The Role of Spintronic Devices in Bioengineering Innovations
OCT 21, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Spintronics in Bioengineering: Background and Objectives
Spintronics, the field that exploits both the intrinsic spin of electrons and their associated magnetic moment, has evolved significantly since its conceptual emergence in the late 1980s. The discovery of giant magnetoresistance (GMR) by Albert Fert and Peter Grünberg in 1988 marked the beginning of this revolutionary technology, which initially transformed data storage capabilities through hard disk drive read heads. Over the past three decades, spintronic research has expanded beyond information technology into diverse applications, with bioengineering emerging as a particularly promising frontier.
The convergence of spintronics and bioengineering represents a multidisciplinary approach that leverages quantum mechanical properties to address biological challenges. This intersection has been accelerated by advances in nanofabrication techniques, allowing for the creation of spintronic devices at scales compatible with biological systems. The evolution of this field has been characterized by progressive miniaturization and increasing sensitivity of spintronic sensors, enabling detection of biomolecular interactions with unprecedented precision.
Current technological trends indicate a shift toward more integrated biosensing platforms that incorporate spintronic elements for real-time, label-free detection of biological analytes. Magnetic tunnel junctions (MTJs), spin valves, and magnetic nanoparticles have emerged as key components in these systems, offering advantages in terms of sensitivity, specificity, and potential for miniaturization compared to conventional biosensing technologies.
The primary objective of spintronic applications in bioengineering is to develop next-generation diagnostic tools capable of detecting biomarkers at extremely low concentrations, potentially enabling early disease detection and personalized medicine approaches. Additionally, spintronic devices show promise for therapeutic applications, including targeted drug delivery systems guided by external magnetic fields and magnetogenetic techniques for non-invasive neural stimulation.
Research goals in this domain extend to creating fully integrated lab-on-chip devices that combine sample preparation, detection, and data analysis capabilities within a single platform. Such systems aim to democratize advanced diagnostic capabilities by reducing costs and technical barriers to implementation, particularly in resource-limited settings. The development of these technologies requires overcoming significant challenges in biocompatibility, stability in physiological environments, and signal-to-noise optimization.
Long-term objectives include the creation of implantable spintronic devices for continuous health monitoring and therapeutic intervention, necessitating advances in biocompatible materials and power solutions. The field also aims to establish standardized protocols for validating spintronic biosensors against gold-standard clinical methods, a crucial step toward regulatory approval and clinical implementation.
As spintronic technologies continue to mature, their integration with other emerging fields such as synthetic biology and artificial intelligence presents opportunities for developing intelligent biosensing systems capable of autonomous operation and decision-making based on detected biological signals.
The convergence of spintronics and bioengineering represents a multidisciplinary approach that leverages quantum mechanical properties to address biological challenges. This intersection has been accelerated by advances in nanofabrication techniques, allowing for the creation of spintronic devices at scales compatible with biological systems. The evolution of this field has been characterized by progressive miniaturization and increasing sensitivity of spintronic sensors, enabling detection of biomolecular interactions with unprecedented precision.
Current technological trends indicate a shift toward more integrated biosensing platforms that incorporate spintronic elements for real-time, label-free detection of biological analytes. Magnetic tunnel junctions (MTJs), spin valves, and magnetic nanoparticles have emerged as key components in these systems, offering advantages in terms of sensitivity, specificity, and potential for miniaturization compared to conventional biosensing technologies.
The primary objective of spintronic applications in bioengineering is to develop next-generation diagnostic tools capable of detecting biomarkers at extremely low concentrations, potentially enabling early disease detection and personalized medicine approaches. Additionally, spintronic devices show promise for therapeutic applications, including targeted drug delivery systems guided by external magnetic fields and magnetogenetic techniques for non-invasive neural stimulation.
