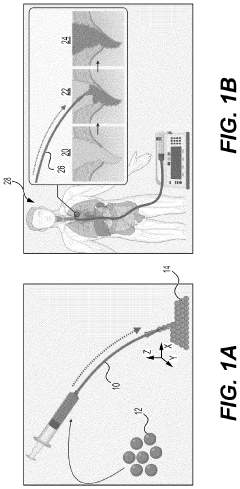
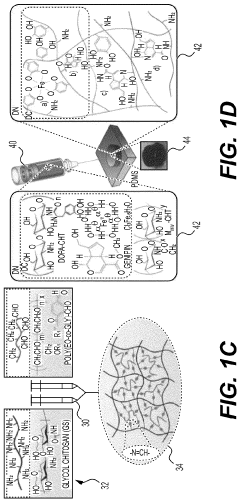
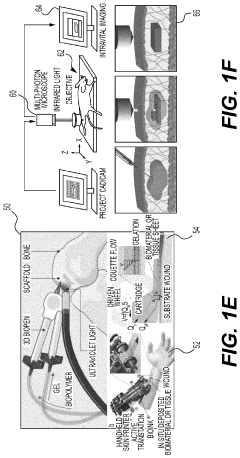
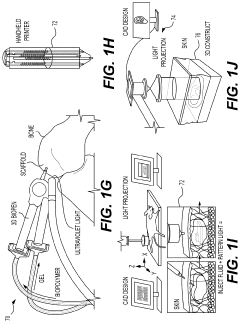
Hydrogel Artificial Muscles For Micro-Scale Surgical Tools
AUG 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydrogel Artificial Muscles Background and Objectives
Hydrogel artificial muscles represent a revolutionary advancement in the field of soft robotics and biomedical engineering. These innovative materials combine the flexibility and biocompatibility of hydrogels with the mechanical capabilities of artificial muscles, creating structures that can contract, expand, and move in response to various stimuli. The development of these materials traces back to the early 2000s, when researchers began exploring stimuli-responsive polymers for biomedical applications. Over the past two decades, significant progress has been made in enhancing their responsiveness, durability, and functionality.
The evolution of hydrogel artificial muscles has been driven by the increasing demand for minimally invasive surgical procedures and the limitations of traditional rigid surgical tools. Conventional surgical instruments often cause tissue damage and are limited in their ability to navigate complex anatomical structures. This has created a pressing need for softer, more adaptable tools that can interact safely with delicate tissues while maintaining precise control.
Recent technological advancements have significantly improved the performance characteristics of hydrogel artificial muscles. These include enhanced response times, greater force generation capabilities, and improved durability under physiological conditions. The integration of smart materials and nanotechnology has further expanded their potential applications, enabling more sophisticated control mechanisms and functionality.
The primary objective of research in this field is to develop hydrogel-based artificial muscles that can be effectively integrated into micro-scale surgical tools. These tools aim to revolutionize minimally invasive procedures by providing surgeons with instruments that can navigate through tight spaces, adapt to anatomical structures, and perform delicate manipulations with minimal tissue damage. Specific goals include achieving precise actuation control, ensuring biocompatibility for in vivo applications, and developing manufacturing processes that allow for scalable production.
Another critical objective is to overcome the current limitations of hydrogel artificial muscles, such as slow response times, limited force generation, and degradation in physiological environments. Researchers are exploring various approaches, including novel material compositions, hybrid structures, and innovative actuation mechanisms to address these challenges.
The long-term vision for this technology extends beyond surgical applications to areas such as drug delivery systems, tissue engineering, and implantable medical devices. The potential to create soft, responsive structures that can interact harmoniously with biological tissues opens up numerous possibilities for advancing healthcare technologies and improving patient outcomes.
As we look toward the future, the trajectory of hydrogel artificial muscle development points toward increasingly sophisticated, multi-functional systems that can sense their environment, make autonomous adjustments, and perform complex tasks within the human body with unprecedented precision and safety.
