Quasicrystal Integration in Medical Device Innovation
Quasicrystal Med Tech Background and Objectives
Quasicrystals, discovered in 1982 by Dan Shechtman, represent a unique class of materials that exhibit long-range order but lack periodicity. This groundbreaking discovery challenged the conventional understanding of crystalline structures and opened up new avenues for materials science and engineering. In the context of medical device innovation, quasicrystals have emerged as a promising frontier due to their exceptional properties and potential applications.
The integration of quasicrystals in medical devices aims to leverage their unique characteristics, such as high hardness, low friction coefficients, and superior corrosion resistance. These properties make quasicrystals particularly attractive for applications in implantable devices, surgical instruments, and diagnostic equipment. The primary objective of this research is to explore and develop novel medical technologies that harness the advantages of quasicrystalline materials to enhance patient outcomes and device performance.
The evolution of quasicrystal technology in the medical field has been marked by significant milestones. Initial research focused on understanding the fundamental properties of quasicrystals and their potential biocompatibility. Subsequent developments have led to the exploration of quasicrystalline coatings for medical implants, aiming to improve wear resistance and reduce bacterial adhesion. Recent advancements have also investigated the use of quasicrystals in drug delivery systems and biosensors.
Current research objectives in this field encompass several key areas. Firstly, there is a focus on optimizing the synthesis and processing of quasicrystalline materials for medical applications, ensuring consistency and scalability. Secondly, efforts are directed towards enhancing the biocompatibility of quasicrystals through surface modifications and compositional adjustments. Thirdly, researchers aim to develop innovative medical devices that fully exploit the unique properties of quasicrystals, such as self-cleaning surfaces for medical equipment or advanced imaging technologies.
The integration of quasicrystals in medical devices also aligns with broader trends in healthcare technology, including the push for miniaturization, increased durability, and improved functionality of medical devices. As such, this research not only contributes to advancing materials science but also has the potential to significantly impact patient care and medical practice.
Looking ahead, the field of quasicrystal integration in medical devices faces both challenges and opportunities. Overcoming manufacturing complexities, ensuring long-term stability in biological environments, and navigating regulatory pathways are key hurdles to be addressed. However, the potential for groundbreaking innovations in areas such as targeted drug delivery, advanced prosthetics, and next-generation diagnostic tools underscores the importance of continued research and development in this exciting field.
Market Analysis for Quasicrystal-Enhanced Medical Devices
The integration of quasicrystals in medical devices represents a significant opportunity for market expansion and innovation in the healthcare industry. The unique properties of quasicrystals, including their exceptional hardness, low friction, and resistance to wear and corrosion, make them particularly attractive for various medical applications. The market for quasicrystal-enhanced medical devices is expected to grow substantially in the coming years, driven by increasing demand for more durable and efficient medical equipment.
One of the primary areas of potential growth is in orthopedic implants. The superior wear resistance and biocompatibility of quasicrystals could lead to longer-lasting joint replacements and other implantable devices. This is particularly relevant given the aging population in many developed countries and the consequent rise in degenerative joint diseases. The market for orthopedic implants is projected to expand significantly, with quasicrystal-enhanced products potentially capturing a substantial share.
Surgical instruments represent another promising market segment for quasicrystal integration. The hardness and low friction properties of quasicrystals could result in sharper, more durable cutting tools and instruments that maintain their edge for longer periods. This would not only improve surgical outcomes but also reduce the frequency of instrument replacement, leading to cost savings for healthcare providers.
In the field of medical imaging, quasicrystals show potential for enhancing the performance of various devices. Their unique atomic structure could be leveraged to improve the resolution and sensitivity of imaging equipment, potentially leading to earlier and more accurate diagnoses. This could be particularly impactful in the rapidly growing market for advanced medical imaging technologies.