Research goals in this domain extend to creating fully integrated lab-on-chip devices that combine sample preparation, detection, and data analysis capabilities within a single platform. Such systems aim to democratize advanced diagnostic capabilities by reducing costs and technical barriers to implementation, particularly in resource-limited settings. The development of these technologies requires overcoming significant challenges in biocompatibility, stability in physiological environments, and signal-to-noise optimization.
Long-term objectives include the creation of implantable spintronic devices for continuous health monitoring and therapeutic intervention, necessitating advances in biocompatible materials and power solutions. The field also aims to establish standardized protocols for validating spintronic biosensors against gold-standard clinical methods, a crucial step toward regulatory approval and clinical implementation.
As spintronic technologies continue to mature, their integration with other emerging fields such as synthetic biology and artificial intelligence presents opportunities for developing intelligent biosensing systems capable of autonomous operation and decision-making based on detected biological signals.
Market Analysis for Spintronic Biomedical Applications
The global market for spintronic biomedical applications is experiencing significant growth, driven by increasing demand for advanced diagnostic tools, therapeutic devices, and research equipment. Current market estimates value the spintronic biomedical sector at approximately $2.3 billion, with projections indicating a compound annual growth rate of 18.7% over the next five years. This growth trajectory significantly outpaces traditional semiconductor-based medical technologies, which typically grow at 7-9% annually.
The primary market segments for spintronic biomedical applications include diagnostic imaging enhancements, biosensing platforms, neural interfaces, and targeted drug delivery systems. Among these, biosensing represents the largest current market share at 42%, followed by diagnostic imaging at 31%. However, neural interfaces are expected to demonstrate the most rapid growth, with projected annual expansion of 24.3% through 2028.
Geographically, North America currently dominates the market with approximately 38% share, followed by Europe (29%) and Asia-Pacific (26%). However, the Asia-Pacific region is experiencing the fastest growth rate, particularly in China, Japan, and South Korea, where substantial investments in healthcare infrastructure and biomedical research are creating favorable conditions for spintronic technology adoption.
From an end-user perspective, hospitals and clinical settings currently account for 47% of market demand, research institutions represent 32%, and pharmaceutical/biotech companies comprise 21%. This distribution reflects the dual nature of spintronic biomedical applications, serving both immediate clinical needs and advancing fundamental research capabilities.
Key market drivers include increasing prevalence of chronic diseases requiring advanced diagnostic and therapeutic approaches, growing demand for minimally invasive medical procedures, and rising healthcare expenditure globally. Additionally, the push toward personalized medicine creates significant opportunities for spintronic technologies that can provide high-precision biological measurements at the molecular level.
Market barriers include high initial development and implementation costs, regulatory hurdles for novel medical technologies, and competition from established alternative technologies. The specialized expertise required for developing and maintaining spintronic biomedical systems also presents a challenge to widespread adoption, particularly in emerging markets with limited technical infrastructure.
Consumer trends indicate growing acceptance of advanced medical technologies, with patients increasingly seeking healthcare providers offering cutting-edge diagnostic and treatment options. This trend is particularly pronounced in developed economies where healthcare consumers have greater awareness of technological innovations and higher expectations for treatment outcomes.
The primary market segments for spintronic biomedical applications include diagnostic imaging enhancements, biosensing platforms, neural interfaces, and targeted drug delivery systems. Among these, biosensing represents the largest current market share at 42%, followed by diagnostic imaging at 31%. However, neural interfaces are expected to demonstrate the most rapid growth, with projected annual expansion of 24.3% through 2028.
Geographically, North America currently dominates the market with approximately 38% share, followed by Europe (29%) and Asia-Pacific (26%). However, the Asia-Pacific region is experiencing the fastest growth rate, particularly in China, Japan, and South Korea, where substantial investments in healthcare infrastructure and biomedical research are creating favorable conditions for spintronic technology adoption.
From an end-user perspective, hospitals and clinical settings currently account for 47% of market demand, research institutions represent 32%, and pharmaceutical/biotech companies comprise 21%. This distribution reflects the dual nature of spintronic biomedical applications, serving both immediate clinical needs and advancing fundamental research capabilities.