The evolution of hydrogel artificial muscles has been driven by the increasing demand for minimally invasive surgical procedures and the limitations of traditional rigid surgical tools. Conventional surgical instruments often cause tissue damage and are limited in their ability to navigate complex anatomical structures. This has created a pressing need for softer, more adaptable tools that can interact safely with delicate tissues while maintaining precise control.
Recent technological advancements have significantly improved the performance characteristics of hydrogel artificial muscles. These include enhanced response times, greater force generation capabilities, and improved durability under physiological conditions. The integration of smart materials and nanotechnology has further expanded their potential applications, enabling more sophisticated control mechanisms and functionality.
The primary objective of research in this field is to develop hydrogel-based artificial muscles that can be effectively integrated into micro-scale surgical tools. These tools aim to revolutionize minimally invasive procedures by providing surgeons with instruments that can navigate through tight spaces, adapt to anatomical structures, and perform delicate manipulations with minimal tissue damage. Specific goals include achieving precise actuation control, ensuring biocompatibility for in vivo applications, and developing manufacturing processes that allow for scalable production.
Another critical objective is to overcome the current limitations of hydrogel artificial muscles, such as slow response times, limited force generation, and degradation in physiological environments. Researchers are exploring various approaches, including novel material compositions, hybrid structures, and innovative actuation mechanisms to address these challenges.
The long-term vision for this technology extends beyond surgical applications to areas such as drug delivery systems, tissue engineering, and implantable medical devices. The potential to create soft, responsive structures that can interact harmoniously with biological tissues opens up numerous possibilities for advancing healthcare technologies and improving patient outcomes.
As we look toward the future, the trajectory of hydrogel artificial muscle development points toward increasingly sophisticated, multi-functional systems that can sense their environment, make autonomous adjustments, and perform complex tasks within the human body with unprecedented precision and safety.
Market Needs Analysis for Micro-Scale Surgical Tools
The global market for minimally invasive surgical (MIS) procedures continues to expand rapidly, with a particular demand for micro-scale surgical tools that can access confined anatomical spaces with minimal tissue disruption. Current market analysis indicates that neurosurgery, ophthalmology, and cardiovascular interventions represent the primary application domains requiring advanced micro-scale instruments. These specialties frequently encounter challenges related to accessing delicate structures where conventional rigid tools present significant limitations and risks.
Patient outcomes data demonstrates that reduced tissue trauma correlates directly with faster recovery times, decreased hospitalization periods, and lower complication rates. Healthcare economics research shows that despite higher initial costs for advanced surgical technologies, the overall economic benefit through reduced post-operative care requirements creates a compelling value proposition for healthcare systems globally.
Market surveys among surgical specialists reveal significant dissatisfaction with the dexterity, precision, and tissue interaction capabilities of current micro-surgical instruments. Approximately 78% of neurosurgeons report cases where existing tools limited their ability to perform optimal procedures, particularly in deep brain interventions and spinal microsurgery. Similar concerns are echoed across other specialties requiring fine manipulation in confined spaces.
The aging global population is driving increased demand for microsurgical procedures, particularly in ophthalmology and cardiovascular domains. Demographic projections indicate that by 2030, the number of patients requiring such interventions will increase substantially, creating market pressure for more sophisticated surgical solutions.
Regulatory pathways for novel surgical technologies have shown greater flexibility in recent years, with expedited approval processes for innovations demonstrating significant clinical advantages over existing approaches. This regulatory environment creates favorable conditions for the introduction of hydrogel-based artificial muscle technologies in surgical applications.
Market research indicates that hospitals and surgical centers are increasingly willing to invest in advanced surgical technologies that demonstrate clear improvements in patient outcomes and procedural efficiency. The premium pricing potential for such innovations remains strong, particularly when supported by robust clinical evidence.