The dental industry also presents significant opportunities for quasicrystal-enhanced devices. From dental implants to cutting and polishing tools, the wear resistance and biocompatibility of quasicrystals could lead to improved products with longer lifespans. As the global dental care market continues to expand, driven by increasing awareness of oral health and cosmetic dentistry, quasicrystal-based innovations could capture a growing market share.
However, the market adoption of quasicrystal-enhanced medical devices faces several challenges. The relatively high cost of production and the need for specialized manufacturing processes may initially limit widespread adoption. Additionally, regulatory hurdles and the need for extensive clinical trials to prove long-term safety and efficacy could slow market penetration.
Despite these challenges, the potential benefits of quasicrystal integration in medical devices are likely to drive continued research and development efforts. As manufacturing processes improve and costs decrease, the market for quasicrystal-enhanced medical devices is expected to expand rapidly. This growth will be further supported by the increasing focus on personalized medicine and the demand for more advanced, durable, and efficient medical technologies.
Current Challenges in Quasicrystal Medical Integration
The integration of quasicrystals into medical devices presents several significant challenges that researchers and engineers must overcome. One of the primary obstacles is the complex nature of quasicrystal structures, which exhibit long-range order but lack periodicity. This unique arrangement makes it difficult to predict and control their properties in medical applications, particularly when interfacing with biological systems.
Manufacturing quasicrystals with consistent quality and reproducibility for medical devices remains a substantial hurdle. The precise conditions required for quasicrystal formation are often difficult to maintain at scale, leading to potential variations in structure and performance. This inconsistency poses risks in medical applications where reliability and predictability are paramount.
Biocompatibility is another critical challenge in quasicrystal integration. While some quasicrystals have shown promising biocompatible properties, extensive research is still needed to fully understand their long-term effects on living tissues. The potential for ion release and surface degradation in physiological environments must be thoroughly investigated to ensure patient safety.
The mechanical properties of quasicrystals, while advantageous in some aspects, can also present challenges. Their inherent brittleness may limit their use in load-bearing medical devices or applications requiring flexibility. Developing composite materials or surface treatments to mitigate these limitations without compromising the unique properties of quasicrystals is an ongoing area of research.
Regulatory approval poses a significant hurdle for quasicrystal-based medical devices. The novelty of these materials in medical applications means that regulatory bodies may require extensive testing and validation processes. Establishing standardized protocols for evaluating the safety and efficacy of quasicrystal-integrated devices is crucial for their widespread adoption in the medical field.
Cost-effective production of quasicrystals suitable for medical applications remains a challenge. Current manufacturing processes can be expensive and energy-intensive, potentially limiting the commercial viability of quasicrystal-based medical devices. Developing more efficient production methods is essential for making these innovative materials accessible for widespread medical use.
Lastly, the integration of quasicrystals with existing medical technologies and materials presents technical challenges. Ensuring compatibility with sterilization processes, imaging techniques, and other standard medical procedures is crucial. Additionally, developing effective methods for bonding quasicrystals to other materials commonly used in medical devices without compromising their unique properties is an area requiring further research and development.
Existing Quasicrystal Integration Solutions
01 Synthesis and production of quasicrystals
Methods for synthesizing and producing quasicrystalline materials, including techniques for controlling composition, structure, and growth conditions. This may involve specific alloy combinations, rapid solidification processes, or other novel manufacturing approaches to create stable quasicrystalline structures.- Synthesis and production of quasicrystals: Various methods for synthesizing and producing quasicrystals, including rapid solidification techniques, melt spinning, and vapor deposition. These processes involve precise control of composition, temperature, and cooling rates to achieve the unique atomic structure of quasicrystals.
- Applications of quasicrystals in materials science: Quasicrystals have unique properties that make them valuable in various applications, such as coatings for improved wear resistance, thermal barriers, and non-stick surfaces. They are also used in composite materials to enhance strength and reduce friction.
- Optical properties and photonic applications of quasicrystals: Quasicrystals exhibit interesting optical properties due to their aperiodic structure. They can be used in photonic devices, such as lasers, optical filters, and light-emitting diodes. Their unique structure allows for the manipulation of light in ways not possible with traditional crystalline materials.