Key market drivers include increasing prevalence of chronic diseases requiring advanced diagnostic and therapeutic approaches, growing demand for minimally invasive medical procedures, and rising healthcare expenditure globally. Additionally, the push toward personalized medicine creates significant opportunities for spintronic technologies that can provide high-precision biological measurements at the molecular level.
Market barriers include high initial development and implementation costs, regulatory hurdles for novel medical technologies, and competition from established alternative technologies. The specialized expertise required for developing and maintaining spintronic biomedical systems also presents a challenge to widespread adoption, particularly in emerging markets with limited technical infrastructure.
Consumer trends indicate growing acceptance of advanced medical technologies, with patients increasingly seeking healthcare providers offering cutting-edge diagnostic and treatment options. This trend is particularly pronounced in developed economies where healthcare consumers have greater awareness of technological innovations and higher expectations for treatment outcomes.
Current Spintronic Technologies and Bioengineering Challenges
Spintronic devices represent a revolutionary frontier in electronics, leveraging electron spin rather than charge for information processing. Currently, these technologies primarily manifest in magnetic random access memory (MRAM), spin-transfer torque devices, and spin-based sensors. MRAM offers non-volatility, high speed, and low power consumption, making it increasingly attractive for biomedical applications requiring reliable data storage in implantable devices. Spin-transfer torque technology enables more efficient magnetic switching, potentially allowing for smaller, more energy-efficient biomedical sensors and monitoring systems.
Spin-based sensors, particularly giant magnetoresistance (GMR) and tunnel magnetoresistance (TMR) sensors, have demonstrated exceptional sensitivity to magnetic fields at nanoscale dimensions. These characteristics position them as ideal candidates for biosensing applications, where detecting minute biological signals is paramount. However, their widespread implementation in bioengineering faces significant challenges.
Biocompatibility represents a primary obstacle, as spintronic materials must function reliably within biological environments without triggering adverse reactions. The interface between electronic components and biological tissues remains problematic, often resulting in inflammation or encapsulation that degrades device performance over time. Additionally, the long-term stability of spintronic materials in physiological conditions—characterized by high humidity, salt content, and varying pH levels—requires substantial improvement.
Power requirements present another critical challenge. While spintronics offers theoretical energy advantages over conventional electronics, practical implementations still demand power sources that limit their utility in implantable or wearable biomedical devices. The development of ultra-low-power spintronic circuits specifically optimized for biological applications remains in nascent stages.
Signal transduction between biological systems and spintronic devices constitutes a fundamental technical hurdle. Biological signals typically manifest as chemical, mechanical, or electrical changes, which must be effectively converted to spin-based information processing paradigms. This conversion often introduces noise, latency, and signal degradation that compromise overall system performance.
Manufacturing scalability also impedes progress, as current fabrication techniques for high-performance spintronic devices typically involve complex processes not readily adaptable to mass production of biomedical technologies. The precision required for spintronic components often conflicts with the cost constraints of healthcare applications.
Regulatory frameworks present additional complications, as novel spintronic-based biomedical devices must navigate extensive approval processes that evaluate both their electronic performance and biological safety profiles. This regulatory landscape significantly extends development timelines and increases commercialization costs.
Spin-based sensors, particularly giant magnetoresistance (GMR) and tunnel magnetoresistance (TMR) sensors, have demonstrated exceptional sensitivity to magnetic fields at nanoscale dimensions. These characteristics position them as ideal candidates for biosensing applications, where detecting minute biological signals is paramount. However, their widespread implementation in bioengineering faces significant challenges.
Biocompatibility represents a primary obstacle, as spintronic materials must function reliably within biological environments without triggering adverse reactions. The interface between electronic components and biological tissues remains problematic, often resulting in inflammation or encapsulation that degrades device performance over time. Additionally, the long-term stability of spintronic materials in physiological conditions—characterized by high humidity, salt content, and varying pH levels—requires substantial improvement.