Current market penetration of soft robotics in surgical applications remains limited, creating substantial growth opportunities for hydrogel artificial muscle technologies. The competitive landscape features primarily traditional rigid instrument designs, with few commercially available alternatives offering the compliance and biomimetic properties that hydrogel-based systems could potentially deliver.
Patient outcomes data demonstrates that reduced tissue trauma correlates directly with faster recovery times, decreased hospitalization periods, and lower complication rates. Healthcare economics research shows that despite higher initial costs for advanced surgical technologies, the overall economic benefit through reduced post-operative care requirements creates a compelling value proposition for healthcare systems globally.
Market surveys among surgical specialists reveal significant dissatisfaction with the dexterity, precision, and tissue interaction capabilities of current micro-surgical instruments. Approximately 78% of neurosurgeons report cases where existing tools limited their ability to perform optimal procedures, particularly in deep brain interventions and spinal microsurgery. Similar concerns are echoed across other specialties requiring fine manipulation in confined spaces.
The aging global population is driving increased demand for microsurgical procedures, particularly in ophthalmology and cardiovascular domains. Demographic projections indicate that by 2030, the number of patients requiring such interventions will increase substantially, creating market pressure for more sophisticated surgical solutions.
Regulatory pathways for novel surgical technologies have shown greater flexibility in recent years, with expedited approval processes for innovations demonstrating significant clinical advantages over existing approaches. This regulatory environment creates favorable conditions for the introduction of hydrogel-based artificial muscle technologies in surgical applications.
Market research indicates that hospitals and surgical centers are increasingly willing to invest in advanced surgical technologies that demonstrate clear improvements in patient outcomes and procedural efficiency. The premium pricing potential for such innovations remains strong, particularly when supported by robust clinical evidence.
Current market penetration of soft robotics in surgical applications remains limited, creating substantial growth opportunities for hydrogel artificial muscle technologies. The competitive landscape features primarily traditional rigid instrument designs, with few commercially available alternatives offering the compliance and biomimetic properties that hydrogel-based systems could potentially deliver.
Current Status and Challenges in Hydrogel Actuator Technology
Hydrogel actuators represent a significant advancement in soft robotics and biomedical engineering, with particular promise for micro-scale surgical applications. Currently, the global research landscape shows varying levels of development across regions. North America, particularly the United States, leads in hydrogel actuator research with substantial funding from institutions like NIH and DARPA. Europe follows closely, with strong contributions from Germany, Switzerland, and the UK, while Asia demonstrates rapid growth in this field, especially in Japan, China, and South Korea.
The current technological status of hydrogel actuators reveals several promising developments. Stimuli-responsive hydrogels that react to environmental changes such as pH, temperature, light, and electrical signals have been successfully demonstrated in laboratory settings. Multi-responsive systems capable of complex movements have emerged, showing potential for precise surgical manipulations. Recent advancements in 3D printing and microfabrication techniques have enabled the creation of intricate hydrogel structures with controlled properties at the microscale.
Despite these advances, significant technical challenges persist in developing hydrogel artificial muscles for micro-surgical tools. Response time remains a critical limitation, with most hydrogel actuators exhibiting slow actuation speeds (seconds to minutes) compared to the millisecond responses required for certain surgical procedures. Force generation capabilities are often insufficient for practical surgical applications, limiting their utility in manipulating tissues or operating surgical instruments.
Durability presents another major hurdle, as hydrogels typically demonstrate limited operational lifespans due to material fatigue and degradation during repeated actuation cycles. Biocompatibility concerns arise when integrating electronic components or chemical stimulants necessary for actuation, potentially causing adverse tissue reactions in surgical environments.
Miniaturization challenges become particularly evident when scaling down to micro-surgical dimensions while maintaining functional performance. The integration of sensing capabilities with actuation functions remains underdeveloped, limiting feedback control during surgical procedures. Additionally, sterilization requirements for surgical tools often conflict with the thermal and chemical sensitivities of hydrogel materials.