- Characterization and analysis of quasicrystalline structures: Advanced techniques for characterizing and analyzing the complex structure of quasicrystals, including electron microscopy, X-ray diffraction, and computational modeling. These methods help in understanding the unique atomic arrangements and properties of quasicrystalline materials.
- Novel quasicrystalline alloys and compositions: Development of new quasicrystalline alloys and compositions with improved properties or specific functionalities. This includes the exploration of different elemental combinations and the fine-tuning of composition to achieve desired characteristics for various applications.
02 Applications of quasicrystals in coatings and surface treatments
Utilization of quasicrystalline materials in coatings and surface treatments to enhance properties such as wear resistance, low friction, and corrosion protection. This includes methods for applying quasicrystalline coatings to various substrates and their use in specific industrial applications.Expand Specific Solutions03 Quasicrystal-based composites and alloys
Development of composite materials and alloys incorporating quasicrystalline phases to achieve unique combinations of properties. This may include metal matrix composites, reinforced polymers, or novel alloy systems designed to leverage the characteristics of quasicrystals.Expand Specific Solutions04 Optical and electromagnetic properties of quasicrystals
Exploration and exploitation of the unique optical and electromagnetic properties of quasicrystals, including their potential use in photonic devices, electromagnetic shielding, or novel optical components. This may involve the design of quasicrystalline structures for specific wavelengths or frequencies.Expand Specific Solutions05 Characterization and analysis techniques for quasicrystals
Advanced methods for characterizing and analyzing quasicrystalline structures, including electron microscopy, X-ray diffraction, and computational modeling techniques. This encompasses tools and processes for studying the unique structural and compositional features of quasicrystals.Expand Specific Solutions
Key Players in Quasicrystal Medical Research
The integration of quasicrystals in medical device innovation is an emerging field in its early stages of development. The market size is relatively small but growing, driven by the potential for enhanced biocompatibility and unique material properties. The technology maturity is still low, with research primarily conducted by academic institutions such as the University of Pennsylvania, Rutgers State University, and the University of California. However, major medical device companies like Medtronic, Boston Scientific, and Abbott Cardiovascular Systems are showing interest, indicating potential for future commercialization. Collaborations between universities and industry players, such as Becton, Dickinson & Co. and Koninklijke Philips NV, are likely to accelerate technological advancements and market adoption in the coming years.
Becton, Dickinson & Co.
Boston Scientific Ltd.
Breakthrough Quasicrystal Medical Applications
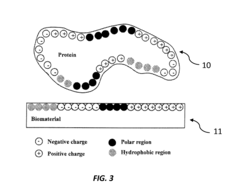
- The development of medical implants with non-equilibrium surface structures created by exposing crystalline materials to high thermal energy or shock, resulting in a non-equilibrium concentration of crystal lattice defects that enhance protein adsorption and osseointegration.
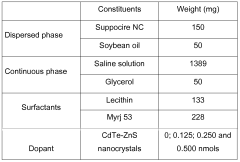
- A nano-emulsion formulation with a continuous aqueous phase and a dispersed oily phase, containing amphiphilic lipids, solubilizing lipids, and cosurfactants, is developed to stabilize nanocrystals, enhance biocompatibility, and facilitate targeted biodistribution by encapsulating them in a stable and stealthy manner.
Regulatory Framework for Quasicrystal Medical Devices
The integration of quasicrystals in medical devices presents a unique regulatory challenge due to their novel properties and potential applications. As these materials gain traction in medical innovation, regulatory bodies worldwide are grappling with the need to establish comprehensive frameworks to ensure safety and efficacy while fostering innovation.
In the United States, the Food and Drug Administration (FDA) is at the forefront of developing guidelines for quasicrystal-based medical devices. The FDA's approach involves a risk-based classification system, where devices incorporating quasicrystals are evaluated based on their intended use and potential risks. Class I devices with low risk may follow a more streamlined regulatory pathway, while Class III devices with higher risk undergo rigorous premarket approval processes.