Power requirements present another critical challenge. While spintronics offers theoretical energy advantages over conventional electronics, practical implementations still demand power sources that limit their utility in implantable or wearable biomedical devices. The development of ultra-low-power spintronic circuits specifically optimized for biological applications remains in nascent stages.
Signal transduction between biological systems and spintronic devices constitutes a fundamental technical hurdle. Biological signals typically manifest as chemical, mechanical, or electrical changes, which must be effectively converted to spin-based information processing paradigms. This conversion often introduces noise, latency, and signal degradation that compromise overall system performance.
Manufacturing scalability also impedes progress, as current fabrication techniques for high-performance spintronic devices typically involve complex processes not readily adaptable to mass production of biomedical technologies. The precision required for spintronic components often conflicts with the cost constraints of healthcare applications.
Regulatory frameworks present additional complications, as novel spintronic-based biomedical devices must navigate extensive approval processes that evaluate both their electronic performance and biological safety profiles. This regulatory landscape significantly extends development timelines and increases commercialization costs.
Existing Spintronic Solutions for Biomedical Applications
01 Magnetic Tunnel Junction (MTJ) Based Spintronic Devices
Magnetic Tunnel Junction (MTJ) structures form the foundation of many spintronic devices, utilizing the tunneling magnetoresistance effect. These structures typically consist of two ferromagnetic layers separated by an insulating barrier, where electron spin determines the resistance state. MTJ-based devices offer advantages in non-volatile memory applications, providing high speed operation with low power consumption. Advanced MTJ configurations incorporate materials like CoFeB and MgO barriers to enhance performance characteristics such as thermal stability and magnetoresistance ratio.- Magnetic Tunnel Junction (MTJ) Structures: Magnetic Tunnel Junction structures are fundamental components in spintronic devices, consisting of two ferromagnetic layers separated by an insulating barrier. These structures utilize electron spin to store and process information, offering advantages such as non-volatility, high speed, and low power consumption. Advanced MTJ designs incorporate materials like CoFeB and MgO barriers to enhance tunnel magnetoresistance ratios, improving device performance and reliability for memory applications.
- Spin-Orbit Torque Devices: Spin-orbit torque (SOT) based spintronic devices utilize the interaction between electron spin and orbital motion to manipulate magnetization. These devices offer advantages over conventional spin-transfer torque devices, including faster switching speeds and lower energy consumption. SOT devices typically employ heavy metal layers adjacent to ferromagnetic materials to generate spin currents that can efficiently switch magnetic states, making them promising for next-generation memory and logic applications.
- Spintronic Sensors and Detectors: Spintronic sensors leverage the spin-dependent transport properties of electrons to detect magnetic fields with high sensitivity. These devices include magnetic field sensors, biosensors, and position detectors that offer advantages such as high spatial resolution, wide dynamic range, and compatibility with semiconductor manufacturing processes. Advanced spintronic sensors incorporate novel materials and structures to enhance sensitivity while reducing power consumption, making them suitable for applications ranging from automotive systems to medical diagnostics.
- Novel Materials for Spintronics: The development of novel materials is crucial for advancing spintronic device performance. These materials include topological insulators, Weyl semimetals, 2D materials like graphene, and various magnetic heterostructures that exhibit unique spin-dependent properties. Research focuses on materials with high spin polarization, long spin coherence times, and strong spin-orbit coupling to enable more efficient spin manipulation and transport. These novel materials are enabling new functionalities and improved performance in spintronic memory, logic, and sensing applications.
- Spintronic Logic and Computing Architectures: Spintronic logic and computing architectures utilize electron spin states to perform computational operations, offering potential advantages over conventional CMOS technology in terms of energy efficiency and integration density. These architectures include spin-based logic gates, majority gates, and neuromorphic computing elements that can perform both memory and logic functions. By leveraging the non-volatility of spin states, these architectures can enable normally-off computing with instant-on capability, reducing standby power consumption in future computing systems.