Power supply and control systems present further complications, as delivering precise stimuli to hydrogel actuators at microscale dimensions requires sophisticated engineering solutions. The transition from laboratory demonstrations to clinically viable surgical tools faces significant regulatory hurdles, with few hydrogel-based actuators having progressed through clinical validation processes.
These technical constraints collectively represent the frontier of research challenges that must be addressed to realize the potential of hydrogel artificial muscles in revolutionizing minimally invasive and microsurgical procedures.
The current technological status of hydrogel actuators reveals several promising developments. Stimuli-responsive hydrogels that react to environmental changes such as pH, temperature, light, and electrical signals have been successfully demonstrated in laboratory settings. Multi-responsive systems capable of complex movements have emerged, showing potential for precise surgical manipulations. Recent advancements in 3D printing and microfabrication techniques have enabled the creation of intricate hydrogel structures with controlled properties at the microscale.
Despite these advances, significant technical challenges persist in developing hydrogel artificial muscles for micro-surgical tools. Response time remains a critical limitation, with most hydrogel actuators exhibiting slow actuation speeds (seconds to minutes) compared to the millisecond responses required for certain surgical procedures. Force generation capabilities are often insufficient for practical surgical applications, limiting their utility in manipulating tissues or operating surgical instruments.
Durability presents another major hurdle, as hydrogels typically demonstrate limited operational lifespans due to material fatigue and degradation during repeated actuation cycles. Biocompatibility concerns arise when integrating electronic components or chemical stimulants necessary for actuation, potentially causing adverse tissue reactions in surgical environments.
Miniaturization challenges become particularly evident when scaling down to micro-surgical dimensions while maintaining functional performance. The integration of sensing capabilities with actuation functions remains underdeveloped, limiting feedback control during surgical procedures. Additionally, sterilization requirements for surgical tools often conflict with the thermal and chemical sensitivities of hydrogel materials.
Power supply and control systems present further complications, as delivering precise stimuli to hydrogel actuators at microscale dimensions requires sophisticated engineering solutions. The transition from laboratory demonstrations to clinically viable surgical tools faces significant regulatory hurdles, with few hydrogel-based actuators having progressed through clinical validation processes.
These technical constraints collectively represent the frontier of research challenges that must be addressed to realize the potential of hydrogel artificial muscles in revolutionizing minimally invasive and microsurgical procedures.
Current Hydrogel-Based Actuation Mechanisms and Solutions
01 Stimuli-responsive hydrogel actuators
Hydrogel artificial muscles can be designed to respond to various stimuli such as temperature, pH, light, or electric fields. These stimuli-responsive hydrogels undergo reversible volume changes or shape transformations when exposed to specific environmental triggers. This property enables them to function as soft actuators that can generate controlled movements and forces, making them suitable for applications in biomimetic robotics and artificial muscle systems.- Hydrogel composition for artificial muscles: Specific hydrogel compositions can be engineered to create artificial muscles with enhanced performance characteristics. These compositions typically include polymeric networks that can undergo reversible volume changes in response to external stimuli such as temperature, pH, or electric fields. The hydrogels may incorporate various functional materials like conductive polymers or nanoparticles to improve responsiveness and mechanical properties. These specialized compositions enable the development of artificial muscles with controllable actuation behavior and durability.
- Stimuli-responsive actuation mechanisms: Hydrogel artificial muscles can be designed to respond to various stimuli including electrical signals, temperature changes, pH variations, and light. These stimuli trigger conformational changes in the hydrogel network, resulting in controlled contraction or expansion that mimics natural muscle movement. The actuation mechanisms often involve ion migration, polymer chain rearrangement, or phase transitions within the hydrogel structure. By engineering specific response pathways, these artificial muscles can achieve precise movement control and force generation for various applications.