The European Union, through its Medical Device Regulation (MDR), has implemented a similar risk-based approach. The MDR emphasizes the importance of clinical evidence and post-market surveillance for novel materials like quasicrystals. Manufacturers must demonstrate compliance with essential requirements, including biocompatibility and long-term stability of quasicrystal components.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) has initiated collaborative efforts with academic institutions to develop specific guidelines for quasicrystal medical devices. These guidelines focus on characterization methods, stability assessments, and biocompatibility testing protocols tailored to the unique properties of quasicrystals.
International harmonization efforts, led by the International Medical Device Regulators Forum (IMDRF), aim to establish global consensus on regulatory requirements for quasicrystal-based medical devices. These efforts include developing standardized testing methodologies and safety evaluation criteria specific to quasicrystalline materials.
Key regulatory considerations for quasicrystal medical devices include material characterization, manufacturing process validation, and long-term stability assessment. Regulatory bodies are particularly focused on understanding the potential for structural changes in quasicrystals under physiological conditions and their impact on device performance and safety.
As the field evolves, regulatory frameworks are expected to adapt to accommodate emerging applications of quasicrystals in medical devices. This may include the development of specialized guidance documents, the establishment of expert panels, and the implementation of accelerated review pathways for breakthrough quasicrystal technologies.
Manufacturers and researchers working with quasicrystal-based medical devices must navigate this complex regulatory landscape by engaging early with regulatory authorities, conducting thorough risk assessments, and generating robust scientific evidence to support their products' safety and efficacy claims.
Biocompatibility and Safety Considerations
The integration of quasicrystals in medical device innovation necessitates a thorough examination of biocompatibility and safety considerations. Quasicrystals, with their unique atomic structure and properties, present both opportunities and challenges in medical applications. The primary concern is ensuring that these materials do not elicit adverse biological responses when in contact with human tissues and fluids.
Biocompatibility testing for quasicrystal-based medical devices must adhere to stringent regulatory standards, such as ISO 10993. This involves a comprehensive series of tests, including cytotoxicity, sensitization, irritation, and systemic toxicity evaluations. The unique surface properties of quasicrystals, particularly their potential for reduced bacterial adhesion, may offer advantages in terms of infection control. However, this must be balanced against the possibility of nanoparticle release and its impact on cellular interactions.
Safety considerations extend beyond immediate biological responses to long-term effects. The stability of quasicrystalline structures in physiological environments is a critical factor. Researchers must investigate the potential for degradation or corrosion of quasicrystal-based materials when exposed to bodily fluids over extended periods. This includes assessing the release of constituent elements and their potential accumulation in organs or tissues.
The mechanical properties of quasicrystals, such as their hardness and wear resistance, may contribute to the durability of medical devices. However, these same properties could pose risks if particulate matter is generated through wear or fragmentation. Careful evaluation of particle size distribution and potential biological effects of such debris is essential.
Immunological responses to quasicrystal-based materials represent another critical area of investigation. While some studies suggest that certain quasicrystalline alloys may exhibit reduced foreign body responses compared to conventional materials, comprehensive immunotoxicity testing is necessary to confirm these findings across a range of potential applications.
The integration of quasicrystals into existing medical device manufacturing processes also raises safety considerations. Compatibility with sterilization methods, such as autoclaving or gamma irradiation, must be thoroughly assessed to ensure that the unique properties of quasicrystals are not compromised, potentially altering their biocompatibility profile.
As research progresses, the development of standardized protocols for evaluating the biocompatibility and safety of quasicrystal-based medical devices will be crucial. This may involve adapting existing testing methodologies or creating new ones specifically tailored to the unique characteristics of quasicrystalline materials. Collaboration between materials scientists, biomedical engineers, and toxicologists will be essential in addressing these complex challenges and realizing the potential of quasicrystals in medical device innovation.