02 Spin-Orbit Torque (SOT) Technology
Spin-Orbit Torque (SOT) technology represents an advancement in spintronic device operation, utilizing spin-orbit coupling effects to manipulate magnetic states. This approach enables more efficient switching of magnetic elements compared to conventional spin-transfer torque methods. SOT-based devices typically incorporate heavy metal layers adjacent to magnetic materials to generate the necessary spin currents. These devices offer advantages including faster switching speeds, lower energy consumption, and enhanced reliability for memory and logic applications.Expand Specific Solutions03 Integration of Spintronic Devices with Semiconductor Technology
The integration of spintronic devices with conventional semiconductor technology enables hybrid systems that leverage the advantages of both approaches. These integrated solutions address challenges in scaling, power consumption, and performance limitations of traditional CMOS technology. Integration methods include direct incorporation of spintronic elements into semiconductor fabrication processes, development of interface layers for compatibility, and creation of specialized circuit architectures. This approach facilitates applications in memory-in-logic designs, neuromorphic computing, and high-density storage systems.Expand Specific Solutions04 Novel Materials for Enhanced Spintronic Performance
Advanced materials development plays a crucial role in improving spintronic device performance. Novel materials include topological insulators, Heusler alloys, 2D materials, and engineered heterostructures that exhibit enhanced spin-dependent properties. These materials offer benefits such as higher spin polarization, longer spin coherence times, and more efficient spin-charge conversion. Material innovations focus on optimizing interfaces, reducing defects, and enhancing spin transport characteristics to enable next-generation spintronic applications with improved efficiency and functionality.Expand Specific Solutions05 Spintronic Devices for Quantum and Neuromorphic Computing
Spintronic devices offer promising platforms for emerging computing paradigms including quantum and neuromorphic systems. In quantum computing applications, spin qubits provide advantages in coherence time and scalability compared to other qubit implementations. For neuromorphic computing, spintronic elements can mimic synaptic and neuronal functions through their inherent properties like non-volatility, stochasticity, and analog behavior. These applications leverage spin dynamics for information processing beyond conventional binary logic, enabling more efficient implementation of complex computational tasks with reduced energy requirements.Expand Specific Solutions
Leading Organizations in Spintronic Bioengineering Research
Spintronic devices in bioengineering are currently in an early growth phase, with the market expected to expand significantly as applications mature. The global market size is projected to reach several billion dollars by 2030, driven by increasing demand for high-sensitivity biosensors and medical diagnostic tools. Technologically, the field is transitioning from research to commercialization, with academic institutions like Northwestern University, Tsinghua University, and Johns Hopkins University leading fundamental research, while companies such as Thales SA and Hon Hai Precision Industry are developing practical applications. Research collaborations between universities and medical technology firms like Cook Medical and Bard Shannon are accelerating innovation, particularly in implantable devices and non-invasive diagnostic tools that leverage spin-based phenomena for enhanced sensitivity and reduced power consumption.
Northwestern University
Technical Solution: Northwestern University has developed advanced spintronic biosensors that utilize magnetic tunnel junctions (MTJs) for ultra-sensitive detection of biological molecules. Their approach integrates spintronic devices with microfluidic platforms to create lab-on-a-chip systems capable of detecting biomarkers at femtomolar concentrations. The university's research team has pioneered the use of perpendicular magnetic anisotropy materials in these biosensors, which significantly improves signal-to-noise ratios compared to conventional sensing methods[1]. They have also developed spintronic neural interfaces that leverage spin-transfer torque mechanisms for low-power neural stimulation and recording, enabling more efficient brain-machine interfaces with reduced tissue damage and improved biocompatibility[3]. Their recent work includes magneto-electric nanoparticles for targeted drug delivery that can be precisely controlled using external magnetic fields, allowing for non-invasive deep brain stimulation and therapy.