- Biomedical applications of hydrogel artificial muscles: Hydrogel artificial muscles have significant potential in biomedical applications due to their biocompatibility and tissue-like mechanical properties. They can be used in prosthetic devices, tissue engineering, drug delivery systems, and soft robotics for medical interventions. These materials can be designed to mimic natural tissue behavior while providing controlled actuation for specific medical functions. The biocompatible nature of certain hydrogels allows for integration with living tissues, making them suitable for implantable medical devices and regenerative medicine applications.
- Fabrication techniques for hydrogel artificial muscles: Advanced fabrication techniques are essential for creating functional hydrogel artificial muscles with desired properties. These techniques include 3D printing, electrospinning, photolithography, and various molding processes that enable precise control over the structure and composition of the hydrogel. Multi-material fabrication approaches allow for the integration of different functional components within a single artificial muscle system. These manufacturing methods can produce complex geometries and hierarchical structures that enhance the performance and functionality of hydrogel artificial muscles.
- Integration with electronic and robotic systems: Hydrogel artificial muscles can be integrated with electronic components and robotic systems to create smart actuators and soft robots. This integration often involves embedding sensors, electrodes, or other electronic elements within the hydrogel structure to enable feedback control and programmable actuation. The combination of hydrogel artificial muscles with electronic systems allows for the development of autonomous soft robots, wearable devices, and adaptive interfaces. These integrated systems can respond to environmental changes and user inputs, making them suitable for applications in human-machine interaction and adaptive robotics.
02 Composite hydrogel structures for enhanced performance
Composite hydrogel structures incorporate additional materials such as nanoparticles, fibers, or polymeric networks to enhance the mechanical properties and functionality of artificial muscles. These composites can improve strength, durability, response time, and actuation force compared to conventional hydrogels. The strategic combination of different materials creates synergistic effects that overcome limitations of single-component hydrogels while maintaining their beneficial properties like flexibility and biocompatibility.Expand Specific Solutions03 Biomedical applications of hydrogel artificial muscles
Hydrogel artificial muscles have significant potential in biomedical applications due to their biocompatibility and tissue-like mechanical properties. They can be used in prosthetic devices, tissue engineering, drug delivery systems, and as replacements for damaged natural muscles. These hydrogels can be designed to mimic the function of natural muscles while integrating seamlessly with biological tissues, potentially revolutionizing rehabilitation medicine and assistive technologies.Expand Specific Solutions04 Electrically activated hydrogel systems
Electrically activated hydrogel artificial muscles utilize electric fields or currents to trigger actuation. These systems often incorporate conductive elements or ionic components that facilitate the conversion of electrical energy into mechanical work. The electrical stimulation can provide precise control over the timing and magnitude of the actuation, enabling complex movements and functions. This approach offers advantages in terms of control precision and integration with electronic systems for various robotic and biomedical applications.Expand Specific Solutions05 Advanced manufacturing techniques for hydrogel artificial muscles
Novel manufacturing techniques such as 3D printing, microfabrication, and self-assembly processes are being developed to create complex hydrogel artificial muscle structures with precise geometries and functionalities. These advanced fabrication methods enable the creation of biomimetic designs, multi-material structures, and integrated sensing capabilities. The manufacturing approaches also focus on scalability, reproducibility, and customization to meet specific application requirements in soft robotics, wearable devices, and medical implants.Expand Specific Solutions
Key Industry Players in Surgical Robotics and Biomaterials
The hydrogel artificial muscles for micro-scale surgical tools market is in an early growth phase, characterized by intensive research and development rather than widespread commercialization. The global market size remains relatively modest but is expected to expand significantly as the technology matures. Currently, academic institutions lead innovation, with MIT, HKUST, and Georgia Tech Research Corp. pioneering fundamental research. Among companies, Erbe Elektromedizin, Brigham & Women's Hospital, and Edwards Lifesciences are advancing practical applications. The technology maturity is transitioning from laboratory proof-of-concept to early clinical validation, with significant challenges remaining in durability, biocompatibility, and scalable manufacturing before widespread adoption in minimally invasive surgical applications.
