions: electromagnetic interference with existing medical equipment, data integrity concerns, and potential impacts on pharmaceutical formulations during manufacturing processes. The FDA's guidance on computerized systems validation (21 CFR Part 11) provides a foundation, but requires adaptation for the unique characteristics of spintronic devices, particularly regarding quantum effects and magnetic field interactions.
Validation protocols should follow a tiered approach beginning with Installation Qualification (IQ) to verify proper hardware setup and electromagnetic shielding. Operational Qualification (OQ) must then evaluate performance under various environmental conditions typical in pharmaceutical facilities, including temperature fluctuations, humidity variations, and exposure to cleaning agents. Performance Qualification (PQ) should focus on long-term reliability and data integrity maintenance.
Critical risk factors requiring specific validation include magnetic field containment, which must be monitored to prevent interference with sensitive pharmaceutical processes or nearby electronic equipment. Data integrity validation protocols must verify that spintronic memory states remain stable under pharmaceutical manufacturing conditions, with particular attention to error rates during read/write operations in environments with vibration or electromagnetic noise.
Continuous monitoring systems represent an essential component of validation frameworks, enabling real-time assessment of spintronic device performance against predetermined specifications. These systems should incorporate automated alerts for deviations that might compromise pharmaceutical product quality or regulatory compliance.
International harmonization of validation approaches presents a significant challenge, as regulatory bodies including the FDA, EMA, and PMDA maintain different requirements for novel technologies in pharmaceutical environments. A risk-based validation strategy aligned with ICH Q9 principles offers the most promising approach for global implementation.
Pharmaceutical companies pioneering spintronic implementations should develop technology-specific validation master plans documenting risk assessment methodologies, acceptance criteria, and ongoing monitoring requirements. These plans should incorporate both standard pharmaceutical validation principles and specialized protocols addressing the unique characteristics of spintronic technologies.
Risk assessment for spintronic implementations must address multiple dimensions: electromagnetic interference with existing medical equipment, data integrity concerns, and potential impacts on pharmaceutical formulations during manufacturing processes. The FDA's guidance on computerized systems validation (21 CFR Part 11) provides a foundation, but requires adaptation for the unique characteristics of spintronic devices, particularly regarding quantum effects and magnetic field interactions.
Validation protocols should follow a tiered approach beginning with Installation Qualification (IQ) to verify proper hardware setup and electromagnetic shielding. Operational Qualification (OQ) must then evaluate performance under various environmental conditions typical in pharmaceutical facilities, including temperature fluctuations, humidity variations, and exposure to cleaning agents. Performance Qualification (PQ) should focus on long-term reliability and data integrity maintenance.
Critical risk factors requiring specific validation include magnetic field containment, which must be monitored to prevent interference with sensitive pharmaceutical processes or nearby electronic equipment. Data integrity validation protocols must verify that spintronic memory states remain stable under pharmaceutical manufacturing conditions, with particular attention to error rates during read/write operations in environments with vibration or electromagnetic noise.
Continuous monitoring systems represent an essential component of validation frameworks, enabling real-time assessment of spintronic device performance against predetermined specifications. These systems should incorporate automated alerts for deviations that might compromise pharmaceutical product quality or regulatory compliance.
International harmonization of validation approaches presents a significant challenge, as regulatory bodies including the FDA, EMA, and PMDA maintain different requirements for novel technologies in pharmaceutical environments. A risk-based validation strategy aligned with ICH Q9 principles offers the most promising approach for global implementation.
Pharmaceutical companies pioneering spintronic implementations should develop technology-specific validation master plans documenting risk assessment methodologies, acceptance criteria, and ongoing monitoring requirements. These plans should incorporate both standard pharmaceutical validation principles and specialized protocols addressing the unique characteristics of spintronic technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!