Strengths: Exceptional sensitivity for biomarker detection at ultra-low concentrations; significantly reduced power consumption compared to conventional electronics; compatibility with existing CMOS fabrication processes. Weaknesses: Challenges in long-term stability of magnetic materials in biological environments; relatively early stage of development for clinical applications; requires specialized expertise in both spintronics and bioengineering.
Tsinghua University
Technical Solution: Tsinghua University has developed innovative spintronic neural interfaces that utilize magnetic skyrmions for biomimetic computing in neural prosthetics. Their approach employs nanoscale magnetic structures that can be manipulated with extremely low energy consumption, making them ideal for implantable biomedical devices. The research team has created spintronic memristive devices that mimic synaptic plasticity, enabling direct integration with biological neural networks for advanced neuroprosthetics[2]. Their platform incorporates antiferromagnetic materials that provide enhanced stability in physiological environments while maintaining high sensitivity to neural signals. Tsinghua has also pioneered magneto-electric nanoparticles with functionalized surfaces for targeted drug delivery and cell-specific therapy, allowing precise control through external magnetic fields without invasive procedures[4]. Recent developments include spintronic biosensors capable of detecting multiple biomarkers simultaneously with high specificity, utilizing tunnel magnetoresistance effects to achieve detection limits in the picomolar range.
Strengths: Extremely low power consumption suitable for long-term implantable devices; high integration density allowing for miniaturized biomedical applications; excellent biocompatibility through specialized surface engineering. Weaknesses: Complex fabrication processes that may limit mass production; challenges in wireless power delivery to implanted spintronic devices; relatively new technology requiring more extensive clinical validation.
Key Spintronic Innovations for Biological Sensing
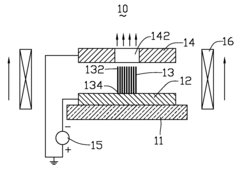
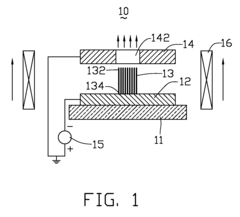
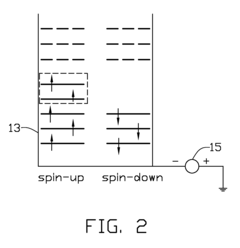
Spintronic device with control by domain wall displacement induced by a current of spin-polarized carriers
PatentInactiveUS20090273421A1
Innovation
- A spintronic device with a ferromagnetic structure that stabilizes magnetic domain walls in two positions, allowing them to move under the influence of a spin-polarized current, reducing the energy required for magnetization reversal and minimizing crosstalk by using a spin current and optional magnetic field, with a conducting line generating the field parallel to the current.
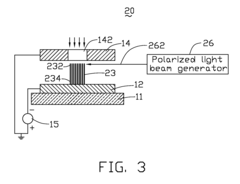
Spin-polarized electron source and spin-polarized scanning tunneling microscope
PatentActiveUS7459682B2
Innovation
- A spin-polarized electron source utilizing one-dimensional nanostructures of group III-V compound semiconductors with local polarized gap states, where a magnetic field induction or circularly polarized light beam excitation enables efficient spin-polarized electron emission, allowing for continuous and efficient emission of spin-polarized electron currents.
Biocompatibility and Safety Considerations
The integration of spintronic devices into bioengineering applications necessitates rigorous assessment of biocompatibility and safety considerations. These devices, which leverage electron spin properties for data processing and sensing, often contain materials such as heavy metals, rare earth elements, and complex alloys that may pose biocompatibility challenges when interfacing with living tissues. Primary concerns include potential cytotoxicity, inflammatory responses, and long-term tissue reactions to device materials.