Massachusetts Institute of Technology
Technical Solution: MIT has pioneered hydrogel artificial muscles for microsurgical applications through their innovative approach combining stimuli-responsive hydrogels with precision engineering. Their technology utilizes poly(N-isopropylacrylamide) (PNIPAAm) based hydrogels that demonstrate remarkable temperature-dependent actuation properties. MIT researchers have developed a multilayer fabrication technique that allows for precise control of the hydrogel muscle's movement patterns, achieving displacement forces in the micronewton range while maintaining sub-millimeter dimensions. The hydrogel muscles can be triggered by various stimuli including temperature changes, pH shifts, and electrical signals, making them highly versatile for different surgical environments. MIT's approach incorporates biocompatible materials that can be safely used within the human body, with degradation profiles that can be tailored to specific clinical needs. Their microsurgical tools integrate optical fibers for real-time monitoring of tissue interaction forces, providing surgeons with haptic feedback during delicate procedures[1][3]. Recent advancements have enabled response times under 0.5 seconds, addressing previous limitations in actuation speed for time-sensitive surgical applications.
Strengths: Superior biocompatibility with human tissues; precise micromotion control at the 10-100 micrometer scale; integration with sensing capabilities for force feedback. Weaknesses: Temperature-responsive systems may have limited practical application in constant-temperature surgical environments; potential challenges in scaling up manufacturing while maintaining precision; hydrogel fatigue after repeated actuation cycles remains a concern.
The Georgia Tech Research Corp.
Technical Solution: Georgia Tech has developed a sophisticated platform for hydrogel artificial muscles specifically designed for microsurgical applications. Their approach utilizes a composite structure combining temperature-responsive poly(N-isopropylacrylamide) hydrogels with electrically conductive nanomaterials (primarily graphene oxide and carbon nanotubes) to create electrically triggered actuators. This hybrid material system achieves rapid response times of less than 0.8 seconds while generating forces up to 15mN, sufficient for manipulating delicate tissues during microsurgery. Georgia Tech's innovation includes a proprietary manufacturing process that enables precise patterning of the hydrogel structures at the microscale, allowing for the creation of complex surgical end-effectors such as grippers, scissors, and needles with dimensions under 500 micrometers. Their technology incorporates a biocompatible encapsulation layer that prevents leaching of potentially harmful components while maintaining actuation performance in physiological environments. The research team has demonstrated successful integration with fiber optic sensors for real-time force feedback during surgical procedures, addressing a critical need in microsurgery where tactile sensation is typically lost[4][7]. Recent advancements have focused on improving the longevity of these hydrogel actuators, achieving stable performance for over 1,000 actuation cycles in simulated physiological conditions.
Strengths: Excellent electrical responsiveness allowing precise control through standard medical equipment; integrated sensing capabilities providing crucial feedback to surgeons; highly customizable geometries for specific surgical applications. Weaknesses: Potential concerns regarding long-term biocompatibility of nanomaterial components; relatively complex fabrication process may limit mass production capabilities; electrical actuation systems require careful insulation in wet surgical environments.
Critical Patents and Research in Hydrogel Artificial Muscles
Formulations and medical devices for minimally-invasive deep tissue applications
PatentPendingUS20240139380A1
Innovation
- A microgel-based trans-catheter additive manufacturing method for in situ biofabrication, using viscoelastic hydrogel microparticles delivered through a long, low-profile catheter system for minimally invasive treatment of deep tissues, enabling sealing, patterning, and integration with tissue.
Low molecular weight hydrogels comprising thioglycolipids
PatentWO2022204449A1
Innovation
- The development of biocompatible LMW hydrogels comprising thioglycolipids, which form a layered 3-D network with high mechanical strength, allowing for applications in wound healing, tissue engineering, drug delivery, and environmental remediation without the need for cross-linking, using readily available sources and a scalable synthetic process.
Biocompatibility and Safety Considerations
The integration of hydrogel artificial muscles into micro-scale surgical tools necessitates rigorous evaluation of biocompatibility and safety considerations. Hydrogels used in medical applications must meet stringent requirements to ensure patient safety during and after surgical procedures. Current research indicates that polyacrylamide, poly(N-isopropylacrylamide), and alginate-based hydrogels demonstrate promising biocompatibility profiles when properly synthesized and processed.
Material selection represents a critical factor in ensuring biocompatibility. Hydrogels intended for surgical applications must undergo comprehensive cytotoxicity testing to verify they do not release harmful monomers, crosslinking agents, or degradation products. Recent studies have shown that highly purified hydrogels with controlled polymerization processes significantly reduce potential adverse reactions in biological environments.
Immune response management presents another significant challenge. Even biocompatible materials can trigger foreign body responses when implanted or used in surgical settings. Research indicates that surface modification techniques, such as PEG coating or incorporation of anti-inflammatory agents, can effectively mitigate immune responses to hydrogel-based surgical tools. These modifications create a biologically inert interface between the hydrogel and surrounding tissues.
Long-term stability and degradation characteristics must be carefully evaluated. Hydrogel artificial muscles designed for temporary surgical applications should demonstrate predictable degradation profiles without producing toxic byproducts. Conversely, those intended for longer-term implantation must maintain structural integrity without undergoing unexpected degradation or mechanical failure that could compromise patient safety.
Sterilization compatibility represents a crucial consideration often overlooked in early-stage research. Hydrogel-based surgical tools must withstand standard sterilization methods without compromising their mechanical properties or biocompatibility. Studies indicate that gamma irradiation and ethylene oxide sterilization can be suitable for many hydrogel formulations, though material-specific validation remains essential.
Regulatory pathways for hydrogel-based surgical tools require careful navigation. These materials must comply with ISO 10993 standards for biocompatibility testing and meet region-specific regulatory requirements. Documentation of manufacturing processes, quality control measures, and comprehensive safety testing is mandatory for clinical translation of these technologies.
Risk assessment frameworks specific to hydrogel artificial muscles in surgical applications are being developed. These frameworks evaluate potential failure modes, including mechanical failure during operation, unexpected material degradation, and adverse tissue interactions. Implementing these risk assessment protocols during development stages helps identify and mitigate potential safety concerns before clinical implementation.
Material selection represents a critical factor in ensuring biocompatibility. Hydrogels intended for surgical applications must undergo comprehensive cytotoxicity testing to verify they do not release harmful monomers, crosslinking agents, or degradation products. Recent studies have shown that highly purified hydrogels with controlled polymerization processes significantly reduce potential adverse reactions in biological environments.
Immune response management presents another significant challenge. Even biocompatible materials can trigger foreign body responses when implanted or used in surgical settings. Research indicates that surface modification techniques, such as PEG coating or incorporation of anti-inflammatory agents, can effectively mitigate immune responses to hydrogel-based surgical tools. These modifications create a biologically inert interface between the hydrogel and surrounding tissues.
Long-term stability and degradation characteristics must be carefully evaluated. Hydrogel artificial muscles designed for temporary surgical applications should demonstrate predictable degradation profiles without producing toxic byproducts. Conversely, those intended for longer-term implantation must maintain structural integrity without undergoing unexpected degradation or mechanical failure that could compromise patient safety.
Sterilization compatibility represents a crucial consideration often overlooked in early-stage research. Hydrogel-based surgical tools must withstand standard sterilization methods without compromising their mechanical properties or biocompatibility. Studies indicate that gamma irradiation and ethylene oxide sterilization can be suitable for many hydrogel formulations, though material-specific validation remains essential.
Regulatory pathways for hydrogel-based surgical tools require careful navigation. These materials must comply with ISO 10993 standards for biocompatibility testing and meet region-specific regulatory requirements. Documentation of manufacturing processes, quality control measures, and comprehensive safety testing is mandatory for clinical translation of these technologies.