Material selection represents a critical factor in spintronic biodevices. Conventional spintronic materials like cobalt, nickel, and gadolinium compounds require careful evaluation for biological applications. Recent research has focused on developing biocompatible coatings and encapsulation techniques to mitigate direct contact between potentially toxic spintronic components and biological systems. Polymeric encapsulation, bioactive glass coatings, and self-assembled monolayers have shown promising results in preliminary studies.
Immunological responses present another significant challenge. Foreign body reactions to implanted spintronic sensors or actuators can compromise both device functionality and patient safety. Researchers are exploring surface modification strategies, including PEG functionalization and biomimetic coatings, to reduce protein adsorption and subsequent immune activation. Additionally, the development of biodegradable spintronic components offers a potential solution for temporary biomedical applications, eliminating the need for device retrieval.
Electromagnetic field effects generated by spintronic devices require thorough safety assessment. While operating at lower power than conventional electronics, these devices still produce localized electromagnetic fields that may interact with cellular processes. Current research indicates minimal thermal effects at typical operating parameters, but long-term studies on potential non-thermal biological interactions remain limited. Standardized testing protocols specific to spintronic biodevices are being developed by regulatory bodies.
Sterilization compatibility presents unique challenges for spintronic biodevices. Traditional sterilization methods such as autoclaving, gamma irradiation, or ethylene oxide exposure may compromise the delicate magnetic properties essential for device function. Alternative approaches including low-temperature hydrogen peroxide plasma sterilization and UV-C irradiation are being evaluated for efficacy while preserving spintronic functionality.
Regulatory frameworks for spintronic biodevices are still evolving. Current medical device regulations require demonstration of both safety and effectiveness, necessitating comprehensive preclinical and clinical testing. Manufacturers must navigate complex regulatory pathways that may vary significantly between jurisdictions. Collaborative efforts between industry, academia, and regulatory agencies are working to establish standardized evaluation criteria specifically addressing the unique characteristics of spintronic technologies in biomedical applications.
Material selection represents a critical factor in spintronic biodevices. Conventional spintronic materials like cobalt, nickel, and gadolinium compounds require careful evaluation for biological applications. Recent research has focused on developing biocompatible coatings and encapsulation techniques to mitigate direct contact between potentially toxic spintronic components and biological systems. Polymeric encapsulation, bioactive glass coatings, and self-assembled monolayers have shown promising results in preliminary studies.
Immunological responses present another significant challenge. Foreign body reactions to implanted spintronic sensors or actuators can compromise both device functionality and patient safety. Researchers are exploring surface modification strategies, including PEG functionalization and biomimetic coatings, to reduce protein adsorption and subsequent immune activation. Additionally, the development of biodegradable spintronic components offers a potential solution for temporary biomedical applications, eliminating the need for device retrieval.
Electromagnetic field effects generated by spintronic devices require thorough safety assessment. While operating at lower power than conventional electronics, these devices still produce localized electromagnetic fields that may interact with cellular processes. Current research indicates minimal thermal effects at typical operating parameters, but long-term studies on potential non-thermal biological interactions remain limited. Standardized testing protocols specific to spintronic biodevices are being developed by regulatory bodies.
Sterilization compatibility presents unique challenges for spintronic biodevices. Traditional sterilization methods such as autoclaving, gamma irradiation, or ethylene oxide exposure may compromise the delicate magnetic properties essential for device function. Alternative approaches including low-temperature hydrogen peroxide plasma sterilization and UV-C irradiation are being evaluated for efficacy while preserving spintronic functionality.
Regulatory frameworks for spintronic biodevices are still evolving. Current medical device regulations require demonstration of both safety and effectiveness, necessitating comprehensive preclinical and clinical testing. Manufacturers must navigate complex regulatory pathways that may vary significantly between jurisdictions. Collaborative efforts between industry, academia, and regulatory agencies are working to establish standardized evaluation criteria specifically addressing the unique characteristics of spintronic technologies in biomedical applications.