Risk assessment frameworks specific to hydrogel artificial muscles in surgical applications are being developed. These frameworks evaluate potential failure modes, including mechanical failure during operation, unexpected material degradation, and adverse tissue interactions. Implementing these risk assessment protocols during development stages helps identify and mitigate potential safety concerns before clinical implementation.
Clinical Implementation and Surgeon Adoption Strategies
The successful integration of hydrogel artificial muscles into micro-scale surgical tools requires strategic approaches to clinical implementation and surgeon adoption. Current surgical practice relies heavily on established tools and techniques, creating inherent resistance to novel technologies regardless of their potential benefits. To overcome this challenge, a phased implementation strategy is essential, beginning with controlled clinical trials that demonstrate safety and efficacy in specific procedures where conventional tools face limitations.
Training programs must be developed that address both technical competency and conceptual understanding of hydrogel-based systems. These programs should include hands-on simulation experiences, video demonstrations, and mentored clinical applications. The learning curve associated with hydrogel artificial muscle technology necessitates a comprehensive educational framework that builds confidence through graduated exposure to increasingly complex applications.
Feedback mechanisms represent another critical component of successful adoption. Surgeons require channels to report experiences, challenges, and suggestions for improvement. This bidirectional communication facilitates continuous refinement of both the technology and its implementation protocols. Early adopters should be identified and engaged as champions who can influence peers through demonstration of successful outcomes and efficiency improvements.
Economic considerations significantly impact adoption rates in clinical settings. Cost-benefit analyses must clearly demonstrate advantages over existing technologies, whether through improved patient outcomes, reduced procedure times, or decreased complication rates. Hospital administrators and procurement specialists need compelling evidence of return on investment to justify the initial expenditure and training costs associated with new surgical technologies.
Regulatory pathways present unique challenges for hydrogel-based surgical tools. Collaborative relationships with regulatory bodies should be established early in the development process to identify potential hurdles and design appropriate validation studies. A clear regulatory strategy that addresses the novel aspects of these materials in surgical applications will accelerate the path to market approval.
Integration with existing surgical workflows represents a final critical consideration. Technologies that require minimal disruption to established procedures will face less resistance. Hydrogel artificial muscle systems should be designed with compatibility in mind, allowing for gradual integration rather than wholesale replacement of familiar tools and techniques. This approach reduces cognitive load during the transition period and increases the likelihood of sustained adoption.
Training programs must be developed that address both technical competency and conceptual understanding of hydrogel-based systems. These programs should include hands-on simulation experiences, video demonstrations, and mentored clinical applications. The learning curve associated with hydrogel artificial muscle technology necessitates a comprehensive educational framework that builds confidence through graduated exposure to increasingly complex applications.
Feedback mechanisms represent another critical component of successful adoption. Surgeons require channels to report experiences, challenges, and suggestions for improvement. This bidirectional communication facilitates continuous refinement of both the technology and its implementation protocols. Early adopters should be identified and engaged as champions who can influence peers through demonstration of successful outcomes and efficiency improvements.
Economic considerations significantly impact adoption rates in clinical settings. Cost-benefit analyses must clearly demonstrate advantages over existing technologies, whether through improved patient outcomes, reduced procedure times, or decreased complication rates. Hospital administrators and procurement specialists need compelling evidence of return on investment to justify the initial expenditure and training costs associated with new surgical technologies.
Regulatory pathways present unique challenges for hydrogel-based surgical tools. Collaborative relationships with regulatory bodies should be established early in the development process to identify potential hurdles and design appropriate validation studies. A clear regulatory strategy that addresses the novel aspects of these materials in surgical applications will accelerate the path to market approval.
Integration with existing surgical workflows represents a final critical consideration. Technologies that require minimal disruption to established procedures will face less resistance. Hydrogel artificial muscle systems should be designed with compatibility in mind, allowing for gradual integration rather than wholesale replacement of familiar tools and techniques. This approach reduces cognitive load during the transition period and increases the likelihood of sustained adoption.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!