Interdisciplinary Collaboration Opportunities
The convergence of spintronics and bioengineering represents a fertile ground for interdisciplinary collaboration that transcends traditional academic and industry boundaries. These collaborations are essential for addressing the complex challenges at the intersection of these fields and accelerating innovation cycles.
Research institutions specializing in materials science and quantum physics can partner with biomedical engineering departments to develop spintronic biosensors with enhanced sensitivity for early disease detection. Such partnerships enable the sharing of specialized equipment, methodologies, and theoretical frameworks that would be prohibitively expensive for single-discipline teams to maintain independently.
Industry-academia collaborations present another promising avenue, where commercial entities provide funding and application-specific requirements while academic partners contribute fundamental research capabilities. Companies like IBM and Intel, with established expertise in spintronics for computing applications, can collaborate with biotechnology firms to adapt these technologies for medical diagnostics and therapeutic delivery systems.
Cross-sector partnerships between healthcare providers and technology developers create valuable feedback loops for spintronic biodevice development. Clinicians can provide insights into practical implementation challenges and patient needs, while engineers can refine devices to meet real-world medical requirements. These collaborations ensure that technological innovations remain grounded in addressing actual healthcare challenges rather than pursuing technology for its own sake.
International research networks focusing on spintronic bioengineering can leverage complementary strengths across different regions. For instance, European expertise in quantum materials could complement Asian leadership in miniaturization and North American strengths in biomedical applications, creating synergistic research ecosystems that accelerate discovery and commercialization.
Funding agencies and policy makers play a crucial role in fostering these interdisciplinary collaborations through dedicated grant programs and regulatory frameworks that encourage cross-disciplinary research. Initiatives like the EU's Horizon Europe and the NIH's Bioengineering Research Partnerships specifically target projects that bridge multiple disciplines, providing essential financial support for high-risk, high-reward collaborative research.
Educational institutions can contribute by developing interdisciplinary training programs that equip the next generation of researchers with competencies spanning spintronics and bioengineering. These programs should emphasize not only technical skills but also communication abilities necessary for effective collaboration across disciplinary boundaries.
Research institutions specializing in materials science and quantum physics can partner with biomedical engineering departments to develop spintronic biosensors with enhanced sensitivity for early disease detection. Such partnerships enable the sharing of specialized equipment, methodologies, and theoretical frameworks that would be prohibitively expensive for single-discipline teams to maintain independently.
Industry-academia collaborations present another promising avenue, where commercial entities provide funding and application-specific requirements while academic partners contribute fundamental research capabilities. Companies like IBM and Intel, with established expertise in spintronics for computing applications, can collaborate with biotechnology firms to adapt these technologies for medical diagnostics and therapeutic delivery systems.
Cross-sector partnerships between healthcare providers and technology developers create valuable feedback loops for spintronic biodevice development. Clinicians can provide insights into practical implementation challenges and patient needs, while engineers can refine devices to meet real-world medical requirements. These collaborations ensure that technological innovations remain grounded in addressing actual healthcare challenges rather than pursuing technology for its own sake.
International research networks focusing on spintronic bioengineering can leverage complementary strengths across different regions. For instance, European expertise in quantum materials could complement Asian leadership in miniaturization and North American strengths in biomedical applications, creating synergistic research ecosystems that accelerate discovery and commercialization.
Funding agencies and policy makers play a crucial role in fostering these interdisciplinary collaborations through dedicated grant programs and regulatory frameworks that encourage cross-disciplinary research. Initiatives like the EU's Horizon Europe and the NIH's Bioengineering Research Partnerships specifically target projects that bridge multiple disciplines, providing essential financial support for high-risk, high-reward collaborative research.
Educational institutions can contribute by developing interdisciplinary training programs that equip the next generation of researchers with competencies spanning spintronics and bioengineering. These programs should emphasize not only technical skills but also communication abilities necessary for effective collaboration across disciplinary boundaries.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!